Agminated presentation of fusion-driven melanocytic neoplasms
Abstract
Background
The conventionally understood pathogenesis of agminated Spitz nevi includes a mosaic HRAS mutation followed by copy number gains in 11p. However, we have recently observed agminated presentations of fusion-driven melanocytic neoplasms.
Methods
We retrieved cases from our database of benign fusion-induced melanocytic neoplasms with an agminated presentation. Both the primary lesion and the secondary lesion were sequenced. TERT-promoter mutational testing and the melanoma fluorescence in situ hybridization assay were also performed.
Results
Three cases were included. Two had a PRKCA fusion (partners ATP2B4 and MPZL1) and one had a ZCCHC8::ROS1 fusion. None of the cases met morphologic or molecular criteria for malignancy. There was no evidence of tumor progression in secondary lesions. The same fusion was identified in the primary and secondary lesions. None of the patients developed evidence of nodal or systemic metastasis.
Conclusions
We present accumulating evidence that fusion-driven melanocytic neoplasms can present with an agminated presentation. The differential diagnosis of an agminated presentation versus a locally recurrent or potentially locally metastatic tumor is critical, and accurate diagnosis has significant prognostic and therapeutic consequences for the patient. As with HRAS mutations, fusion-driven melanocytic tumors may have an agminated presentation.
1 INTRODUCTION
Agminated melanocytic nevi are characterized by the presence of multiple cutaneous clustered nevi.1-3 These lesions can arise on an underlying nevus spilus, normal-appearing background skin, after radiation or chemotherapy, and in immunosuppressed states.4-10 A commonly recognized pattern of agminated nevi is that of agminated Spitz nevi (ASN).11-19 The World Health Organization defines Spitz nevi as melanocytic nevi with characteristic spitzoid cytomorphologic features harboring an HRAS mutation/gain or a kinase fusion involving ALK, ROS1, NTRK1-3, RET, MET, BRAF, or MAP3K8.20-23 ASN have been shown to occur in the setting of nevus spilus as a result of a mosaic HRAS mutation, involving the entire area of the nevus spilus, along with copy number gains of 11p in the focal spitzoid proliferations.14, 16, 20, 24, 25
However, there is limited knowledge regarding the pathophysiology of ASN outside of the setting of nevus spilus, and the potential for genomic fusions to play a role in agminated nevi.11, 15, 26 In this study, we present the clinical, histologic, and molecular findings of three cases of agminated melanocytic neoplasms harboring genomic fusions and therefore propose that genomic fusions can play a role in the development of agminated Spitz or other fusion-mediated melanocytic neoplasms.
2 MATERIALS AND METHODS
2.1 Case selection
Study approval and waiver of consent for the use of deidentified health information were obtained through our University's Institutional Review Board (STU00001127). Our dermatopathology database was searched between 2017 and 2022 for melanocytic neoplasms with kinase fusion tumors identified through next-generation sequencing (NGS) at the time of clinical care. Only cases with multiple lesions at the time of presentation or with a localized recurrence after a wide local excision were included. In total, three cases were identified that met the above criteria.
2.2 Genomic sequencing
For all three cases, the primary lesions were sequenced at the time of clinical care. The recurrent lesion for Case 2 had sequencing performed retrospectively for research purposes. In Case 3, multiple lesions were sequenced at the time of the initial presentation. These cases were sequenced using the Tempus xT Panel and Fusion Plex Pan Solid Tumor v2. DNA and mRNA whole transcriptome sequencing was performed on the primary lesions from Cases 1 and 2 using Illumina HiSeq 4000 System with Tempus xT assay. The Tempus xT DNA sequencing platform included a 648 gene panel.27, 28 The primary lesion from Case 3 and the recurrent lesion from Case 2 were sequenced using Fusion Plex Pan Solid Tumor v2 assay. This panel detects fusion transcripts involving 142 genes associated with various cancers. The final sequencing occurred on the ION S5xl (Thermo Fisher Scientific).
2.3 RT-PCR
Case 3 underwent RT-PCR on one of the agminated lesions of the primary tumor. The MPZL1::PRKCA fusion and involved exons in the primary lesion were determined through the Fusion Plex pipeline software. Exon sequences were obtained from the genome browser, Ensembl (https://useast.ensembl.org/index.html, accessed on November 2022) to design fusion-specific primers. RT-PCR using SYBER green and melt curve analysis was performed using the primary tumor as a positive control.
2.4 Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) studies were performed as an additional test for melanoma at the time of clinical diagnosis in the primary lesion of Case 1. This analysis was performed as previously described,29-31 using probes targeting 6q23, 6p25, Cep6, 7p11, 8q24, 9p21, and 11q13. The recurrent lesion for Case 1 was assessed for a rearrangement of ROS1 (6q22) by FISH testing performed on the formalin-fixed paraffin-embedded (FFPE) tissue section. Deparaffinization and pretreatment of FFPE slides were done using the automated VP2000 Processor (Abbott Molecular) prior to running the FISH protocol following manufacturer's recommendation. The dual-color break-apart probe set from Kreatech (Leica Biosystems) designed to detect chromosomal rearrangements of ROS1 consists of 5′ and 3′ portions of the ROS1 gene labeled with SpectrumOrange (red) and SpectrumGreen (green), respectively. An abnormal result is determined if the percentage of cells with a separation of the 5′ and 3′ ROS1 due to a rearrangement is above the defined cut-off value for a positive result (15%) at 95% confidence.
2.5 TERT-promotor mutation
In all cases, TERT-promoter mutational analysis was assessed at the time of clinical care. In Cases 1 and 2, the primary lesions were tested using the Tempus xT. TERT-promotor mutations were analyzed by digital droplet PCR (ddPCR) for the primary lesion of Case 3 and the recurrence for Case 1. Primers and probes from Bio-Rad were specific for the two most common TPMs: c.-124C>T C228T and c.-146C>T C250T; Chr5:1295228 and Chr5:1295250, respectively. Poisson statistics and limited dilution of the target DNA partition into droplets allowed for the detection and quantification of the mutated allele burden without the need for a standard curve or a reference gene. Data were analyzed using the Bio-Rad QX200 droplet digital PCR system and Quanta Soft analysis software. The variant allele frequency (VAF) was determined by the following formula: VAF = (# of MUT events)/(# of MUT events + # of WT events) × 100. The sensitivity limit was set to 0.1%, which required a minimum of 150 copies/mL.
3 RESULTS
3.1 ROS1 fusion: Case 1
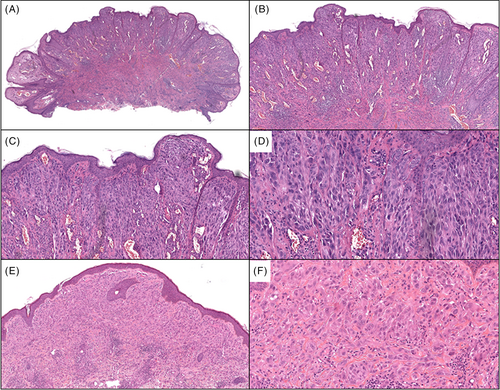
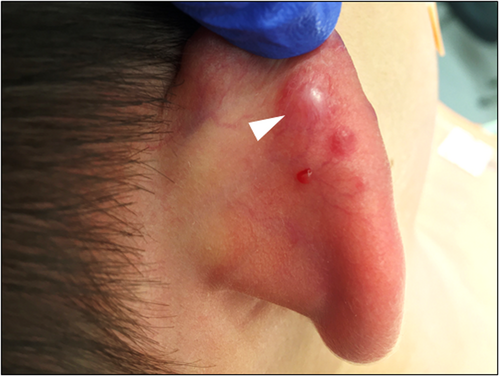
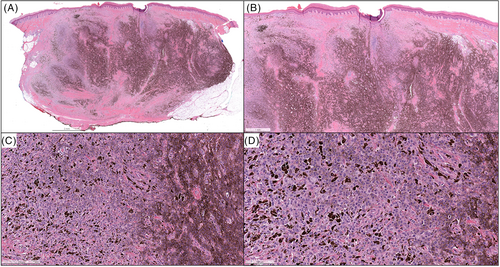
A 6-year-old male presented with a rapidly enlarging 15 mm well-defined red nodule on the right superior ear with a clinical impression of pyogenic granuloma (Table 1). Morphologically (Figure 1A–D), the lesion showed a compound polypoid and exophytic architecture with a junctional component surmounted by epithelial hyperplasia. Larger nests of melanocytes were present superficially and there was good maturation with descent. Cytology was epithelioid and spindle shaped with convincingly spitzoid features. The mitotic index was 3/mm2. PRAME immunohistochemistry (IHC) was negative. Whole exome RNA-sequencing identified a ZCCHC8::ROS1 gene fusion. FISH studies for melanoma were negative. TERT-promoter mutation and CDKN2A mutations assessed by NGS studies were negative. The tumor was excised with negative margins and a final diagnosis of ROS1 fusion atypical Spitz tumor was given. About 5 months later, the patient presented with four new red papules inferior and outside of the surgical scar (Figure 2), showing highly similar histopathologic (Figure 1E, F) features to the original biopsy. ROS1 break-apart FISH studies performed on the second lesion also identified a break-apart in the ROS1 gene. ddPCR showed no evidence of a TERT-promoter mutation. The lesion was re-excised with clear margins. Fourteen months after the initial recurrence, the patient presented with another red papule outside the original re-excision site. A wide excision was performed with clear margins and no evidence of distant disease was found in CT scans. An additional 4 months of follow-up showed no evidence of clinical recurrence thus far.
| Case | Age | Gender | Location | Clinical impression | Diagnosis | Follow-up (months) | Recurrence | Fusion |
|---|---|---|---|---|---|---|---|---|
| 1 | 6-year old | M | Right ear | 15 mm well-defined red nodule. R/o pyogenic granuloma | AST | 23 | Yes, four new red papules at 5 months after excision and one new red papule at 19 months after excision | ZCCHC8::ROS1 |
| 2 | 4-month old | F | Left foot | Multiple blue–black nodules. Thrombosed vascular malformation vs. atypical melanocytic neoplasm | PFMT-PEM | 18 | Yes, additional 2 mm solitary grayish blue papule at 13 months after excision | ATP2B4::PRKCA |
| 3 | 42-year old | F | Left shin | Several pigmented brown papules. Malignant melanoma with in-transit metastasis | PFMT-PEM | 2 | No | MPZL1::PRKCA |
| Mean follow-up time: 14.3 months | ||||||||
- Abbreviations: AST, atypical Spitz tumor; PFMT-PEM, PRKCA fusion melanocytic tumor-pigmented epithelioid melanocytoma.
3.2 PRKCA fusion: Cases 2 and 3
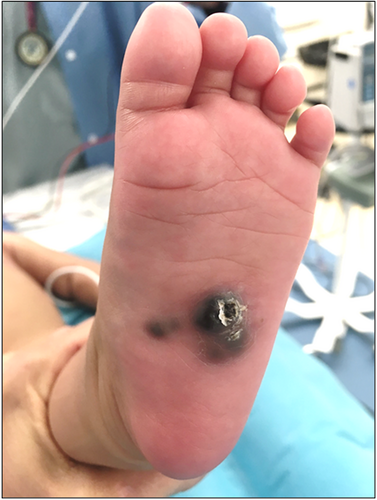
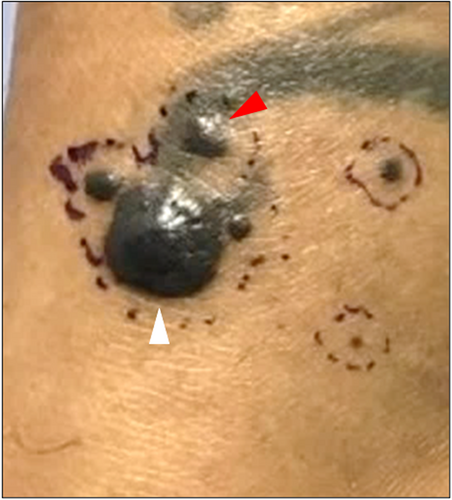
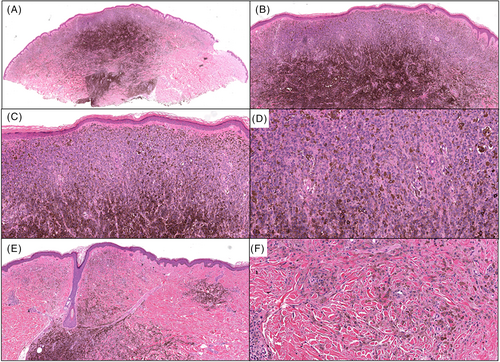
In Case 2, a 4-month-old female presented with multiple blue–black nodules on the left plantar surface (Figure 3) present since birth, while in Case 3, a 42-year-old female presented with several pigmented papules located on the left distal shin (Figure 4). The larger brown papule for Case 3 had been present for years with additional clustered pigmented lesions arising in the last year. The clinical impression for Cases 2 and 3 was thrombosed vascular malformation versus atypical melanocytic neoplasm and malignant melanoma with in-transit metastasis, respectively. The histology for Cases 2 (Figure 5) and 3 (Figure 6) were similar. Both cases showed predominately dermal melanocytic proliferations of pigmented epithelioid melanocytes of intermediate size forming a solid cellular nodule centrally. The cells were highly monotonous with vesicular nuclei, prominent nucleoli, and pigmented cytoplasm. In the more peripheral regions, there were smaller aggregates of similar epithelioid melanocytes as well as some dendritic melanocytes with melanophages separated by dense bundles of collagen. The agminated lesions in both cases showed identical histology with no signs of tumor progression. Whole exome RNA-sequencing identified an ATP2B4::PRKCA fusion for Case 2 and an MPZL1::PRKCA gene fusion on two of the lesions that underwent sequencing for Case 3. Pathogenic variants in TERT-promoter were not identified in NGS studies for Case 2 and in ddPCR for Case 3. In both cases, the tumor was completely resected with clear margins, with a final diagnosis of a PRKCA fusion melanocytic tumor that currently falls under the rubric of pigmented epithelioid melanocytoma. Approximately 13 months later, the patient from Case 2 presented with an additional 2 mm solitary grayish blue papule outside the scar from the original re-excision. This lesion showed similar histopathologic features to the primary lesion. Mutational profiling by NGS studies on the new lesion also identified an ATP2B4::PRKCA fusion. The lesion was re-excised with clear margins and there has been no evidence of clinical recurrence for an additional 5 months. For Case 3, the patient has had no evidence of recurrence during a brief follow-up period of 2 months.
4 DISCUSSION
For many years, mutations in HRAS were the only known pathogenic driver for Spitz neoplasms.32 Since then, HRAS has also been recognized as a driver for nevus spilus and investigators have shown that while the entire nevus spilus carries an HRAS mutation, agminated Spitz arising in a nevus spilus also have copy number gains in 11p, where the HRAS gene resides.14, 16, 20, 24, 25 Since these discoveries, much has been learned about the pathogenesis of Spitz including the fact that a significant majority of Spitz, as well as some other classes of melanocytic neoplasms such as PRKCA fusion melanocytic nevi/tumors, are the result of genomic fusions.20, 21, 33-35 However, there have been very few studies investigating whether fusions can play a role in agminated presentations of melanocytic neoplasms.
Understanding whether genomic fusions can play a role in the development of agminated Spitz has significant clinical consequences. Spitz tumors can cause great anxiety for clinicians and pathologists. Pathologists often have trouble definitively excluding the possibility of a malignancy. While the overwhelming majority of these cases have an excellent prognosis, they are often reported as tumors of uncertain malignant potential. The presence of a multiplicity of lesions or recurrence of a lesion outside the scar in the setting of a lesion of uncertain malignant potential may be interpreted as an indicator of malignancy with the recurrence being a localized metastasis. This raises a highly disparate potential differential diagnosis of benign agminated Spitz nevus/tumor versus clinical Stage 3 melanoma with local regional metastasis, which has a 5-year survival rate of 69% (i.e., AJCC eighth edition N2c).36 Considering the great disparity in prognosis and optimal therapy, being able to optimally distinguish the agminated disease from Spitz melanoma with local recurrence is critical.
In this study, we identified three cases in which the differential diagnosis included agminated Spitz, agminated PRKCA fusion melanocytic nevus/tumor, or melanoma with local recurrence. In all three cases, we favor the diagnosis of a fusion-based agminated melanocytic neoplasm. The concept of the presence of mosaicism of a gene fusion giving rise to multiple identical tumors has been demonstrated by other investigators both in cutaneous and non-cutaneous tumors. There have been several reports of gene fusion mosaicism related to renal cell carcinoma (RCC) development involving a chromosome 3 translocation.37-41 Bodmer et al. reported a familial case of RCC involving a t(2;3)(q35;q21) chromosomal translocation with mosaicism.37, 38 One patient developed three different anatomically distinct tumors on two different occasions, all of which were found to have a translocation-derived der(3) chromosome loss. Subsequent sequencing of the VHL tumor suppressor gene revealed distinct mutations in two of the three tumors, suggesting the tumors developed independently and not as metastases of a primary tumor. Non-cutaneous gene fusion mosaicism has also been demonstrated in acute lymphoblastic leukemia (ALL) including TEL::AML1, KMT2A::AFF1 (previously known as MLL::AF4) and BCR::ABL1 rearrangements.42-45 Interestingly, the TEL::AML1 (now referred to as ETV6::RUNX1) gene aberration has been shown to be prenatal in origin and is detectable in blood spots of 1% of randomly selected newborns.46-51 However, only approximately 1% of newborns harboring the translocation develop ALL,44, 52 suggesting that embryonic somatic mosaicism allows for the proliferation of mutations that can lead to neoplasia.
There are various other studies supporting the concept of gene fusions in ASN. Gene fusion mosaicism in ASN has been discussed in two reported GOPC::ROS1 positive cases11, 15 and in one case with a complex three-way rearrangement involving TRPM1, PUM1, and LCK.26 Goto et al. described two patients with clustered Spitz neoplasms harboring a GOPC::ROS1 fusion detected in the tumoral cells as well as in the normal-appearing basal melanocytes located in the interlesional epidermis.11 Similarly, the presence of a translocation in the melanocytes of the tumor and surrounding normal epidermis was also observed in a novel TRPM1::PUM1::LCK ASN case.26 Robertson et al. reported a case of facial ASN involving a GOPC::ROS1 kinase fusion.15 Sequencing results revealed a GOPC::ROS1 genetic rearrangement affecting chromosome 6 that was not present in DNA extracted from blood, suggesting that the Spitz nevi emerged from cutaneous genetic mosaicism.
In all three of our cases, identical gene fusions involving protein kinases were identified in both the primary and secondary lesions. In Case 1, the secondary lesions occurred following the excision of the initial lesion, while in Cases 2 and 3 the patient presented with multiple lesions. In all cases, there were no signs of tumor progression between the primary and secondary lesions. None of the primary lesions met histologic criteria for malignancy as the cytology was relatively bland, there was no evidence of ulceration, epidermal consumption or subcutaneous involvement. In the secondary lesions, the histologic grade was comparable with no evidence of greater cytologic atypia, increased mitotic count, necrosis, or other features typical of metastatic lesions. At the genomic level, the secondary lesions also lacked features suggestive of tumor progression. In all the tested cases, the primary and secondary lesions showed no evidence of TERT-promoter mutations and were negative for chromosomal copy number aberrations by the melanoma FISH assay.
All the patients continue to be followed clinically and none of them have had evidence of lymph node or systemic involvement and they all remain disease-free following re-excision. Overall, we believe the studies from Goto et al.11, 26 in conjunction with our current study, support the concept that some agminated melanocytic neoplasms including agminated Spitz or agminated PRKCA fusion melanocytic nevi/tumors are the result of mosaic gene fusions. Longer follow-ups and a larger study with additional cases will further help support this hypothesis. However, we believe that since the consequence of overdiagnosis of an agminated Spitz or PRKCA fusion melanocytic nevi/tumors can result in considerable overtreatment of patients with benign lesions, it is important to report these findings at this time. We believe that this is another example of how understanding molecular genomics and the pathogenesis of various subtypes of melanocytic neoplasms can result in considerable advances in our diagnostic acumen and improve patient quality of care.
ACKNOWLEDGMENTS
This work was supported by the IDP Foundation.
CONFLICT OF INTEREST STATEMENT
Dr. Pedram Gerami has served as a consultant for Castle Biosciences and has received honorarium for this. Other authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by of our Institutional Review Board (IRB) (STU00001127). Given the retrospective chart review design, a waiver of HIPPA authorization was granted for research purposes and informed consent was not required for this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




