Mucin as a diagnostic clue in dermatopathology
Abstract
Mucins are high-molecular weight glycoproteins typically found in normal skin in small amounts. There are several reports regarding different types of cutaneous mucinosis. In this report, we discuss mucins in dermatopathology as a diagnostic clue of some well-known entities and some less frequent cutaneous diseases. We also emphasize mucin as a sign in the differential diagnosis between conditions that show histopathological overlap. Lastly, we discuss the locations and circumstances in which mucin could be considered almost normal or physiological.
Mucins, proteoglycans and mucinoses
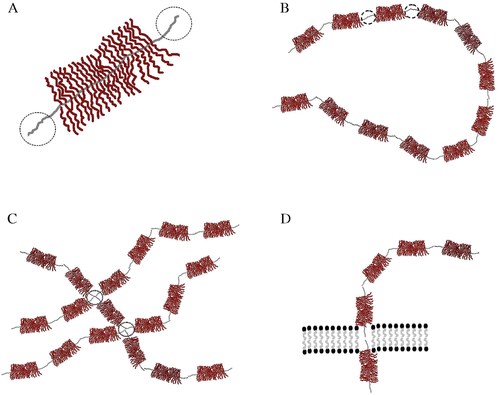
Mucins are high-molecular weight glycoproteins. Although texts usually insist that ‘mucin,’ ‘proteoglycan’ and ‘glycoprotein,’ are interchangeable terms, ‘proteoglycan’ commonly refers to molecules in which the carbohydrate component represents the vast majority of the molecule (up to 95%). Some other authors prefer to restrict the term ‘mucin’ to refer only to the intracellular glycoproteic molecules and the term ‘proteoglycan’ to refer to the mucins that are free in the stroma. Proteoglycans are made up of a central protein core to which many chains of polysaccharides are attached (Fig. 1A). Mucins are classified into families according to the sequence of the proteic core, which has carboxyl and hydroxyl terminal endings. Units made up of a central proteic core plus branches of polysaccharides can join together by covalent bonds to give rise to a complex lineal structure (Fig. 1B). However, the proteic areas close to the covalent bond are devoid of carbohydrates, which allows hydrophobic bonds to also be established between lineal complex chains to form ultra-complex nets of lineal molecules (Fig. 1C). Also, the hydrophobic parts of the molecules are easily integrated into the cell membrane to form the transmembrane mucins (Fig. 1D). The glycosylated part of stromal mucins is made up of glycosaminoglycans (GAG). The main GAGs are hyaluronic acid, chondroitin sulfate, dermatan sulfate, keratin sulfate and heparan sulfate. The dermis is rich in hyaluronic acid and in chondroitin sulfate.1
Mucinoses are a group of conditions in which mucin accumulate in the skin, in a focal or in a diffuse pattern. Among the focal mucinoses, the main conditions are myxoid cyst, cutaneous focal mucinosis, cutaneous mucinosis of infancy, self-healing cutaneous mucinosis, papular and nodular mucinosis associated with lupus erythematosus, acral persistent papular mucinosis and lichen mixedematosus (the discrete papular form). The diffuse forms include the generalized variant of lichen myxedematosus, the scleredema, the reticular erythematous mucinosis, the generalized myxedema and the pretibial myxedema. Follicular mucinosis is usually classified as a separate entity, which includes the recently described nevoid follicular mucinosis2 as well as the urticaria-like follicular mucinosis.
Some authors include additional forms of mucinoses, such as the myxoma and the angiomyxoma, under the category of neoplastic mucinoses.3
Mucin and proteoglycans in normal skin
From a structural point of view, mucins and proteoglycans can be divided into three categories: epithelial, endothelial and stromal. The epithelial group comprises 15 members (MUC1–8, MUC12, MUC13, MUC15–17, MUC19 and MUC20), which are subclassified into two subtypes: membrane-bound and secreted mucins.4-7 The endothelial group includes CD34, CD45RA, GlyCAM-1 and PSGL-1 (CD162).8 The stromal group is mainly represented by dermatan sulfate, chondroitin sulfate and hyaluronic acid.
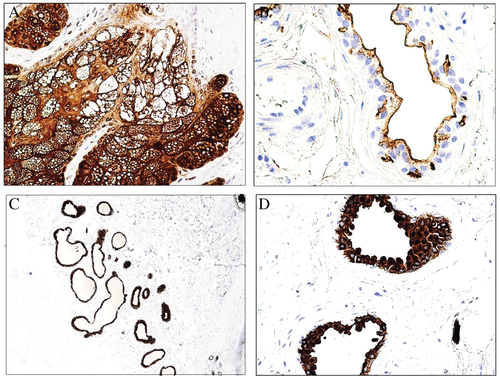
The normal epidermis does not express any of the epithelial mucins of the families MUC1, MUC2, MUC5AC or MUC6, but weakly expresses MUC4. MUC1 is expressed by the luminal surfaces of the eccrine and apocrine glands as well as by the secretions of the sebaceous glands and weakly by the cytoplasm of the apocrine glands (Fig. 2). The marker is mainly expressed by the secretory portion of the glands. The ducts are either negative or weakly positive. MUC1 is also expressed by Merkel cells and dermal lymphocytes.
The reticular dermis has small amounts of stromal mucins, and they are difficult to see with hematoxylin-eosin staining, depending on the balance staining of each laboratory. However, in our experience this perception can be highly deceptive, and confirmation with special histochemical stains is recommended.
Mucins can be prominent in the papillary and adventitial dermis (Fig. 3) as well as in the hair papilla of anagen hair. They are mainly non-sulfated acid mucins (such as hyaluronic acid). However, under certain reactive circumstances (a reparative wound, for instance), sulfated mucins can appear (chondroitin and dermatan sulfates).9
Mucin in the reticular dermis can be found on the legs as a person ages. However, this is not a physiological condition, and it is usually associated with cutaneous stasis changes. In stasis changes, the papillary dermis shows prominent fibrosis and expansion with mucin deposition, which extends to the dermoepidermal junction with no Grenz zone. This is accompanied by angiodysplasia and hemosiderin deposition10 (Fig. 4). Patients are usually older (most are above age 60),10, 11 although patients in their thirties have been described.10 These cases are not associated with Graves' disease. On the contrary, pretibial myxedema associated with Graves' disease shows a Grenz zone, whereas mucin is mainly deposited in the upper half of the reticular dermis (although the lower papillary dermis and the lower reticular dermis can also be involved in some cases) (Fig. 5). Angioplasia and hemosiderin deposits are not common in pretibial myxedema.
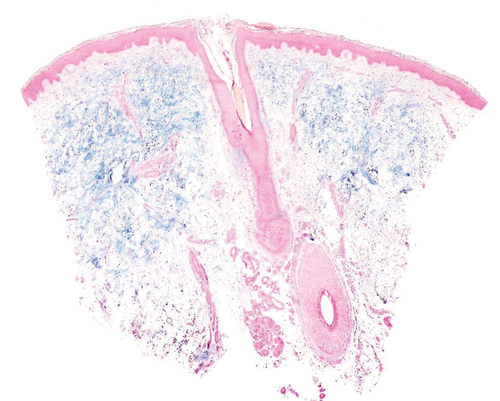
Because stasis dermatitis usually shows a prominent vascular component, one of the differential diagnoses to consider might be superficial angiomyxoma, which also shows prominent mucin dermal deposits (Fig. 6).11 However, the clinical presentation of the latter is usually different, with lesions confined to the head, neck or trunk.12, 13 Moreover, the mucin usually extends to the hypodermis, and a sparse inflammatory infiltrate containing lymphocytes and neutrophils is evident. Up to 30% of cases show entrapped epithelial components such as keratinous cysts or nests of basaloid cells.12, 13
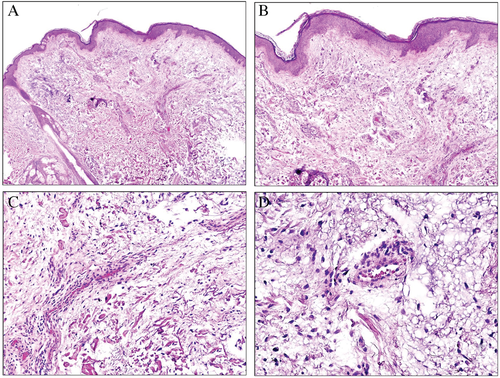
Also there is a condition described as obesity-associated lymphedematous mucinosis, (rongioletti obesity associated) in which pretibial mucinosis is associated with morbid obesity and lymphoedematous features of the legs. Clinically, it presents with semitranslucent pretibial papules and/or nodules and sometimes vesicles. The biopsy shows a hyperkeratotic and atrophic epidermis with oedema of the superficial dermis and mucin deposition. This is accompanied by angioplasia in the upper part of dermis and fibrosis in the reticular dermis. The interesting point is that the condition is partly reversible if the individuals lose weight. Additional studies, however, are necessary to rule out that the condition is not a mere form of mucinosis related to stasis.
Granuloma annulare
Granuloma annulare is a classic disease in which stromal deposits of mucin are prominent. The mucin is evident in the center of the granuloma, primarily in large amounts. Mucin deposits are commonly found in the different variants of granuloma annulare, such as perforating or elastolytic.
There are two variants of granuloma annulare in which evidence of mucin deposits in the granuloma can be especially helpful. One is deep granuloma annulare14 (Fig. 7). This variant is also known as a pseudorheumatoid nodule,15 a term that makes reference to its morphologic similarities to a rheumatoid nodule. However, deep granuloma annulare does not have the same clinical connotation as a rheumatoid nodule: no clinical association has been proven between deep granuloma annulare and rheumatic disease or any of the collagen autoimmune diseases.16-19 In some texts, it is claimed that the morphology of rheumatoid nodules is identical to that of deep granuloma annulare. However, other authors have noted certain important differences; for example, deep granuloma annulare tends to be made up of several individual granulomas that show a tendency for confluence (Fig. 7A). As with other variants of granuloma annulare, the center of such a granuloma commonly shows a significant amount of mucin (Fig. 7B), which contrasts with the absence (or presence in very small amounts) of mucin in rheumatoid nodules. The latter instead shows a more dense eosinophilic type of central necrosis. Also, in deep granuloma annulare, a significant amount of eosinophils in the inflammatory infiltrate has been described (Fig. 7C). Lastly, prominent small blood vessels are common in the periphery of deep granuloma annulare (Fig. 7D).
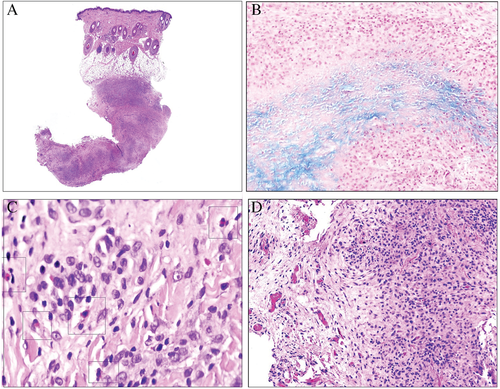
The other variant of granuloma annulare in which mucins can have diagnostic importance is interstitial granuloma annulare. This variant is not always easy to show because histiocytes are distributed in a scattered form in the dermis (Fig. 8).20-22 This pattern can mimic that of a xanthoma, Kaposi sarcoma,23 or mycobacterium infection.24 In these cases the accompanying mucin deposits can help to reinforce a diagnostic suspicion of the disease. Heparan sulfate has been demonstrated in the interstitium of the inflammatory infiltrate of granuloma annulare.25 In difficult cases the histiocytic nature of the infiltrate can be corroborated with the appropriate immunohistochemical markers.26, 27
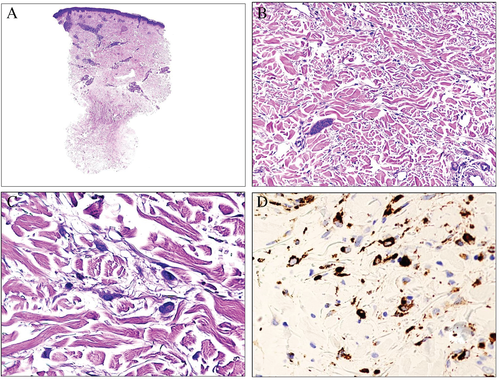
Lupus erythematosus and its imitators
Owing to several clinical and histopathological imitators, dermal deposits of mucin are very helpful in the appropriate context to reinforce a diagnosis of lupus erythematosus. Deposits of mucin can appear early in the disease in cases of acute lupus and become prominent in cases of either subacute or chronic lupus erythematosus28, 29 (Fig. 9).
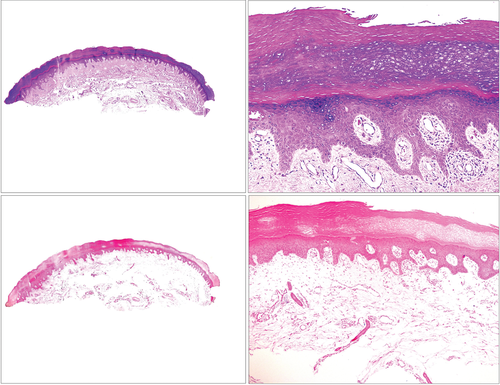
Polymorphous light eruption can clinically mimic lupus erythematosus: lesions appear on sunlight-exposed sites, and patients with this condition are predominately female. In contrast to lupus erythematosus, there are no mucin deposits in the reticular dermis. Moreover, polymorphous light eruption often involves edema of the papillary dermis, which can sometimes be very prominent (Fig. 10). In an experimentally induced study conducted in 1987, the authors showed how slight papillary edema was already evident a few hours after irradiation, but they did not find mucin at any stage of the disease.30 Although polymorphous light eruption can present in several histologic variations – including plaque type, erythema multiforme type, hemorrhagic type, insect bite type, ictus type or vesiculobullous type – mucin is not found in any of these variants.30
Dermatomyositis can histopathologically mimic lupus erythematosus to the point that many texts emphasize that a differential diagnosis between acute cutaneous lesions of systemic lupus erythematosus and dermatomyositis cannot properly be done unless an appropriate clinical correlation is made.31 This morphological overlap includes the dermal deposit of mucin, which can be found in both diseases.31 McNiff et al.32 found differences in the pattern of distribution of plasmacytoid dendritic cells in dermatomyositis and in lupus erythematosus, but these findings could not be reproduced by other investigations.33
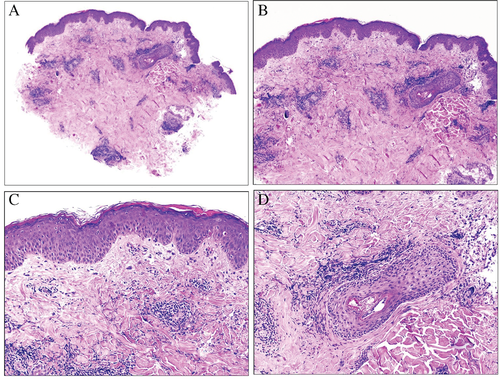
One less-investigated aspect of dermatomyositis is the morphology of Gottron's papules, primarily because they are rarely biopsied because of their clinical identification as such. In the literature, the main changes described are hyperkeratosis, hypergranulosis, acanthosis and a perivascular chronic inflammatory infiltrate that can sometimes spill over the basal layers of the epidermis and is accompanied by cytoid bodies. It has been showed that lymphangiectases are also a component of Gottron's papules34 (Fig. 11). Nevertheless, to the best of our knowledge, mucin has not been investigated in Gottron's papules. We have had the opportunity to investigate mucin deposits with Alcian blue in the dermis from a biopsy of a Gottron's papule from a 64-year-old woman with dermatomyositis, and we have not seen any deposits (Fig. 11).
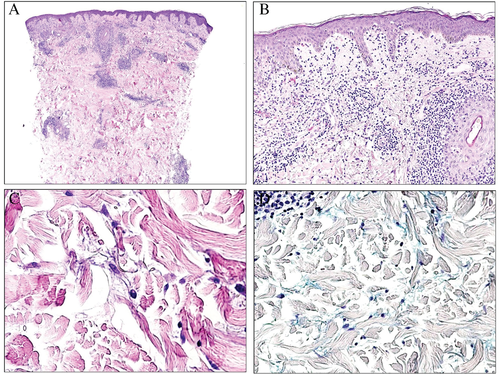
Equestrian cold panniculitis is another disease that can histopathologically mimic lupus erythematosus, including large amounts of interstitial patchy or pandermal mucin (Fig. 12).35 The condition is included among the so-called cold panniculitis, which are a form of perniosis. Although in some of these conditions, mucin can be evidenced in the dermis, the clinical presentation is usually very different. In equestrian cold panniculitis, for instance, the lesions present as violaceous plaques involving the superolateral aspects of the thighs of young women who have been exposed to cold temperatures.36 Pernio – considered as a form of lupus erythematosus by some authors – presents as papules, vesicles or even ulcerations on acral parts, such as nose, ears or toes. Some histopathologic additional clues are sometimes evidenced. For instance, equestrian cold panniculitis shows dense inflammatory lymphocytic infiltrate permeating the walls of the blood vessels, which also often show endothelial swelling (Fig. 12D).36-41 Pernio can sometimes present with thrombi in the blood vessels.
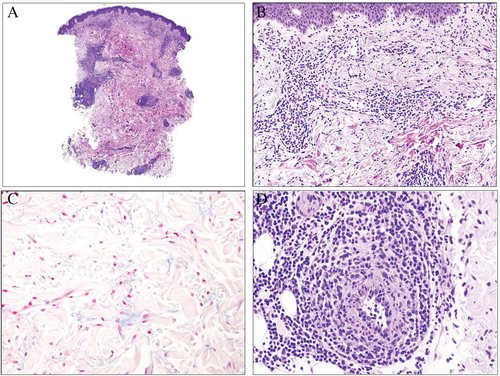
Bloom's syndrome is a rare genodermatosis of autosomal recessive inheritance that presents clinically with dwarfism and telangiectatic erythema of the face.42 The histopathological findings in the skin are similar to that of lupus erythematosus, including superficial and deep perivascular and periadnexal lymphocytic inflammatory infiltrate with moderate amounts of mucin in the dermis.43 However, immunofluorescence studies on Bloom's syndrome have shown no IgG, IgA, C3 or C5b-9 skin complexes, and serologic assays for antinuclear antibodies, double-stranded DNA, autoantibodies and complement levels are within normal limits.43
Paget's disease
Mammary Paget's disease (PD) is most often associated with an underlying ductal carcinoma, whereas the association of extramammary PD with a carcinoma is not so certain. In fact, a primary form of extramammary PD is distinguished from a secondary form. Primary extramammary PD is thought to arise either from the intraepithelial part of the cutaneous adnexal glandular epithelium or from Toker cells. In secondary extramammary PD, the associated neoplasm can sometimes be distant from the breast, such as in the genitourinary or gastrointestinal tracts.44-46
PD can easily be suspected with routine hematoxylin-eosin staining, but its confirmation usually requires immunohistochemical studies to distinguish it from melanoma or Bowen's disease. Paget cells express cytokeratins (in contrast with melanoma) and are cytokeratin-7 positive (in contrast with Bowen's disease). Immunohistochemistry can also help in the conundrums of mammary vs. extramammary PD and primary vs. secondary extramammary PD.
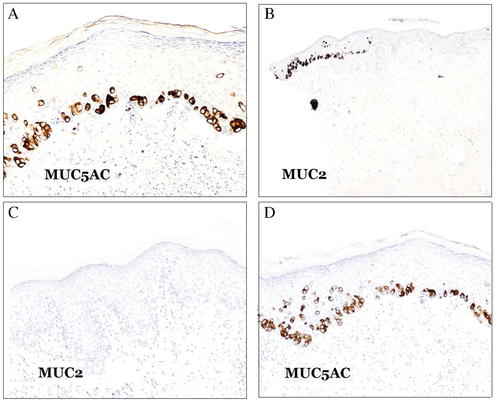
The three main types of mucin used in the differential diagnosis of PD are MUC1, MUC2 and MUC5AC, which are also known as mammary, intestinal and gastric surface-type mucins, respectively. MUC1 is also known as epithelial membrane antigen (EMA) or polymorphic epithelial mucin (PEM), and it is encoded by the MUC1 gene. Most anti-MUC1 antibodies target the tandem repeats array domain of the extracellular alpha subunit.47-49
Mammary PD is commonly negative for MUC5AC, whereas extramammary PD usually expresses the marker50 (Fig. 13A). MUC2 is negative in mammary PD as well as in primary extramammary PD, whereas secondary extramammary PD usually expresses MUC2 (Fig. 13B). Perhaps cases with MUC5AC+/MUC2+ merit clinical investigation for a hidden underlying carcinoma in addition to immunohistochemical investigation with markers traditionally suggestive of certain anatomical sites (CDX2 and CK20 for the gastrointestinal tract; PSA for the prostate; and WT-1, PAX8, CA-125 and hormonal receptors for the genital tract). It is remarkable that PD in the breast is not equivalent to mammary PD. When PD in the breast is associated with an underlying breast carcinoma, it usually shows an MUC2−/MUC5AC− phenotype, whereas the phenotype is usually MUC2−/MUC5A+ in cases without underlying breast carcinoma (extramammary primary-type PD) (Fig. 13C,D). Some of these cases have presented with multiple lesions involving not only the nipple but also the genital area.51
Pseudomyxoma cutis
Pseudomyxoma cutis was presented by Terada52 as a novel entity. Their study describes a cutaneous metastasis from an anal mucinous adenocarcinoma. However, cutaneous metastases from mucinous adenocarcinomas have been previously reported in the literature,53, 54 and the use of a novel term does not seem justified for such metastases.
In addition, cases of pseudomyxoma peritonei with subsequent cutaneous fistulae55, 56 have also been described.
Cutaneous metastasis from a mucinous adenocarcinoma should be distinguished from a primary cutaneous mucinous carcinoma, which usually presents with high rates of local recurrence but low chances of distant metastasis.57 The latter usually presents in elderly patients as a single lesion located on the head and neck.57 In this sense, immunohistochemistry can help in distinguishing a metastasis from a primary cutaneous mucinous adenocarcinoma. D2-40 has proved to be useful in identifying lymphatic invasion, which is indicative of a metastasis,55 and some studies have suggested that p63 immunoexpression could be indicative of a primary cutaneous tumor instead of metastasis.58, 59 Cytokeratin (CK) 5/6 immunoexpression has also been presented as suggestive of a primary cutaneous carcinoma.60 Kazakov et al.61 presented the largest series on mucinous carcinomas and concluded that the best clue suggesting a primary cutaneous neoplasia was evidence of an in situ component.
Mucin in less frequent cutaneous conditions
Necrotizing infundibular crystalline folliculitis is a rare entity that may or may not be related to the hair follicles.62-64 This disease usually presents as waxy, yellowish papules. Histopathologically, the papules correspond to necrotic material with abundant mucin and needle-shaped organic birefringent crystals, sometimes related to the infundibulum and sometimes to transepidermal elimination. Prominent dermal mucin deposits have also been described.64
Erythromelalgia is a somewhat mysterious and often histopathologically deceptive condition in which patients suffer from episodic burning erythema and burning from acral sites. Biopsy in these cases often does not show any conclusive morphological findings. Recently, a case of erythromelalgia was presented in which large mucin deposits were evident inside the walls of dermal arterioles as well as in a perivascular location.65
Cases of follicular mucinosis-associated with alopecia areata have been published on only two occasions.66, 67 Curiously, both cases presented with a very high CD4:CD8 ratio in the inflammatory infiltrate (over 20), which is unusual for alopecia areata.
Myxoid variant of anaplastic large cell lymphoma has rarely been reported in the literature.68 In 1990, Chan et al. reported a lesion on the leg of a 45-year-old man with a sarcomatoid pattern mimicking a myxoid malignant fibrous tumor but expressing Ki-1 antigen.69 Since then, very few additional reports have been published.68 These cases show atypical lymphoid tumoral cells in a myxoid background, accompanied by a mixed inflammatory infiltrate.68
Conclusions
We have presented an approach to some aspects of mucins in dermatopathology that are not often discussed in literature, such as immunohistochemical use in the diagnosis of certain conditions, including some rare and controversial diseases.




