Perineal hernia repair: Multicentre comparative analysis of mesh-only versus mesh combined with tissue flap
Saskia I. Kreisel and Dite L. C. de Jong shared first authorship.
Abstract
Aim
Surgical techniques for perineal hernia repair after abdominoperineal resection have evolved over time. Synthetic mesh repair is currently the preferred technique, but recurrence rates are high. The aim of this study is to compare the outcomes of mesh-only repair with combined mesh and tissue flap repair.
Method
Between 2006 and 2022, patients who underwent perineal hernia repair with synthetic mesh or any mesh combined with tissue flap were retrospectively identified from three referral centres. The primary endpoint was recurrent perineal hernia.
Results
Seventy-two patients with primary perineal hernia were included, of whom 58 underwent mesh-only repair and 14 mesh with flap repair. Postoperative perineal wound complications occurred in 21% in both groups. Meshes were explanted solely within the mesh-only group (n = 3). There were no technical flap failures. Recurrence of perineal hernia occurred in 34% (n = 20/58) of mesh-only patients and in 14% (n = 2/14) of mesh with flap patients (p = 0.12), during a median follow up of 53 months [interquartile range (IQR) 20–81 months] and 24 months (IQR 14–58 months), respectively. Time to recurrent hernia was a median of 16 months (IQR 3–31 months). Crossover to mesh with flap after failed mesh-only repair was successful in 4/4 patients.
Conclusion
Mesh combined with tissue flap repair of a perineal hernia seems more effective than synthetic mesh-only repair, based on an absolute 20% difference in recurrence rate. There was a lack of statistical power due to this being a low-volume type of surgery, even in this largest published series so far.
What does this paper add to the literature?
The literature on perineal hernia repair consists of small case series, while our study includes a large multicentre cohort comprising 72 patients. This paper describes results of mesh with flap repair, a relatively novel technique, which appears beneficial in treatment of perineal hernia when compared with the use of mesh only.
INTRODUCTION
Perineal hernia can develop following an abdominoperineal resection (APR), and usually occurs after a median of 8–11 months [1-3]. Perineal hernia can have a significant impact on a patient's quality of life due to discomfort during sitting and walking, urogenital dysfunction and small bowel obstruction [4-7].
The reported incidence of perineal hernia after primary perineal wound closure varies widely, ranging up to 30% [1, 2]. It is important to note that the true incidence of perineal hernia might be even higher due to the underreporting of asymptomatic patients. The development of a perineal hernia is influenced by several risk factors, including preoperative radiotherapy, extralevator resection (which leads to a broader perineal defect at the pelvic floor level), wound infection and the technique used for perineal wound closure [1, 2].
Treatment of a perineal hernia ranges from a nonsurgical approach, such as utilizing supportive garments, to perineal reconstruction. It remains uncertain which technique for repairing perineal hernia yields the most favourable outcome as relevant literature is scarce. A systematic review from 2023 revealed 29 studies, which in total reported on 325 patients who underwent 347 repairs [8]. Synthetic meshes were preferred over biological meshes for their suggested superior success rates and lower cost [9]. Nevertheless, both types of meshes still exhibit significant recurrence rates [10].
Promising outcomes were observed with a novel surgical approach combining mesh repair with a tissue flap based on initial experience in a small series [9]. This comparative multicentre cohort study aimed to evaluate perineal hernia repair using synthetic mesh only or mesh combined with a tissue flap, with recurrence rate as primary outcome.
METHOD
Patients
This study describes an extended experience in perineal hernia repair with follow-up update following previous publications [9, 11, 12]. Furthermore, we combined the experiences from three institutes, which enabled a direct comparative analysis at an individual patient level between two surgical techniques.
Consecutive patients who underwent a perineal hernia repair were included from 2006 (Catharina Hospital, Eindhoven) or 2014 (Amsterdam University Medical Centre (UMC)) until 2022. A third cohort (2021–2022) from the Erasmus Medical Centre (MC) comprised an extension of the Amsterdam UMC cohort after a consultant surgeon switched clinics.
Patients with a symptomatic primary perineal hernia who underwent surgery at one of these three institutes with either a synthetic mesh or a mesh (synthetic or biological) combined with a tissue flap were included in the main comparative analysis. A symptomatic perineal hernia was defined as recognizable perineal complaints along with perineal bulging found during physical examination and/or descent of abdominal contents below the line between the coccyx (or distal sacrum) and the perineal body on radiological imaging. Asymptomatic hernias, detected incidentally on imaging, were not considered as an indication for surgical repair. Patients who were referred with recurrent perineal hernia after failed primary repair in another hospital were analysed separately. Patients were included irrespective of the type of APR and irrespective of underlying disease. Those who underwent pelvic exenteration as index surgery or perineal hernia repair using a biological mesh-only repair were excluded. We have previously published on this technique, and stopped performing biomesh repairs thereafter [11]. The medical ethics boards in all three hospitals were consulted, and if deemed necessary an opt-out letter was sent.
Surgical technique
The type of hernia repair was almost exclusively determined by the institution and year of surgery. Synthetic mesh-only repair has been standard of care in the Catharina Hospital, Eindhoven during the whole study period. Before the study period (2003–2006), a partially absorbable mesh and Prolene 2.0 sutures were used in eight cases, which all failed. Since 2006, the standard approach has been transperineal in the prone position with fixation of a large nonabsorbable propylene mesh with sufficient overlap under high tension to the sacrotuberal ligaments laterally, to the urogenital diaphragm ventrally and to the gluteus maximus muscle and coccygeal bone laterally. A suction drain is placed according to the surgeon's preference. Finally, layered closure of subcutaneous fat and skin is performed. Videos showing this technique were provided in a previous publication [12]. The technique of synthetic mesh-only repair since 2014 at Amsterdam UMC was similar to the Eindhoven technique, except for a more inward fixation of the mesh to the remnants of the levator muscle laterally.
Combined mesh with tissue flap repair in Amsterdam UMC and Erasmus MC was also performed using a perineal approach in the prone position. Several types of fasciocutaneous tissue flaps were initially used on indication if tensionless closure over the mesh was not possible, and gluteal turnover flap has become the standard technique since 2019. After fixation of the mesh, a gluteal turnover flap can be performed with one of the buttocks as the donor site. A half-moon-shaped skin island with a maximum width of 2.5 cm is created by making an additional curved incision on one side of the wound. Subsequently, the subcutaneous fat is divided in a 45° angle towards the gluteus fascia. The skin is de-epithelialized. A redon suction drain is routinely placed. The flap is then positioned on top of the mesh and the dermis of the flap is stitched to the opposite fixation line of the mesh laterally. The perineal subcutaneous fat of the opposite site where the flap is harvested is mobilized from the gluteus fascia if indicated for tensionless closure over the flap, followed by layered closure of fat and skin. This technique has previously been described and illustrated [9, 13].
Data collection
Patient records were retrospectively reviewed for baseline characteristics. Intraoperative details, surgical approach, type of mesh, type of flap and operating time were collected. Also, data were retrieved on hospital stay, perineal wound complications and recurrent perineal hernia. In the event of a recurrence, data were collected regarding date of diagnosis, radiological imaging and re-repair. Missing data were handled using a pairwise deletion strategy.
Outcome
The primary outcome was recurrent perineal hernia, which was defined as recognizable perineal complaints along with perineal bulging found during physical examination and/or descent of abdominal contents below the line between the coccyx (or distal sacrum) and the perineal body on radiological imaging. Secondary outcomes were intraoperative complications, length of hospital stay, postoperative perineal wound complications and reinterventions, long-term complications related to the mesh (i.e. explantation, fistula, small bowel obstruction) and treatment of recurrent perineal hernia.
Statistical analysis
Categorical data were compared using the chi-square test and numerical data with the independent t-test or Mann–Whitney U-test according to distribution. Kaplan–Meier curves including the log-rank test were constructed to estimate the cumulative proportion of recurrent perineal hernias. The level of statistical significance was set at a p-value of <0.05. Statistical analysis was performed using SPSS software for Windows version 28 (IBM Corp, Armonk, NY).
RESULTS
Patients
Between 2006 and 2022, 107 consecutive patients underwent a perineal hernia repair. Patients were excluded because of biological mesh closure (n = 19), primary closure (n = 2), exenteration as index surgery (n = 6) and refused consent (n = 1). In total, 79 patients were included, seven of whom had undergone initial perineal hernia repair at a referring hospital. Of the 72 patients who underwent primary perineal hernia repair at one of our three centres, 58 had mesh-only perineal hernia repair and 14 had mesh with flap repair. Included patients underwent APR for rectal cancer in 88% of cases (63/72; Table 1). In the mesh with flap group, patients had more often undergone previous pelvic surgery (p = 0.04), more often had anal cancer (p = 0.01) and more often underwent extralevator APR (p = 0.01), which was performed more often by laparoscopy (p < 0.001) and involved more additional resection (p = 0.04). Mesh-only patients were more frequently treated with any type of radiotherapy (p = 0.03). The rate of perineal wound complications after index APR was significantly higher in the mesh with flap group (64% vs. 22%, p = 0.009).
| Mesh only (n = 58), n (%) | Mesh + flap (n = 14), n (%) | p-value | |
|---|---|---|---|
| Referral | 11/58 (19) | 5/14 (36) | |
| Gender, female | 31/58 (53) | 10/14 (71) | 0.22 |
| Age (years). mean ± SD | 68 ± 9 | 66 ± 9 | 0.38 |
| BMI (kg/m2), mean ± SD | 26 ± 3 | 27 ± 5 | 0.30 |
| ASA ≥ 3 | 7/58 (7) | 3/14 (21) | 0.71 |
| Active or previous smoking | 21/58 (36) | 7/14 (50) | 0.27 |
| Medical history, yesa | 15/58 (26) | 6/14 (43) | 0.18 |
| Diabetes | 6/58 (10) | 3/14 (21) | |
| COPD | 3/58 (5) | 3/14 (21) | |
| Cardiac disease | 12/58 (21) | 3/14 (21) | |
| Previous pelvic surgery (other than APR) | 20/58 (35) | 9/14 (64) | 0.04 |
| Neoadjuvant radiotherapy | |||
| None | 3/56 (5) | 3/14 (21) | 0.03 |
| Short-course radiotherapy | 11/56 (20) | 1/14 (7) | |
| Chemoradiotherapyb | 40/56 (71) | 7/14 (50) | |
| Radiotherapy for anal cancer | 2/56 (4) | 3/14 (21) | |
| Missing | 2/58 (3) | 0/14 (0) | |
| Primary disease | |||
| Rectal cancer | 54/58 (94) | 9/14 (64) | 0.01 |
| Anal cancer | 2/58 (3) | 3/14 (22) | |
| Otherc | 2/58 (3) | 2/14 (14) | |
| Type of APR | |||
| Extralevator | 15/47 (32) | 6/7 (86) | 0.01 |
| Conventional | 27/47 (57) | 0/7 (0) | |
| Intersphincteric | 5/47 (11) | 1/7 (14) | |
| Missing | 11/58 (19) | 7/14 (50) | |
| Abdominal approach APR | |||
| Open | 46/53 (87) | 1/9 (11) | <0.001 |
| Laparoscopic | 7/53 (13) | 8/9 (89) | |
| Missing | 5/58 (9) | 5/14 (36) | |
| Additional resection during APRa | 14/52 (27) | 7/11 (64) | 0.04 |
| Coccygectomy or sacrectomy | 4/52 (8) | 0/11 (0) | |
| (Partial) vagina wall resection | 5/25 (20) | 3/8 (38) | |
| Uterus extirpation | 3/25 (12) | 0/6 (0) | |
| Adnex extirpation | 4/25 (16) | 0/8 (0) | |
| Other | 2/52 (4)d | 5/11 (45)e | |
| Missing | 6/58 (10) | 3/14 (21) | |
| Omentoplasty | 47/54 (87) | 8/12 (67) | 0.17 |
| Missing | 4/58 (7) | 2/14 (14) | |
| Perineal closure after APR | |||
| Primary closure | 53/58 (92) | 13/14 (93) | 0.75 |
| Biological mesh | 2/58 (3) | 0/14 (0) | |
| Flap closure | 3/58 (5)f | 1/14 (7)g | |
| Uterus extirpation before or during APR | 8/31 (26) | 4/10 (40) | 0.35 |
| Perineal wound complication <30 days after APR | 12/54 (22) | 7/11 (64) | 0.009 |
| Missing | 4/58 (7) | 3/14 (21) | |
- Note: Data are presented as absolute numbers (proportions), unless stated otherwise.
- Abbreviations: APR, abdominoperineal resection; ASA, American Society of Anesthesiologists; BMI, body mass index; COPD: chronic obstructive pulmonary disease; SD, standard deviation.
- a More than one of the variables below may be applicable to the same patient.
- b Of which two patients did not receive chemotherapy.
- c Crohn's disease (n = 2), vulvar cancer, pararectal synovial sarcoma.
- d Partial prostate, pelvic rim and seminal vesicles.
- e Lateral lymph node dissection (n = 2), proctocolectomy, vulvectomy, seminal vesicles.
- f Vertical rectus abdominis muscle flap (n = 2), gracilis flap.
- g Gluteal turnover flap.
Perineal hernia repair
A perineal-only approach was carried out in 97% (56/58) of the mesh-only group and in 100% (14/14) of the mesh with flap group. The performed flaps included the gluteal turnover flap (n = 7), unilateral VY plasty (n = 3), bilateral VY plasty (n = 2) and both inferior (n = 1) and superior (n = 1) gluteal artery perforator flap. In one patient with VY plasty, a gluteal turnover flap was used to close the perineum after APR. In the mesh with flap group, every patient had at least one perineal vacuum drain, while this was done in 26% (15/58) of mesh-only patients (p < 0.001). The duration of surgery was a median of 56 min [interquartile range (IQR) 44–70 min] for mesh-only reconstruction, which was significantly shorter than the 167 min (IQR 103–240 min) for mesh with flap reconstruction (p < 0.001; Table 2).
| Mesh-only (n = 58), n (%) | Mesh + flap (n = 14), n (%) | p-value | |
|---|---|---|---|
| Time between APR and diagnosis of PH (months), median (IQR) | 13 (8–39) | 5 (2–14) | 0.03 |
| Missing | 8/58 (14) | 2/14 (14) | |
| Time between APR and primary PH repair (months), median (IQR) | 20 (12–49) | 17 (8–34) | 0.34 |
| Approach to PH repair | |||
| Transperineal | 56/58 (97) | 14/14 (100) | 0.65 |
| Abdominal, open | 3/58 (3) | 0/14 (0) | |
| Type of synthetic permanent mesh | |||
| Polypropylene | 35/58 (60) | – | |
| Composite | 22/58 (38) | – | |
| Not specified | 1/58 (2) | – | |
| Type of mesh | |||
| Synthetica | – | 8/14 (57) | |
| Biologicalb | – | 6/14 (43) | |
| Perineal vacuum drain | 15/58 (26) | 14/14 (100) | <0.001 |
| Two or more drains | 1/58 (2) | 4/14 (29) | |
| Intraoperative complicationc | 1/58 (2) | 1/14 (0) | 0.35 |
| Duration of surgery (min), median (IQR) | 56 (44–70) | 167 (103–241) | <0.001 |
- Note: Data are presented as absolute numbers (proportions), unless stated otherwise.
- Abbreviations: APR, abdominal perineal resection; IQR, interquartile range; PH, perineal hernia.
- a Polypropylene (n = 5), composite (n = 3).
- b Strattice (n = 5), not specified.
- c Mesh-only: extensive adhesiolysis along with serosa injury requiring suturing. Mesh + flap: injury to the ureter occurred because of disturbed anatomy with the bladder herniated through the pelvic floor.
An intraoperative complication occurred in one patient in the mesh-only group, involving minor injuries to the serosa during extensive adhesiolysis; these were sutured. In the mesh with flap group, one intraoperative complication occurred, consisting of a ureteral injury for which a nephrostomy was placed.
Outcome after perineal hernia repair
A postoperative perineal wound complication occurred in 21% (12/58) of the mesh-only patients and in 21% (3/14) of the mesh with flap patients (p = 0.60). The infective complications of mesh-only repairs were wound infection (n = 6) and presacral abscess (n = 4), and noninfective complications were seroma (n = 4) and dehiscence (n = 1). In mesh with flap patients, no infective complications were reported; however, three patients developed perineal wound dehiscence.
In the mesh-only group, infective postoperative complications required antibiotic therapy (n = 7), radiological abscess drainage (n = 4) and surgical abscess drainage (n = 3), which are Clavien–Dindo grade 2, 3a and 3b complications, respectively. For the mesh with flap group, all three perineal wound dehiscences were closed by secondary wound healing without any reintervention (Clavien–Dindo grade 1). The postoperative perineal complications required readmission in four mesh-only patients, whereas this was not indicated in mesh with flap patients. Finally, no flap failures or donor site complications were observed. The median length of hospital stay was 2 days (IQR 1–3 days) and 7 days (IQR 3–8 days) in mesh-only and mesh with flap patients, respectively (p = 0.003).
Most of the long-term complications in the mesh-only group comprised infection of the mesh (n = 3), along with fistula formation between the perineal skin and the mesh in two patients and an isolated fistula between perineal skin and the gluteal region (n = 1; Table 3). All infected meshes were explanted (n = 3), and the fistula trajectory (perineum to gluteal region) was incised followed by filling with gentamycin-impregnated mesh and beads (n = 1).
| Mesh-only (n = 58), n (%) | Mesh + flap (n = 14), n (%) | p-value | |
|---|---|---|---|
| Length of hospital stay, days (median, IQR) | 2 (1–3) | 7 (3–8) | 0.003 |
| Perineal wound complication ≤30 daysa | 12/58 (21) | 3/14 (21) | 0.60 |
| Infectiousa | 9/58 (16) | 0/14 (0) | 0.13 |
| Wound infection | 6/58 (10) | 0/14 (0) | |
| Presacral abscess | 4/58 (7) | 0/14 (0) | |
| Noninfectious | 5/58 (9) | 3/14 (21) | 0.18 |
| Seroma | 4/58 (7) | 0/14 (0) | |
| Dehiscence | 1/58 (2) | 3/14 (21) | |
| Flap failure | – | 0/14 (0) | |
| Reintervention due to perineal wound complication ≤30 daysa | 11/58 (19) | 0/14 (0) | 0.08 |
| Antibiotic therapy | 7/58 (12) | 0/14 (0) | |
| Radiological abscess drainage | 4/58 (7) | 0/14 (0) | |
| Surgical abscess drainage | 3/58 (5) | 0/14 (0) | |
| Otherb | 1/58 (2) | 0/14 (0) | |
| Readmission | 4/58 (7) | 0/14 (0) | |
| Pelvic complication >30 daysa | 5/58 (9) | 2/14 (14) | 0.41 |
| Infection of mesh | 3/58 (5) | 0/14 (0) | |
| Fistula formation | 3/58 (5) | 1/14 (7) | |
| Seroma | 1/58 (2) | 0/14 (0) | |
| Small bowel obstructionc | 1/58 (2) | 0/14 (0) | |
| Vaginal wall defect, exposing mesh | 0/58 (0) | 1/14 (7) | |
| Requiring surgical reintervention | 4/58 (7) | 1/14 (7) | |
| Explantation of mesh | 3/58 (5) | 0/14 (0) |
- Note: Data are presented as absolute numbers (proportions), unless stated otherwise.
- a More than one of the variables below may be applicable to the same patient.
- b Approximating the perineal wound dehiscence using skin staplers.
- c Only small bowel obstruction that is related to the mesh of recurrent perineal hernia.
In the mesh with flap group, one patient with Crohn's disease suffered from a recurrent fistula between the posterior vaginal wall and presacral space, for which a resection was performed while the bilateral VY plasty remained sufficient. In another mesh with flap patient there appeared to be a small defect of the posterior vaginal wall where the mesh was immediately palpable, which was treated conservatively.
Recurrence of perineal hernia
The median duration of follow-up was 53 months (IQR 20–81 months) after mesh-only repair, and 24 months (IQR 14–58 months) after mesh with flap repair (p = 0.11; Table 4). A recurrent symptomatic perineal hernia occurred in 34% (n = 20/58) of the mesh-only patients and in 14% (n = 2/14) of the mesh with flap patients (p = 0.12). The overall median interval to recurrent perineal hernia was 16 months (IQR 3–31 months). Using Kaplan–Meier analysis, the recurrence-free survival rate at 30 months was 69% and 77%, respectively (p = 0.348; Figure 1).
| Mesh-only (n = 58), n (%) | Mesh + flap (n = 14), n (%) | p-value | |
|---|---|---|---|
| Recurrence of perineal hernia | 20/58 (34) | 2/14 (14) | 0.12 |
| Imaging performed | 12/20 (60) | 1/2 (50) | 0.66 |
| Secondary perineal hernia repair | 14/20 (70) | 2/2 (100) | |
| Total number of perineal hernia repairs per patient | |||
| One | 44/58 (76) | 12/14 (86) | 0.53 |
| Two | 6/58 (10) | 2/14 (14) | |
| Three | 6/58 (10) | 0/14 (0) | |
| Four | 2/58 (3) | 0/14 (0) | |
| Total duration of follow-up (months)a | 53 (20–81) | 24 (14–58) | 0.11 |
- Note: Data are presented as absolute numbers (proportions), unless stated otherwise.
- a Counting from the date of primary perineal hernia repair.
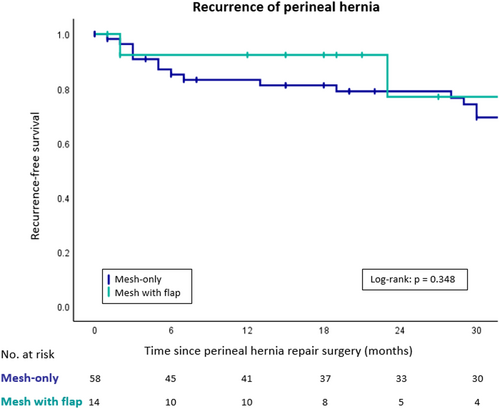
In the mesh-only group, 14 of 20 patients with a symptomatic recurrent perineal hernia underwent a second repair. These secondary repairs consisted of mesh only in 12 patients (by adding another synthetic permanent mesh or a refixation of the previous mesh) and mesh with flap in two patients, resulting in 8/12 and 0/2 re-recurrent perineal hernias, respectively (Figure S1). Success rates of a third and fourth hernia repair, which were all mesh-only, were 4/8 and 0/2, respectively.
In the mesh with flap group, both patients with a symptomatic recurrence underwent a second perineal hernia repair. In the first patient, a new biological mesh was placed because of contamination in the presence of a fistula, after which the previously performed bilateral VY plasty was resutured to the mesh. The other patient was treated with replacement of the synthetic permanent mesh. This patient developed a re-recurrent perineal hernia for which no surgical intervention has been performed.
All referred patients with recurrent perineal hernia after failed repair in another hospital (n = 7) underwent mesh-only secondary hernia repair at Amsterdam UMC or Catharina Hospital; these were successful in 3/7 patients (Figure S2). The success rate of a third hernia repair was 1/3 for mesh-only and 1/1 for mesh with flap, and corresponding success rates were 1/2 and 1/1, respectively, for a fourth hernia repair.
Overall, crossover from mesh-only to mesh with flap repair was successful in all four patients [two crossovers in the comparative cohort of primary repairs (n = 72) and two crossovers in the referred patients with recurrent perineal hernia (n = 7)].
DISCUSSION
This multicentre comparative cohort study demonstrates a lower recurrence rate after primary perineal hernia repair using a mesh and flap compared with synthetic mesh-only repair (14% vs. 34%). Although this is the largest documented cohort of perineal hernia repair so far this difference did not reach statistical significance due to the low volume of this type of surgery. Furthermore, crossover from mesh-only repair to mesh with flap re-repair in the case of recurrent perineal hernia was successful in all four patients. Infective postoperative perineal wound complications occurred exclusively in the mesh-only group, resulting in radiological and surgical reinterventions, along with three mesh explantations in the long term. Within the mesh with flap repair group, only perineal wound dehiscences have been seen so far; these were all closed by secondary wound healing. Furthermore, no flap failures nor donor site morbidity were observed. These are promising results that warrant evaluation in larger series.
Several perineal hernia reconstruction methods are described in the literature. A systematic review with literature search until December 2021 revealed 19 studies that reported on 172 patients [10]. The following pooled recurrence rates for three different techniques were found: 39% (95% CI 27%–52%) after biological mesh closure (n = 60), 29% (95% CI 21%–39%) after synthetic mesh closure (n = 93), 37% (95% CI 14%–67%) after tissue flap reconstruction only (n = 9) and 9% (95% CI 1%–45%) after tissue flap reconstruction combined with mesh (n = 10). The highest recurrence rate after biological mesh closure might be related to the use of resorbable mesh, but synthetic meshes still have high recurrence rates, indicating that fixation of the mesh is probably the Achilles heel of the technique. The present study even revealed a 34% crude recurrence rate using synthetic mesh. The study with the highest weight in pooled analysis (n = 29) was a previous publication from one of our centres, the Catharina Hospital, Eindhoven. The technique adopted since 2006 appeared to be successful in 20 of 21 patients (95%) at that time with relatively short follow-up [12]. These 21 patients have been updated and included in the present series. This update revealed another six recurrences, emphasizing the importance of adequate length of follow-up.
The systematic review also showed that tissue flap-only repair is similarly ineffective as mesh-only repair, while combined mesh with flap repair revealed the lowest failure rate (9%) [10]. Eight of the 10 patients who underwent mesh with flap repair in the review are also included in the present study, with updated follow-up. The findings are still promising, and in addition we now demonstrate that mesh with flap repair can be also effectively applied after a failed mesh-only technique.
Baseline differences, proportions of anal cancer, extralevator APR and additional resection during APR were larger in the mesh with flap group. Also, more laparoscopic APRs were performed within this group, limiting the formation of adhesions that usually prevents the small bowel from descending [2]. Furthermore, more mesh with flap patients had previously had a hysterectomy, which reduces pelvic support with increased risk of vaginal prolapse and development of a perineal hernia [9]. Lastly, significantly more postoperative perineal wound complications after index APR were observed in the mesh with flap group, which further increases the risk of perineal hernia in this patient group [14]. Given these findings, the initial characteristics of the mesh with flap patients were worse than those of the mesh-only patients. This strengthens our conclusion on the potential superiority of mesh with tissue flap reconstruction over mesh-only repair.
The disadvantages of the combined technique are a longer duration of surgery and prolonged length of hospital stay. The five additional days of admission may be attributed to the initial imposition of bedrest, a practice we have gradually abandoned. In contrast, postoperative complications seem to be mild after mesh with flap repair. The observed perineal dehiscences could be due to some traction on the midline after gluteal turnover flap, as there is less skin to close after harvesting the flap. However, these perineal wound dehiscences recovered without any reintervention or mesh explantation. It can be imagined that a tissue flap covering the implanted mesh has some protective effect, as illustrated by the difference in postoperative infective complications (16% after mesh only versus 0% after mesh with flap). This might be explained by the fact that the tissue flap is sutured onto the mesh, filling up the dead-space between the mesh and the perineal skin and thereby preventing the accumulation of fluid with the inherent risk of secondary infection. Routine placement of at least one perineal vacuum drain might also have contributed to the prevention of these complications—this was done in only 26% of the mesh-only patients.
Regarding the suggested lower recurrence rate, we assume that the flap provides additional support to the mesh. Complete coverage of the bridging mesh by well-vascularized tissue might also improve ingrowth of the mesh. A better wound healing process can strengthen the neo-pelvic floor with better support of the abdominal organs, thereby reducing the number of recurrent perineal hernias.
Our study has several limitations, mostly related to the retrospective cohort study design. The number of patients included in the mesh with flap group is still low. The duration of follow-up was relatively short after mesh and flap repair, despite this being an update of a previously published initial experience. Nevertheless, the median 24 months follow-up in this group was longer than the median time to developing a recurrent perineal hernia. Because of the rarity of perineal hernia it is difficult to substantially increase experience with a novel repair technique within a reasonable time frame. The inclusion of patients with multivisceral resections introduces a potential bias, as these procedures are a known risk factor for development of perineal hernia. Similarly, included patients underwent different types of perineal closure during index surgery. However, their inclusion ensures that the findings are representative of the diverse spectrum of patients encountered in clinical practice. Finally, there was also heterogeneity among applied techniques, due to the different gluteal flaps that were used for filling the dead space.
By multicentre collaboration we have, to the best of our knowledge, created the largest cohort on this topic [10]. This has enabled us to provide clinically relevant advice on treatment for future perineal hernia patients. Based on the observed differences in recurrence rates between the two repair techniques, we advocate the use of mesh with flap reconstruction as the primary treatment principle for patients with a primary perineal hernia as well as those with a recurrent perineal hernia.
CONCLUSION
In patients with a perineal hernia, adding a tissue flap to the mesh repair of the pelvic outlet seems to results in a lower recurrence rate compared with synthetic mesh-only repair. This combined repair also seems to be effective as a salvage treatment after a failed mesh-only repair. Furthermore, perineal wound complications in mesh with tissue flap patients were minor. These findings must be confirmed in larger series.
AUTHOR CONTRIBUTIONS
Saskia I. Kreisel: Conceptualization; investigation; writing – original draft; methodology; formal analysis; data curation. Dite L. C. de Jong: Conceptualization; investigation; writing – original draft; methodology; formal analysis; data curation. Roel Hompes: Conceptualization; supervision; writing – review and editing. Jacobus W. A. Burger: Supervision; writing – review and editing. Simon W. Nienhuijs: Supervision; writing – review and editing; conceptualization. Cornelis Verhoef: Supervision; writing – review and editing. Gijsbert D. Musters: Supervision; writing – review and editing; conceptualization. Pieter J. Tanis: Supervision; writing – review and editing; conceptualization.
FUNDING INFORMATION
No funding has been received by any author in relation to this article.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
The medical ethics board was consulted in all participating hospitals, and all gave their approval. Therefore, this study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
PATIENT CONSENT STATEMENT
An opt-out letter was sent if deemed necessary by the medical ethics board.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
No material reproduced from other sources was used in this article.
CLINICAL TRIAL REGISTRATION
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




