Protocol for a multi-centre observational and mixed methods pilot study to identify factors predictive of poor functional recovery after major gastrointestinal surgery and strategies to enhance uptake of perioperative optimization
Optimizing the care and treatment pathways for older patients facing major gastrointestinal surgery (OCTAGON)
Trial registration ID: NCT04545125
Funding information
This study has been funded by an educational grant from Bowel Research UK (formerly the Bowel Disease Research Foundation) (awarded February 2019). The funder had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data or decision to submit results.
Abstract
Introduction
National datasets report large variations in outcomes from older people (≥65 years) between different UK surgical units. This implies that not all patients receive the same level of care or access to resources, such as rehabilitation or allied health professional input. This might impact functional decline.
Aims
Our aim is to evaluate the baseline status of older patients facing major gastrointestinal surgery and the impact of variation in perioperative assessment and provision of perioperative support on functional outcomes. Patients’ experiences and views of assessment and optimization will be explored via integrated qualitative semi-structured interviews.
Methods and analysis
This multi-centre, pilot cohort study will include patients ≥65 years presenting via both elective and emergency pathways at three to five South Yorkshire NHS hospitals (Clinical Trials registration NCT04545125). The primary outcome is functional recovery measured using the World Health Organization Disability Assessment Schedule 2.0 at 6 weeks post-operation. Secondary outcomes include feasibility, quality of life, length of stay and complication rate. An opportunistic sample size of 120 has been estimated and will inform the design of a future, adequately powered study. For the qualitative study, 20–30 semi-structured patient interviews will be undertaken with patients from the cohort study to explore experiences of assessment and optimization. Interviews will be digitally recorded, transcribed verbatim and analysed according to the framework approach.
Ethics and dissemination
This study has been approved by the National Health Service Research Ethics Committee and is registered centrally with Health Research Authority. It has been adopted by the National Institute for Health Research Portfolio scheme. Dissemination will be via international and national surgical and geriatric conferences.
INTRODUCTION
Background
As the population ages, there are increasing numbers of older people presenting with gastrointestinal (GI) conditions amenable to major surgery. However, both under-investigation and under-treatment of older people are common, with rates of surgery declining with age [1-3]. There are large variations in outcomes in older people across different surgical units in the UK that are not wholly attributable to differences in socioeconomic circumstances or comorbidity. This implies that not all older patients receive the same level of care or access to resources [4-7]. In GI surgery, there is some concern that patients in centres with low elective surgery rates could be inappropriately denied the benefits of operative intervention including disease control and symptom improvement. This may lead to higher rates of emergency admission and intervention [3, 8]. Conversely, in centres with high rates of elective surgery, patients may be inappropriately subjected to the morbidity or mortality of surgery when there is limited or no benefit. This disparity is accentuated when older patients require emergency surgery [4, 9-11].
Adverse factors associated with ageing include comorbidity, polypharmacy, malnutrition, cognitive impairment, dependency and frailty, all of which are associated with increased all-cause mortality in the general population [12] and reduced quality of life following hospital admission [13]. There is also a natural decline in cardiorespiratory fitness with age. Major surgery in all patients leads to a short-term decrease in cardiorespiratory fitness and functional capacity [14]. However, in older adults this contributes towards long-term disability and loss of independence [15, 16]. Many older patients never regain their previous level of functioning after major surgery [16, 17].
Stratification and optimization of risk in the older population is a challenge. Guidelines for GI cancers focus on diagnosis and staging rather than assessment of suitability for treatment in this patient group [18-20]. Other guidance, such as that for diverticular disease, advises against the use of age in treatment decisions but does not offer alternative stratifiers [21]. Consequently, the process of assessment varies considerably between surgeons and surgical units [22]. Subjective assessment of functional status is a frequent aspect of surgical and anaesthetic assessments [23] but it has been suggested that this may result in misclassification of high-risk patients as low risk [24, 25]. In the elective setting, cardiopulmonary exercise testing (CPET) is gaining acceptance as an objective measure of cardiorespiratory fitness with the ability to predict complications and mortality after major abdominal surgery [24, 26, 27] however, it is not universally available [28]. Malnutrition is known to be a poor predictor of outcomes but many patients are not screened prior to treatment decisions [29]. Frailty, the state of enhanced vulnerability to minor stressors, has been shown to be predictive of poor outcomes and increased care needs post-discharge in both the elective [30] and emergency surgical settings [9, 10, 31] but still requires integration into surgical pathways in many hospitals [11]. Perioperative risk assessment, combining clinical assessment, objective tests and available risk calculators, must be individualized in both the elective and emergency settings to enable targeted optimization.
Optimization of outcomes in older patients with comorbidities and frailty requires multi-professional input which is often lacking [32, 33]. Many of the adverse factors associated with ageing may be modifiable if they are identified early in the patient pathway and by using evidence-based strategies and interventions. Strategies shown to be effective, particularly in the emergency setting, include senior clinician involvement, early cross-sectional imaging, timely access to theatres and risk-stratified decisions on postoperative destination (i.e., intensive care department vs. surgical ward) [11]. Interventions include multimodal exercise, nutrition and psychological interventions, termed ‘prehabilitation’ [34], comprehensive geriatric assessment and intervention, enhanced recovery after surgery protocols and delirium prevention pathways, for example. However, there is limited evidence of how these interventions are applied in current surgical practice and their effects on the functional recovery of older patients [35].
This study will objectively measure the functional recovery of older adults after a range of major GI operations using validated questionnaires completed by the patients with support from the research team. Evaluation of outcomes from the patient's perspective (patient reported outcomes, PROs) is acknowledged to be important in surgical research, particularly in older adults. Many studies in this area collect PROs as secondary outcomes [36, 37] and increasingly integrated patient interviews are being used to facilitate process evaluation [38].
The study will determine what optimization strategies are used in practice to mitigate against identified risk factors. It will also observe the functional trajectory of some patients who are deemed ‘unfit’ for major operative intervention. Barriers and facilitators to perioperative optimization and experiences of patients will be explored through semi-structured interviews. Mixed methods synthesis of results will enable qualitative data to help explain some of the differences observed in the observational study.
The research questions are as follows.
- Is it feasible to use questionnaires to comprehensively baseline assess older patients presenting via elective and emergency pathways?
- Can validated questionnaires be used to assess functional recovery in GI surgical patients?
- What baseline characteristics of patients are associated with poor functional outcome?
- What preoperative, perioperative and postoperative optimization strategies are used in practice and what are their effects on functional outcomes? (exploratory question only)
- What are the views of patients about the assessment process for major GI surgery?
- What are patients’ experiences of preoperative, perioperative and postoperative optimization strategies?
Objectives
The objectives are to determine:
- which baseline characteristics of older patients with GI pathology amenable to major surgery are predictive of poor postoperative functional recovery measured using PROs;
- whether certain baseline characteristics mean that an individual is more likely to undergo a risk-adapted procedure or conservative management;
- the degree and causes of variation in the patient pathway with respect to assessment and perioperative support across a region;
- the views of older patients who have undergone elective and emergency surgical management regarding enhanced perioperative support measures and fitness/risk assessment.
METHODS
This protocol has been prepared according to the SPIRIT-PRO guidelines [39]. It is registered on the clinicaltrials.gov database (NCT04545125) and is funded by an educational grant from Bowel Research UK (formerly the Bowel Disease Research Foundation).
Aims
The aims are to assess the functional recovery of older patients following elective and emergency GI surgery and the impact of variation in baseline health status and provision of perioperative support at different hospitals within a region.
Study design
This is a prospective, multi-centre, mixed methods observational pilot cohort with integrated qualitative patient evaluation.
Study schematic
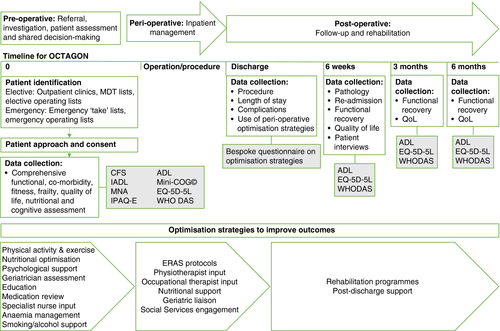
An overview of the design is presented in Figure 1.
Eligibility criteria
Inclusion
Those eligible for recruitment include patients aged 65 years or older with a diagnosis of GI pathology amenable to curative elective, urgent or emergency major GI surgery (surgical eligibility criteria are presented in Table 1). Participants may undergo major surgery, a risk-adapted procedure or conservative management due to patient wishes, comorbidities or fitness. Participants must have the mental capacity to consent and be able to understand written and spoken English due to insufficient resources to support translation services and the PRO nature of data collection.
| Inclusion | Exclusion | |
|---|---|---|
| Elective |
Malignant Colon, rectal, gastric, oesophageal and pancreatic cancers, hepatocellular carcinoma, colorectal liver metastases, sarcoma, cholangiocarcinoma Non-malignant Complicated diverticular disease, complex abdominal wall hernias, Crohn's disease, ulcerative colitis, complicated gallstone disease (planned open or CBD exploration), reflux disease (fundoplication) |
Planned laparoscopic treatment of uncomplicated gallstone disease, uncomplicated groin hernia, laparoscopic appendicectomy |
| Emergency |
Malignant Obstructing/symptomatic colon, rectal or gastric cancer, reoperations for complications of previous elective surgery (these will be included in elective) Non-malignant Adhesional small bowel obstruction, obstructed hernias, bowel ischaemia, gastric/duodenal perforation, colonic perforation, peritonitis, large bowel obstruction, volvulus, complicated diverticulitis, Crohn's disease, ulcerative colitis |
Trauma, appendicitis, pancreatitis |
- Abbreviation: CBD, common bile duct.
Exclusion
Individuals with unresectable disease due to location, invasion or dissemination will be excluded as any surgery would not be with curative intent (surgical management is less common and aims of treatment in these patients are very different to the general GI surgical population). Individuals with permanent or transient lack of capacity (e.g., due to delirium) will not be able to take part unless the delirium develops after enrolment in the study.
Surgery for major trauma or primary gynaecological, vascular or urological disease is excluded.
Primary outcome
The primary outcome is functional recovery at 6 weeks measured using the World Health Organization Disability Assessment Schedule 2.0 (WHO DAS v2.0) [40] which has been validated as a qualitative measure of functional recovery in surgical populations [41, 42]
Secondary outcomes
- Feasibility of recruiting and retaining older elective and emergency surgical patients to a questionnaire-based study, including adherence to outcome assessment schedule (this will help to inform the design of future studies)
- Health related quality of life at 6 weeks (measured using the EQ-5D-5L [43])
- Length of hospital stay (days)
- Postoperative complications (including type and Clavien–Dindo grade of complication [44])
- Overall survival (including time to and cause of death)
- Rate of use and type of perioperative assessment tools such as CPET, 6-min walk test
- Rate of and type of perioperative support such as formal prehabilitation programmes, physical activity interventions, nutritional support etc.
Participant recruitment
Patients will be identified at multidisciplinary team meetings, surgical outpatient clinics, from elective operating lists and on call ‘take’ lists for elective and emergency presentations respectively and screened for eligibility. Patients will be approached by the local principal investigator (PI), delegated clinician or nursing study team members with the appropriate good clinical practice training. All potentially eligible patients will be recorded on the local screening log. Recruitment at Sheffield Teaching Hospitals commenced on 9 September 2020; the study is in set-up phase at the other sites. Study visits will be coordinated with usual clinical appointments or conducted by telephone or post to reduce the burden on patients. See Table 2 for a summary of the study timeline.
| Baseline (first clinic to day 0/operation) | Discharge | 6 weeks postoperative or after decision to not operate ± 2 weeks | 3 months post-operation or post-decision | 6 months post- operation or post-decision | |
|---|---|---|---|---|---|
| Consent | x | ||||
| Demographics, comorbidity, polypharmacy | x | ||||
| Questionnaires | ADL, IADL, EQ-5D-5L, CFS, MNA, IPAQ-E, Mini-Cog, WHO DAS | Bespoke questionnaire (see Appendix S1–S3) | EQ-5D-5L, ADL, WHO DAS | EQ-5D-5L, ADL, WHO DAS | EQ-5D-5L, ADL, WHO DAS |
| CPET/6 MWT results (if available) | x | ||||
| Optimization strategies | x | ||||
| Operation details | x | ||||
| Postoperative details | x | ||||
| Complications | x | ||||
| Pathology | x | ||||
| Survival | x | x | x | x |
- Abbreviations: ADL, Activities of Daily Living; CFS, Clinical Frailty Scale; CPET, Cardiopulmonary Exercise Testing; 6 MWT, 6-min walk test; IADL, Instrumental Activities of Daily Living; IPAQ-E, International Physical Activity Questionnaire—Elderly; MNA, Mini Nutritional Assessment; WHO DAS, World Health Organization Disability Assessment Schedule 2.0.
Data collection
At baseline, demographics, type of referral, preoperative assessment date and admission details will be collected for all patients. Comorbidities will be collected using the Charlson Comorbidity Index, a validated measure of prognostic impact of multiple chronic illnesses. Polypharmacy is defined as five or more regular medications. Preoperative blood tests relevant to the emergency and elective presentations will be collected. A detailed functional, nutritional and fitness assessment will be carried out using a number of validated questionnaires (Barthel's Activities of Daily Living [ADL], Lawton and Brody's Instrumental Activities of Daily Living, Clinical Frailty Scale [45], Mini Nutritional Assessment [46], International Physical Activity Questionnaire—Elderly [47], Mini-Cog© [48], WHO DAS and EQ-5D-5L) for the patient to complete themselves or with assistance from the research team and review of patient records (e.g., for CPET results). The order of administration of baseline questionnaires will be standardized as follows:
- Activities of Daily Living
- Instrumental Activities of Daily Living
- EQ-5D
- International Physical Activity Questionnaire—Elderly
- WHO DAS v2.0
The following questionnaires are completed by the research team at baseline in the following order:
- Clinical Frailty Scale
- Mini-COG
- Mini Nutritional Assessment
Members of the patient and public involvement (PPI) group have been consulted to ensure that the number of questionnaires is acceptable to patients and does not represent a significant burden. Validated questionnaires will be used in accordance with their respective user manuals. PRO measure instruments are summarized in Table 3.
| PRO measure | Number of domains | Number of items | Instrument scaling and scoring |
|---|---|---|---|
| EQ-5D | 5 measuring overall health status (utility measure) plus visual analogue scale | 5 | Simple scoring: 1–5 per question with 1 being ‘no problems’ and 5 being ‘unable to’ or ‘extreme’. The individual scores are not added together but interpreted using the guidelines. The visual analogue scale is scored from 0 to 100 with 0 being ‘worst health imaginable’ and 100 being ‘best health’ to give a quantitative measure of the patient's overall perception of health |
| WHO DAS v2.0 | 6 | 12 | Simple scoring: 0–4 per question with 48 maximum score representing the highest level of disability |
| ADL | 8 | 10 | Simple scoring: 0–2 or 3 per question with maximum score 20. Lower score represents higher level of dependency |
- Abbreviations: ADL, Activities of Daily Living; PRO, patient reported outcome; WHO DAS v2.0, World Health Organization Disability Assessment Schedule version 2.0.
At hospital discharge, patients will be asked to complete a questionnaire (see Appendix S1–S3) regarding preoperative, perioperative and postoperative optimization. Elective patients will be asked whether they participated in any form of prehabilitation (exercise, nutrition, psychological, geriatric), attended ‘surgery school’ or attended for transfusion, iron infusion, physiotherapy appointment, smoking cessation services or dietitian review and whether this was self-directed or arranged by the hospital. Elective and emergency patients will be asked about perioperative and postoperative optimization and specialty reviews (e.g., geriatrician, cardiology). The hospital records will be used to determine operative details, postoperative complications (using the Clavien–Dindo classification system), length of hospital stay and discharge arrangements.
Follow-up
At 6 weeks post-operation/procedure or decision not to operate the final pathology result, survival and readmission rate will be determined. Follow-up questionnaires (ADL, EQ-5D-5L and WHO DAS) will be completed to assess functional recovery at 6 weeks, 3 months and 6 months post-operation/procedure. These time-points have been chosen to look at postoperative recovery over time [17]. In addition, follow-up questionnaire results will be compared to baseline results. The order of administration of follow-up questionnaires will be standardized as follows:
- EQ-5D
- ADL
- WHO DAS v2.0
Follow-up questionnaires will be completed by telephone with the patient. If a patient does not answer the telephone the researchers will attempt two more times before reporting the item as missing. Patients will then be contacted at their next follow-up time-point.
Integrated qualitative study
Qualitative patient interviews will be integrated with the quantitative study. Interviews will be semi-structured to enable an exploration of different themes with reference to a pre-prepared interview prompt sheet and the patients’ responses to the bespoke questionnaire. Interviews will focus on views on perioperative support measures, perceived barriers and facilitators to implementing these and mode of delivery. Perceptions of fitness and risk assessment and what this means to individuals will also be explored.
Maximal variation sampling will be used to select patients who have undergone different methods of assessment and optimization and across the full age spectrum (stratified recruitment to age groups 65–75, 75–85 and >85 years). From previous work in the field it is anticipated that approximately 20–30 interviews will be required, aiming for 10 in each age cohort across elective and emergency presentations. Participants will be identified from the cohort study if they have given consent for this aspect of the study. All interviews will be carried out by the chief investigator (CI) of the study, will be digitally recorded and transcribed verbatim. Interview transcripts will be anonymized prior to analysis and original recordings deleted.
Interview data analysis using the framework approach [49]will occur alongside recruitment, and recruitment will cease on achievement of data saturation. Three or four transcripts (10% total) will be double coded by an experienced qualitative researcher from the research team (MB) to ensure credibility and dependability of the qualitative findings.
Analysis of the qualitative and quantitative aspects will occur in tandem. This will enable the patient interviews to be used to explore the effects of different baseline health status and optimization strategies on patient attitudes and responses. They will also be used to explore whether patients with worse functional outcomes have different experiences to those with better outcomes. Triangulation of qualitative and quantitative data will enable us to develop a comprehensive understanding of the findings [50]. Findings from this study could help to inform prioritization of services.
Protocol amendments
Protocol amendments will be approved by the Health Research Authority and communicated to all local PIs and research and development teams by the study team.
Data management and monitoring
All data will be handled in accordance with the General Data Protection Regulation 2018 principles. All patients will be given a unique ID number which will be used in the database rather than their National Health Service (NHS) number (i.e., pseudo-anonymized). Data will be collected and recorded by hospital staff or members of the hospital research team on paper-based case report forms which will then be entered into a secure server running the Research Electronic Data Capture (REDCap) system based at the University of Sheffield [51]. REDCap allows collaborators to enter and store data in a secure system. Data will be monitored for quality and completeness by the study team. Missing data will be sought until they are received, confirmed as not available, or the study is at analysis. PRO data will not be monitored during the study to inform clinical care as this is an observational study only. Access to the entire study dataset will only be available to co-investigators of the study as detailed in the ethics application.
Statistical analysis and power calculation
Descriptive analyses will be performed to describe the recruited population of patients aged 65 years and over undergoing major GI surgery via emergency and elective pathways at different surgical units within a region. Descriptive analyses will also be used to detail the optimization pathways reported by patients and documented in their medical records. Univariate correlation analyses will be used to look for relationships between baseline variables and functional outcomes, but these will be exploratory only. As this is a pilot study, feasibility of recruiting from this patient population will be assessed by recording the number of people who decline participation. Adherence to the follow-up schedule will also be used to guide the design of future studies.
An opportunistic sample size of 120 has been estimated over the 6-month study recruitment period based on the number of patients undergoing major surgery at each of the units. Each surgical unit in South Yorkshire performs between 70 and 300 major elective GI resections per year and 114–300 emergency laparotomies per year, of which at least 50% will be over the age of 65 [11]. A high uptake rate of this simple, questionnaire-based study is anticipated based on a recent study of frailty in emergency laparotomy patients [10] and a postoperative study of quality of life after emergency laparotomy [52]. One aim of the study is to capture variation in practice; a sample of 120 should permit this, especially when spread across three or more centres. Methods to use pilot study data to estimate sample size for trials vary. A sample size of 120 exceeds the proposed size of 60–100 proposed by Teare et al. [53] to estimate effect size for continuous outcomes. It should also be compatible with other methods of effect size estimation [54].
DISCUSSION
Optimizing treatment pathways of older adults undergoing major GI surgery is of importance with growing waiting lists and constraints on NHS resources. Patients are likely to be waiting longer for their surgical treatment to commence; therefore efforts need to be made to maximize the use of this time to ensure that patients are as prepared as possible before any surgery. The pandemic has led to huge changes to the way in which the NHS works and has driven digital innovations; patients may not meet their surgeon face-to-face until late in the surgical pathway.
The introduction of numerous different interventions in different pathways makes conduct of randomized trial designs challenging in perioperative care, as was demonstrated in the EPOCH trial [29]. This study will observe current practice in optimization practice across a diverse range of NHS hospitals providing elective and emergency GI surgery. Mixed methods data on how and why interventions are effective will be used to drive local service improvement and to inform the design of future trials. If this pilot study is successful, future studies by the research team aim to integrate translational elements, such as biomarker studies, to measure and monitor frailty in this population.
We are not aware of any similar questionnaire-based studies in this patient population. The primary outcome measure, the WHO DAS v2.0, has been used as an outcome measure in urological trials, but not in GI surgery. The feasibility of collecting baseline and outcome data through questionnaires in this population may assist in the development of low-cost assessment processes for patients in both clinical and research contexts. The study will also provide much needed data to inform the design of future studies with a focus on patient centred outcomes, rather than clinical end-points.
ETHICS AND DISSEMINATION
Ethical approval
Ethical approval for this study was granted by an NHS Research Ethics Committee via the proportionate review system. Health Research Authority Approval has been obtained. This was granted by the Health and Care Research Wales (HCRW) Research Committee on 4 May 2020 (REC Reference 20/SC/0076).
All participating units must obtain approval from their local Research and Development Department consistent with the guidance from the Health Research Authority. It is the responsibility of the local PI to ensure that the relevant approvals are in place prior to commencing data collection.
Patient and public involvement
Patient and public involvement is integral to this study. The role of the PPI group is reported here according to the GRIPP2-SF guidelines [55]. The main aim of the PPI group so far has been to ensure that the research question is of relevance to the lay person and to review the study protocol. They also reviewed the patient-facing materials, which included checking that the number and time taken to complete the questionnaires was not much of a burden for patients. This resulted in a reduction in the number of baseline questionnaires and to changing one of the questionnaires to a shorter version. The CI met with the PPI group twice regarding this study and also communicated with them via email. One member is on the study steering group. This group will also be involved in disseminating the study findings. Future PPI involvement for a study of this nature might benefit from a speciality specific PPI group, such as those with experience of GI surgery, to facilitate dissemination of results.
Dissemination
Results from the study will be analysed after completion of data collection at all sites. Results from individual sites will be fed back to the local PIs for dissemination at hospital level. The PPI representatives will also be involved in disseminating to patient groups. Findings will be submitted to regional and national conferences in surgery, geriatrics or perioperative care. Manuscript(s) will also be prepared for publication in peer-reviewed journal(s).
Collaborators at participating hospitals with significant input, particularly regarding recruitment and follow-up of patients, will be eligible for collaborative authorship according to published guidelines [56].
CONFLICT OF INTERESTS
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
SLD and LW conceived the project. SLD, LW, SB, TRW, ML and SM initiated the study design. MB assisted in qualitative study design. SLD, LW and MB are responsible for the PRO content of the study protocol. JG will be taking over coordination and delivery of the study in April 2021. All authors contributed to refinement of the study protocol and approved the final paper.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.




