Comparable kidney transplant outcomes in selected patients with a body mass index ≥ 40: A personalized medicine approach to recipient selection
Abstract
Introduction
Many kidney transplant (KT) centers decline patients with a body mass index (BMI) ≥40 kg/m2. This study's aim was to evaluate KT outcomes according to recipient BMI.
Methods
We performed a single-center, retrospective review of adult KTs comparing BMI ≥40 patients (n = 84, BMI = 42 ± 2 kg/m2) to a matched BMI < 40 cohort (n = 84, BMI = 28 ± 5 kg/m2). Patients were matched for age, gender, race, diabetes, and donor type.
Results
BMI ≥40 patients were on dialysis longer (5.2 ± 3.2 years vs. 4.1 ± 3.5 years, p = .03) and received lower kidney donor profile index (KDPI) kidneys (40 ± 25% vs. 53 ± 26%, p = .003). There were no significant differences in prevalence of delayed graft function, reoperations, readmissions, wound complications, patient survival, or renal function at 1 year. Long-term graft survival was higher for BMI ≥40 patients, including after adjusting for KDPI (BMI ≥40: aHR = 1.79, 95% CI = 1.09–2.9). BMI ≥40 patients had similar BMI change in the first year post-transplant (delta BMI: BMI ≥ 40 +.9 ± 3.3 vs. BMI < 40 +1.1 ± 3.2, p = .59).
Conclusions
Overall outcomes after KT were comparable in BMI ≥40 patients compared to a matched cohort with lower BMI with improved long-term graft survival in obese patients. BMI-based exclusion criteria for KT should be reexamined in favor of a more individualized approach.
1 INTRODUCTION
According to recent estimates, more than 42% of adults in the United States (US) meet criteria for obesity, with 9.2% meeting criteria for severe obesity as defined by a body mass index (BMI) of 40 kg/m2 or greater.1, 2 The highest rate of obesity is among non-Hispanic black adults. Increases in mean BMI have been even steeper among those with end stage renal disease (ESRD) compared to the US population as a whole in recent years.3 The kidney transplant waitlist in the US includes more than 90 000 patients listed for transplant and greater than 40% of those patients have a BMI of 30 kg/m2 or greater, placing them in the “obese” category. Nearly 18% have a BMI of 35 kg/m2 or greater.4
Obese patients continue to face decreased access to kidney transplant. BMI ≥40 kg/m2 is commonly used by many US transplant centers to decline patients for kidney transplant evaluation and listing. Published evidence has linked high BMI to a higher proportion of adverse outcomes following kidney transplantation including inferior patient and graft survival, higher incidence of delayed graft function, and greater rates of wound infections and incisional hernias.5-11
However, while high BMI recipients may face greater risks than their lower BMI counterparts, many studies have demonstrated improved survival for these patients compared to remaining on dialysis, and complications arising from obesity in the peri-operative period may be managed successfully without lasting sequelae.12-15 A comparison of 1-year post-transplant survival rates showed a 46%–66% reduction in mortality in obese patients compared to waitlisted patients depending on whether a deceased donor or living donor transplant was performed.16
BMI is a crude measure of body size and habitus, which does not take into account the distribution of muscle and excess fat. Using this metric alone as an exclusionary factor for transplant listing not only ignores mounting evidence that those patients with a higher BMI can have acceptable long-term outcomes, but also neglects more granular evaluation and selection practices. At our center, a strict BMI cutoff is not utilized for kidney transplant listing. Our approach is to use computed tomography (CT)-based criteria for selection, with image-based measurement of the depth of the iliac vessels and the angle and width of the pelvis to assess technical risk of performing a kidney transplant in the overweight or obese candidate. Herein, we report our retrospective experience using this method in the transplant evaluation process and compare post-transplant outcomes between patients with a BMI ≥40 kg/m2 to a matched BMI < 40 kg/m2 cohort.
2 METHODS
2.1 Study design
We performed a single center retrospective analysis of adult kidney transplant alone recipients transplanted at our center between 2006 and 2020. During this period, we performed kidney transplants on a total of 84 patients with a BMI ≥40 kg/m2. These patients were matched to a cohort of 84 patients with a BMI of < 40 kg/m2. Matching was performed based on recipient age, gender, race, diabetes status, and type of donor (living or deceased).
2.2 Recipient characteristics
Additional recipient characteristics compared between study groups included duration of dialysis, calculated panel reactive antibody (cPRA) level prior to transplant, and whether the recipient had undergone a previous kidney transplant.
2.3 Donor characteristics
Donor characteristics analyzed included living or deceased donor, donor age, Kidney Donor Profile Index (KDPI), donation after cardiac death (DCD), and cold ischemic time (CIT). KDPI, DCD status, and CIT were compared for deceased donor transplants only.
2.4 Assessment of body habitus
At our center, all patients undergo pre-transplant non-contrast abdominal and pelvic CT scanning as part of their standard work-up. In addition, iliac duplex ultrasonography is performed in many patients based on risk factors or evidence of vascular disease to evaluate patency and disease burden in the iliac vasculature. These screening studies provide us with an excellent “roadmap” for surgical planning. Physical examination is useful in assessing (1) the presence and size of a pannus, (2) whether the patient “flattens out” when placed in the supine position, and (3) how much of the body weight is posterior. In patients with a large pannus, the medial portion of the transplant incision is intentionally placed well above the symphysis pubis and pannus in order to promote wound healing. This results in an incision that is more transverse than vertical. On rare occasions, we may send the patient in consideration for panniculectomy, particularly if there is an exceedingly thick subcutaneous layer (>6–8 cm). However, in most cases, panniculectomy may not be helpful in influencing transplant suitability in the absence of weight loss because of the internal anatomy.
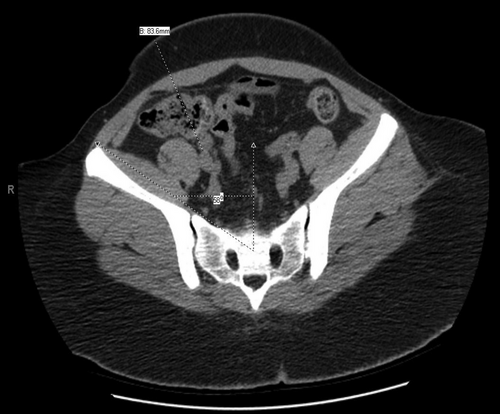
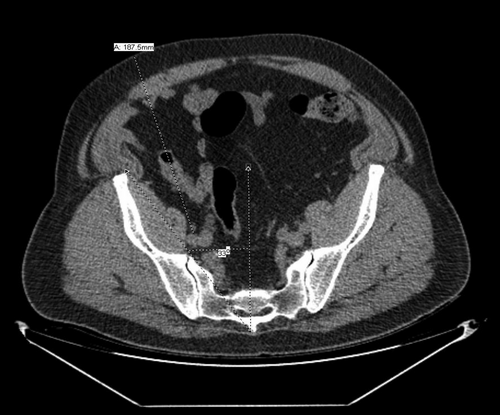
Measurements of external iliac vascular depth and pelvic angle on CT scan imaging permit a level of quantitation beyond external physical assessment, which may not be fully revealing. Female patients tend to have shallower iliac vessels and wider pelvic angles whereas the typical male android pelvis tends to be narrower with deeper vessels in the setting of obesity. Using cross-sectional CT imaging, we identify a target just below the common iliac bifurcation in the proximal to mid-external iliac artery and vein. We then use the “measurements” function to draw a straight line from the skin through the lateral edge of the rectus muscle to the site between the external iliac artery and vein to determine “iliac depth” for transplant (Figures 1 and 2). Historically, we consider an iliac depth of >18 cm to be a technical contraindication to kidney transplantation and recommend weight loss. We additionally determine the “pelvic angle” at this same level based on cross-sectional CT scan imaging. As seen in Figures 1 and 2, the pelvic angle is a measure of the width of the ilium compared to midline. A pelvic angle of ≤32 degrees is considered narrow, 33–38 degrees is moderate, and >38 degrees is wide. In our experience, pelvic angle is as important as iliac depth in determining transplant suitability. The ability to gain medial and lateral exposure in the depths of the pelvis can be critical to the success of transplantation. These CT metrics are used to determine technical feasibility of transplantation irrespective of BMI. In Figure 1, a patient with a BMI = 45 kg/m2 has an extremely wide pelvis and shallow iliac depth measured at 8.3 cm, which is favorable for transplantation irrespective of BMI. Figure 1 also provides a helpful demonstration of a patient with a more posterior distribution of fat. In Figure 2, a patient with a BMI = 36 kg/m2 has a narrow pelvis and deep vessels with depth of iliac bifurcation measured at approximately 19 cm (“steep and deep”). This would typically represent a contraindication to transplant irrespective of the BMI. Figure 2 also demonstrates a patient who has predominantly a visceral or internal distribution of fat compared with subcutaneous distribution, which results in a greater challenge of achieving adequate exposure at the time of transplant. However, in our experience, a BMI > 45 kg/m2 is often a relative contraindication and a BMI ≥48 kg/m2 is an absolute contraindication to transplantation because at these extremes the CT metrics typically are unfavorable.
2.5 Surgical technique
Fascia closure was performed in both groups according to standard clinical practice with approximately half of patients receiving a 2-layer fascial closure (if no prior surgical procedure in that location) versus 1-layer closure with a running, synthetic, slowly absorbable, monofilament suture such as #1 PDS according to individual surgeon's preferred technique. Retention sutures were not utilized at the time of the initial surgical procedure. Generally, all patients received a single active closed drain placed adjacent to the kidney in the retroperitoneal space and exiting through a separate stab incision in the flank, either a 19 round silicone (Blake) drain or a 10 French flat (Jackson-Pratt) drain. In < 10% of cases in each cohort, a second active closed drain was placed in the subcutaneous space. Closed-incision negative pressure therapy dressings similarly were used in less than 10% of patients with no differences according to BMI group. As such, this study lacked power to assess any impact of these adjunctive wound closure therapies on wound outcomes.
2.6 Post-operative outcomes
Peri-operative outcomes compared included incidence of delayed graft function (DGF), defined as dialysis for any reason within the first 7 days of transplant, hospital length of stay (LOS) for the transplant admission, re-operation and re-admission rates at 30 and 90 days post-transplant, and wound complications, which is a composite measure including hematoma, superficial or deep wound infection, dehiscence within 90 days post-transplant, and incisional hernia within 1 year post-transplant. Additionally, we compared 1-year post-transplant outcomes including: weight and BMI change at 1 year, incidence of post-transplant diabetes, hemoglobin A1c level, patient and graft survival, and serum creatinine and estimated glomerular filtration rate (eGFR) according to the chronic kidney disease (CKD)-EPI calculation. Finally, we compared long-term graft and patient survival rates for each cohort, according to time since transplant.
2.7 Statistical analysis
Continuous variables were described with means and standard deviations (SD) and were compared according to Students t-tests and one-way ANOVA. Categorical variables were described according to proportions with percentages and compared according to Chi-square and Fisher's exact tests. Matching was performed according to propensity score methods using nearest neighbor with caliper = .2 with no replacement according to recipient variables outlined above. Patient and graft survival rates were compared utilizing Kaplan-Meier curves and log-rank tests. Cox proportional hazards regression was performed among deceased donor kidney transplants alone to compare survival after controlling for KDPI given noted differences between groups. A two-sided p-value of < .05 was considered to be significant. All analyses were performed with STATA software (version 15.1, College Station, TX, USA).
2.8 Ethics approval
This study was reviewed and approved by the Institutional Review Board of Wake Forest Baptist Medical Center (IRB#00084139).
3 RESULTS
3.1 Recipient characteristics
The matched cohorts each included 84 patients. Characteristics for which patients were matched (age, gender, race, diabetes status, and living vs. deceased donor) did not significantly differ between groups after matching and are shown in Table 1. Approximately half of patients were Black and 5% Hispanic in each group. Average BMI in the ≥40 group was 42 ± 2 versus 28 ± 5 kg/m2 in the BMI < 40 group (p < .01). Average weight was 111 ± 19 kg in BMI ≥40 group compared to 79 ± 16 kg in the BMI < 40 group (p < .01). BMI ≥40 patients spent significantly more time on dialysis than those with a BMI < 40 (BMI ≥40: 5.2 ± 3.2 years vs. BMI < 40: 4.1 ± 3.5 years; p = .03) and were less likely to have had a previous kidney transplant (BMI ≥40: 6% vs. BMI < 40: 17%; p = .03). Actual time on waitlist was not significantly different according to BMI group (BMI ≥40: 2.1 ± 1.8 years vs. BMI < 40: 2.8 ± 3.0 years; p = .11) A cPRA level > 80% was present in 14% of BMI ≥40 patients compared to 19% of BMI < 40 patients (p = .41) (Table 1).
| BMI≥40 | BMI < 40 | ||
|---|---|---|---|
| (n = 84) | (n = 84) | p-value | |
| Recipient characteristics | |||
| Agea, mean ± SD (years) | 48 ± 13 | 48 ± 13 | .97 |
| Female | 65% | 67% | .87 |
| Race/ethnicity | |||
| White | 41% | 48% | .76 |
| Black | 52% | 47% | |
| Hispanic | 6% | 4% | |
| Other | 1% | 1% | |
| BMI (kg/m2) | 42 ± 2 | 28 ± 5 | <.01 |
| Weight (kg) | 111 ± 19 | 79 ± 16 | <.01 |
| Diabetes | 39% | 35% | .52 |
| Dialysis time, mean ± SD (years) | 5.2 ± 3.2 | 4.1 ± 3.5 | .03 |
| Waitlist time, mean ± SD (years) | 2.1 ± 1.8 | 2.8 ± 3.0 | .11 |
| cPRA > 80% | 14% | 19% | .41 |
| Previous transplant | 6% | 17% | .03 |
| Donor characteristics | |||
| Living donor | 10% | 7% | .58 |
| Donor age, mean ± SD (years) | 35 ± 15 | 42 ± 15 | .01 |
| KDPI, mean ± SD (%)b | 40 ± 25 | 53 ± 26 | .003 |
| KDPI ≥ 85%b | 4% | 13% | .04 |
| DCDb | 30% | 24% | .41 |
| CIT, mean ± SD (h)b | 23 ± 11 | 27 ± 16 | .05 |
- a Recipients matched according to age, gender, race, diabetes, donor type (living vs. deceased).
- b KDPI, DCD, CIT for deceased donor kidneys only.
3.2 Donor characteristics
Donor age was significantly younger in the BMI ≥40 cohort (BMI ≥40: 35 ± 15 years vs. BMI < 40: 42 ± 15 years; p = .01). Percentage of living donors within each cohort was similar (BMI ≥40: 10% vs. BMI < 40: 7%; p = .58). Among deceased donors in each cohort, mean KDPI was significantly lower in the BMI ≥40 cohort (BMI ≥40: 40 ± 25% vs. BMI < 40: 53 ± 26%; p = .003), and the percentage of kidneys with a KDPI > 85% was significantly lower in the BMI ≥40 group (BMI ≥40: 4% vs. BMI < 40: 13%; p = .04). The proportion of DCD donors was not significantly different between groups (BMI ≥40: 30% vs. BMI < 40: 24%; p = .41) (Table 1).
3.3 Patient outcomes
DGF was not significantly different between groups (BMI ≥40: 35% vs. BMI < 40: 24%; p = .13). LOS for the transplant admission was also not different between groups (BMI ≥40: 5.7 ± 4.4 days vs. BMI < 40: 5.6 ± 2.4 days; p = .91). Within the first 30 and 90 days, re-operation rates were similar between groups (BMI ≥40: 6% vs. BMI < 40: 7%; p = .76 at 30 days and BMI ≥40: 11% vs. BMI < 40: 13%; p = .63 at 90 days). Re-admission rates were also similar within the first 30 and 90 days after transplant for both groups (BMI ≥40: 32% vs. BMI < 40: 38%; p = .42 at 30 days and BMI ≥40: 56% vs. BMI < 40: 58%; p = .76 at 90 days). Wound complication rates (composite measure) were comparable between groups (BMI ≥40: 14% vs. BMI < 40: 13%; p = .82) (Table 2).
| BMI≥40 | BMI < 40 | ||
|---|---|---|---|
| (n = 84) | (n = 84) | p-value | |
| Delayed graft function | 35% | 24% | .13 |
| Length of stay for transplant admission: mean ± SD (days) | 5.7 ± 4.4 | 5.6 ± 2.4 | .91 |
| Reoperation in 1st 30 days | 6% | 7% | .76 |
| Reoperation in 1st 90 days | 11% | 13% | .63 |
| Readmission in 1st 30 days | 32% | 38% | .42 |
| Readmission in 1st 90 days | 56% | 58% | .76 |
| Wound complication (compositea) | 14% | 13% | .82 |
| Weight change in 1st year, mean ± SD (kg) | 2.5 ± 9.1 | 3.0 ± 8.9 | .74 |
| BMI change in 1st year, mean ± SD | .9 ± 3.3 | 1.1 ± 3.2 | .59 |
| Post-transplant (new onset) diabetes | 9% | 4% | .28 |
| HbA1c at 1 year, mean ± SD | 6.9 ± 1.7 | 5.9 ± 1.4 | .003 |
| Patient survival at 1 year | 98% | 98% | .98 |
| Graft survival at 1 year | 95% | 90% | .23 |
| Serum creatinine at 1 year, mean ± SD | 1.7 ± 1.1 | 1.8 ± 1.0 | .54 |
| eGFR at 1 year, mean ± SD | 44 ± 15 | 39 ± 15 | .06 |
- a Composite measure included wound hematoma, superficial or deep wound infection, dehiscence within 90 days post-transplant, and incisional hernia within 1 year post-transplant.
Among first year outcomes, weight gain was not significantly different between groups (BMI ≥40: +2.5 ± 9.1 kg vs. BMI < 40: +3.0 ± 8.9 kg; p = .74). Change in BMI was also comparable (BMI ≥40: +.9 ± 3.3 kg/m2 vs. BMI < 40: +1.1 ± 3.2 kg/m2; p = .59). Incidence of new onset post-transplant diabetes was not significantly different between cohorts (BMI ≥40: 9% vs. BMI < 40: 4%; p = .28). HgbA1c level (for all patients) at 1 year was higher in the BMI ≥40 group (BMI ≥40: 6.9 ± 1.7% vs. BMI < 40: 5.9 ± 1.4%; p = .003). Serum creatinine was similar at 1 year between cohorts (BMI ≥40: 1.7 ± 1.1 mg/dl vs. BMI < 40: 1.8 ± 1.0 mg/dl; p = .54), as was eGFR (BMI ≥40: 44 ± 15 ml/min/1.73m2 vs. BMI < 40: 39 ± 15 ml/min/1.73m2; p = .06) (Table 2).
3.4 Survival outcomes
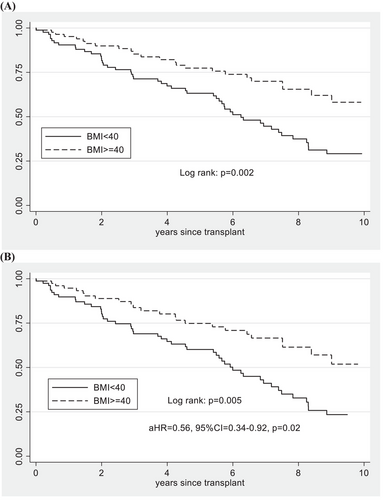
Patient and graft survival rates at 1 year were both comparable between cohorts (1-year patient survival: BMI ≥40: 98% vs. BMI < 40: 98%; p = .98 and 1-year graft survival BMI ≥40: 95% vs. BMI < 40: 90%; p = .23) (Table 2). Long-term patient survival was also comparable in both unadjusted (log rank: p = .09) and after adjusting for KDPI among deceased donor kidney transplants (Ref: BMI < 40: aHR = .73, 95%CI = .36–1.51, p = .4), but did show evidence of a trend towards higher patient survival in the BMI ≥40 group after 6 years following transplant (Figure 3). The kidney graft survival rate was higher in BMI ≥40 patients in both unadjusted survival among all kidney transplants (log rank: p = .002) and after adjusting for KDPI among deceased donor kidney transplants (Ref: BMI < 40: aHR = .56, 95%CI = .35–.92, p = .02) (Figure 4). When examining only DCD kidney transplant recipients, there were no differences in patient survival (log rank: p = .69) or kidney graft survival (log rank: p = .31) according to BMI group.

4 DISCUSSION
In this study, we present a retrospective review of our single center experience using CT scan-based criteria for selection of renal transplant candidates based on technical and anatomical considerations rather than strict BMI-based criteria. Using measurements of pelvic angle and iliac vessel depth as selection criteria, our data suggest that in appropriately selected candidates with a BMI of ≥40 kg/m2, outcomes are acceptable and overall equivalent to those with a BMI < 40 kg/m2 despite a mean BMI difference of 12 kg/m2 between cohorts.
While morbid obesity has historically been viewed as a contraindication for kidney transplantation, practice patterns and guidelines are evolving. The “Kidney Disease: Improving Global Outcomes” (KDIGO) guidelines from 2020 suggest that obese patients have their body habitus reviewed by a transplant surgeon and not be denied access to transplantation solely on the basis of obesity.17 Despite these recommendations, the transplant community continues to struggle with unequal access to transplantation for obese patients, with obese patients remaining on the waiting list for longer periods of time once they are listed or placed on a “temporarily inactive status” (status 7) and never achieving reactivation while waiting to achieve a weight loss target.18, 19 There is also evidence for unequal transplantation rates in obese men versus obese women, with men reaching a much higher BMI before their rates of transplantation decline.20 Furthermore, in the US, the prevalence of obesity is higher in multiple minority groups, which can compound the inequities in transplant access for these already disadvantaged groups.2, 21, 22
In this cohort, BMI ≥40 kg/m2 patients spent a longer time on dialysis. Of interest, while dialysis time was significantly longer in the BMI ≥40 kg/m2 patients, time on waitlist was not different and in fact the mean waiting time trended lower in the BMI ≥40 kg/m2 patients, thus suggesting that access to the list is a main contributor and barrier to transplant. This trend of lower access to transplantation for the most overweight patients is consistent with prior reports.18 Obese patients are less likely to be referred to a transplant center or listed for transplant; and in many of these patients, obesity was cited as the sole contraindication.23 These patients were more often younger, female, and Black.23 In our study, nearly two-thirds of our patients in the BMI > 40 kg/m2 group were female and more than half were minorities. Once listed, obese patients have longer median wait times in studies based on the national registry data and are more likely to be bypassed on the list when an organ became available.18 Decisions to bypass obese patients are complex and multi-factorial, but must involve considerations of donor suitability for this group of patients. We have previously published our experience with achieving acceptable post-transplant outcomes with “marginal” donor kidneys, which demonstrated the importance of matching estimated donor nephron capacity or mass with projected recipient need based on avoiding large mismatches between donor and recipient age and size.24
Concerns remain about higher risks and inferior outcomes for high BMI recipients compared to their lower BMI counterparts. However, in our study, we did not find any differences in the rates of DGF, LOS, re-operation or re-admission, wound complications, or patient and graft survival rates. In fact, we found that long-term graft survival was improved in the BMI ≥40 kg/m2group, which is likely related to lower KDPI donors utilized in this group. Interestingly, however, this improvement in long-term graft survival remained significant even after adjusting for KDPI. Additionally, we noted that the 1-year eGFR of those patients with a BMI ≥40 trended towards a higher value compared to those with BMI < 40 kg/m2 (eGFR 44 vs. 39 ml/min/1.73m2, p = .06).
Our findings are commensurate with the growing, more modern body of evidence that overwhelmingly supports transplantation as the therapy of choice for ESRD patients, including those who are overweight or obese.16, 25, 26 Sureshkumar at al. reported on outcomes of mate kidneys transplanted into patients classified into four BMI strata, with the upper 2 strata consisting of patients with a BMI of 30–35 and > 35 kg/m2.27 They noted comparable patient and overall graft survival rates between these two groups, but the odds of DGF did increase as recipient BMI increased. Other studies have also found an association between recipient obesity and DGF after renal transplant.25, 28 In addition, both DGF and recipient obesity have been linked to a higher incidence of wound complications in multiple studies.
In our study, patients with a BMI ≥40 kg/m2 were transplanted with kidneys from donors who were younger and had lower KDPI scores based on our center's donor selection practice patterns for higher weight patients to match estimated donor nephron capacity or mass with projected recipient needs. As such, there were few KDPI > 85% donor kidneys in the BMI ≥40 kg/m2 group. Careful donor selection is paramount to achieving excellent outcomes in any recipient group but may be particularly important in the BMI ≥40 kg/m2 patients. For example, judicious consideration of donor and preservation factors known to increase the likelihood of DGF (such as longer warm ischemia and CIT) may help mitigate the propensity to DGF in obese recipients. In our high BMI cohort, mean CIT was 4 h less than the control BMI matched group, so the combination of shorter CIT and lower KDPI donors in the high BMI cohort may have contributed to the comparable rates of DGF in the two groups. In addition, 10% of BMI ≥40 kg/m2 recipients underwent a living donor transplant. We believe it is important to note that living donor transplantation was technically feasible and successful in selected obese recipients (albeit requiring careful consideration of the donor and recipient vascular anatomy) as typically living donors represent the optimal quality donor for most patients. Thus, when donors and recipients are both well selected, outcomes in the BMI ≥40 kg/m2 group can be similar to those in the BMI < 40 kg/m2 group. Furthermore, as noted above these comparable outcomes between BMI ≥40 kg/m2 and BMI < 40 kg/m2 transplanted patients are in addition to a potential survival benefit when comparing BMI ≥40 kg/m2 to waitlisted (not-transplanted) patients such as the 50% reduction in mortality hazard identified by Gil et al.16
However, improving access to transplantation requires more than a “one size fits all” approach. KDIGO guidelines suggest that obese patients be offered weight loss strategies prior to kidney transplantation, but durable and healthy medical weight loss is difficult to achieve prior to transplant for many reasons.17, 29, 30 Bariatric surgery represents an important and efficacious option to achieve pre-transplant weight loss and is successful in improving several obesity-associated conditions.31 However, consideration should be given to the higher risks associated with ESRD patients. Turgeon et al., reported an increasing rate of peri- and post-operative complications after bariatric surgery with increasing CKD stage.32 Additionally, the nutritional, mineral, and drug absorption implications of bariatric surgery (such as Roux-en-Y gastric bypass) must be considered in this patient population already limited to a restrictive diet.30 Sheetz et al., reported that although short-term outcomes may show a slight increase in mortality at 1 year following bariatric surgery versus no surgery in patients with CKD, the 5-year outcomes demonstrated lower mortality and a higher incidence of actually receiving a kidney transplant, which is linked not only to increased quantity but also quality of life.33 Freeman et al. described their single center's experience with laparoscopic gastric sleeve in waitlisted ESRD patients and reported minimal complications with excellent excess weight loss and reduction in comorbidities.34 There is other encouraging evidence for pre-transplant bariatric surgery, and attention to pre-transplant weight loss continues to be warranted in order to add more deserving patients to the waitlist and improve the likelihood of excellent technical outcomes following kidney transplantation.35 Finally, robotic kidney transplantation has gained traction as an opportunity to perform technically feasible and safe transplants in morbidly obese recipients at selected centers.36-38 This modality can allow for successful transplantation in some obese individuals who do not meet imaging-based criteria for transplant eligibility such as those outlined in this study and are unable to achieve meaningful weight loss prior to transplant. In sum, different modalities should be considered according to an individual patient's characteristics and weight loss is appropriate to encourage in almost all obese kidney transplant candidates. However, delaying transplant and the associated survival benefit is likely not warranted in patients meeting the anatomical criteria herein given the ability to achieve excellent and comparable outcomes to non-obese recipients regardless of BMI.
5 LIMITATIONS
Study limitations include that this was a retrospective review of a single center's experience spanning many years. The decision to list and transplant a patient with a BMI ≥40 kg/m2 is based on numerous medical, psychosocial, financial, and other surgical factors during committee review independent of pelvic anatomy and body habitus. Thus, there are ample opportunities for selection bias. Control group matching was performed in order to address for more easily identifiable differences between obese and non-obese cohorts. Some of these differences would typically predict higher risk outcomes in obese patients such as greater proportion of African-American patients and patients with diabetes in the BMI ≥40 kg/m2. Interestingly, other differences might favor the BMI ≥40 kg/m2 cohort such as younger age when compared to all lower BMI patients. Consequently, we acknowledge the likelihood of selection bias that occurs when evaluating and listing obese patients. For example, high BMI patients may be declined as kidney transplant candidates and deemed higher risk or less “robust” merely because of obesity. These decisions may include aspects not easily identifiable, and thus not controllable according to standard matching techniques or statistical adjustment. Additionally, careful selection of donors as evidenced by lower KDPI values and mitigating risk factors for DGF such as avoiding long CIT in the setting of deceased donor transplantation are likewise integral in achieving the excellent and comparable outcomes seen in this BMI ≥40 kg/m2 group.
Additionally, < 10% of patients underwent non-standardized use of wound closure adjuncts including subcutaneous surgical drains or closed-incision negative pressure dressings precluding the ability to assess their impact on wound complication rates. Published evidence suggests that surgical drains do not affect wound complication rates in kidney transplantation.39 Low quality evidence suggests that closed incision negative pressure dressings may reduce seroma and wound infection rates, highlighting an area worthy of further study.40
6 CONCLUSION
In conclusion, we present our center's experience using CT imaging-based criteria for determining anatomic suitability for kidney transplantation irrespective of BMI. Historically, a BMI ≥40 kg/m2 has been a contraindication to listing at many kidney transplant centers. In fact, many of the patients in this study were referred to us after being refused for transplantation at other centers. However, with the availability of current imaging techniques, we believe that using BMI alone as a contraindication to kidney transplantation is not warranted. This study provides evidence that well-selected obese patients with a BMI ≥40 kg/m2, when transplanted with kidneys from an appropriate donor, can have successful outcomes including comparable rates of patient survival and peri-operative complications and perhaps even improved long-term graft survival when compared to a matched lower BMI cohort. Taken as a whole, these results strongly advocate for increased use of more nuanced and personalized criteria to determine which obese patients are suitable for listing for a kidney transplant.41
AUTHOR CONTRIBUTIONS
Marie L. Jacobs: Concept/design, Data collection, Data analysis/interpretation, Statistics, Drafting article, Critical revision of article, Approval of article. ORCID: 0000-0003-3396-1417. Karanpreet Dhaliwal: Concept/design, Data collection, Data analysis/interpretation, Statistics, Drafting article, Critical revision of article, Approval of article. David I. Harriman: Concept/design, Data collection, Data analysis/interpretation, Statistics, Critical revision of article, Approval of article.
CONFLICTS OF INTEREST
No disclosures or conflicts.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




