Host cell membrane microdomains and fungal infection
Funding information: Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Grant/Award Numbers: E-26/202.696/2018, E-26/202.760/2015, E-26/202.809/2018; National Institutes of Health, Grant/Award Number: R21 AI124797; Pasteur-Roux-Cantarini; Institut Pasteur; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Grant/Award Number: Finance Code 001; Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Numbers: 311179/2017-7, 311470/2018-1, 408711/2017-7
Abstract
Lipid microdomains or lipid rafts are dynamic and tightly ordered regions of the plasma membrane. In mammalian cells, they are enriched in cholesterol, glycosphingolipids, Glycosylphosphatidylinositol-anchored and signalling-related proteins. Several studies have suggested that mammalian pattern recognition receptors are concentrated or recruited to lipid domains during host-pathogen association to enhance the effectiveness of host effector processes. However, pathogens have also evolved strategies to exploit these domains to invade cells and survive. In fungal organisms, a complex cell wall network usually mediates the first contact with the host cells. This cell wall may contain virulence factors that interfere with the host membrane microdomains dynamics, potentially impacting the infection outcome. Indeed, the microdomain disruption can dampen fungus-host cell adhesion, phagocytosis and cellular immune responses. Here, we provide an overview of regulatory strategies employed by pathogenic fungi to engage with and potentially subvert the lipid microdomains of host cells.
Take Away
- Lipid microdomains are ordered regions of the plasma membrane enriched in cholesterol, glycosphingolipids (GSL), GPI-anchored and signalling-related proteins.
- Pathogen recognition by host immune cells can involve lipid microdomain participation. During this process, these domains can coalesce in larger complexes recruiting receptors and signalling proteins, significantly increasing their signalling abilities.
- The antifungal innate immune response is mediated by the engagement of pathogen-associated molecular patterns to pattern recognition receptors (PRRs) at the plasma membrane of innate immune cells. Lipid microdomains can concentrate or recruit PRRs during host cell-fungi association through a multi-interactive mechanism. This association can enhance the effectiveness of host effector processes. However, virulence factors at the fungal cell surface and extracellular vesicles can re-assembly these domains, compromising the downstream signalling and favouring the disease development.
- Lipid microdomains are therefore very attractive targets for novel drugs to combat fungal infections.
1 INTRODUCTION
The presence of lipid microdomains in the membrane was first suggested by Simon and Van Meer in the MDCK cell (Simons & Van Meer, 1988). Since then, their existence and participation in vital cellular processes, including protein sorting, membrane trafficking and signal-transduction events, have been confirmed (Simons & Ikonen, 1997). Lipid microdomains are enriched in sphingolipids, cholesterol and specific proteins. The lipid moiety of sphingolipids and sterols are connected through hydrogen bonds and hydrophobic interactions resulting in a highly ordered structure (Mukherjee & Maxfield, 2004; Simons & Ikonen, 1997). The presence of glycans covalently attached to sphingolipids can also mediate cis interactions, promoting lateral association with other glycosphingolipids and proteins (D'Angelo, Capasso, Sticco, & Russo, 2013). The major proteins enriched in lipid microdomains include glycosylphosphatidylinositol (GPI) anchored proteins and palmitoylated proteins, making these domains organised platforms for signal transduction (Komura et al., 2016; Westerlund, Grandell, Isaksson, & Slotte, 2010). They are continuously dynamic, and upon stimulus, can coalesce in larger complexes, aggregating different raft-associated proteins and lipids in intimate association with the cytoskeleton (Gulbins, Dreschers, Wilker, & Grassmé, 2004). Consequently, several receptors and signal transduction proteins accumulate in these regions, increasing signalling efficiency. The internalisation of pathogens seems to be one of the events potentially mediated by lipid microdomains (Bukrinsky, Mukhamedova, & Sviridov, 2020).
The host-microbe association is a highly dynamic mechanism that involves multiple interactions. In this context, the combination of molecules gathered in the host cell platforms during the cellular response directly impacts the fate of the invaders. It is important to mention that the fungal plasma membrane also contains lipid microdomains (Farnoud, Toledo, Konopka, Del Poeta, & London, 2015). In these organisms, the microdomains have been correlated with protein sorting, cell budding and growth, biofilm formation, drug resistance and infectivity, as extensively reviewed by Farnoud et al. (2015). The presence of virulence factors, such as phospholipase B and superoxide dismutase in lipid rafts from Cryptococcus neoformans (Siafakas, Wright, Sorrell, & Djordjevic, 2006) and the requirement for their integrity during the infection of macrophages by Histoplasma capsulatum (Tagliari et al., 1818) suggest that fungal lipid microdomains are also determinant components during the interaction with host cells. Nonetheless, this review aims to discuss the participation of lipid microdomains from host cells during the interaction and invasion of pathogenic fungi.
2 LIPID RAFTS AND THE INNATE IMMUNE RESPONSE TO FUNGAL INFECTIONS
The major pattern recognition receptors (PRRs) involved with the recognition of fungi are Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) (Kirkland & Fierer, 2020). PPRs are expressed in many cell types involved in the innate immune response, and, upon activation, they trigger downstream signalling transduction pathways that lead to phagocytosis, microbial killing and cytokine production. Among TLRs, TLR-2/1, TLR2/6 and TLR4 (surface localization) and TLR3/7 and 9 (endosomal localization) are involved in fungal responses (Biondo et al., 2012; Kasperkovitz, Cardenas, & Vyas, 2010; Miyazato et al., 2009; Netea, Van De Veerdonk, Verschueren, Van Der Meer, & Kullberg, 2008; Rubino et al., 2012). Transmembrane CLRs linked to antifungal immunity include dectin-1, dectin-2, dectin-3, mannose receptor, DC-SIGN, Mincle and galectin-3 (Patin, Thompson, & Orr, 2019).
With the important exception of encapsulated pathogenic fungal species (e.g., C. neoformans and Cryptococcus gattii), the cell wall establishes the first contact of these organisms with PRRs. The precise identification of receptors to fungal pathogen-associated molecular patterns (PAMPs) is challenging since the fungal cell wall is a complex and dynamic structure (Kang et al., 2018; Patin et al., 2019), where diversity of PAMPs, such as glucans, chitin, mannans and mannoproteins, can be exposed to phagocytes PRRs displayed by phagocytes. The cell wall inner layer is relatively conserved among different species, but the outer layers can differ substantially and variably affect the host cell response (Gow, Latge, & Munro, 2017).
The involvement of co-receptors during fungal recognition is associated with the amplification of the immune response. The initial mechanism of cooperation occurs during the engagement of fugal cells to their receptors, where one receptor can capture and present the fungal particle to a co-receptor (Hoebe et al., 2005). In addition, the signalling response mediated after the engagement could be integrated intracellularly (Dennehy et al., 2008). PRRs can cluster on the host cell surface in response to the abundance and presence of distinct ligands, strengthening the immune response. Indeed, the lack of PRRs co-stimulation seems to be the mechanism used by the dematiaceous fungus Fonsecaea pedrosoi to reduce the host immune response, resulting in a chronic infection called chromoblastomycosis (da Glória et al., 2011). Although leukocytes recognise F. pedrosoi conidia through Mincle, this association is not sufficient to promote a protective inflammatory response (da Glória et al., 2011). However, the co-stimulation with TLR agonists potentiated the inflammatory response, indicating that a collaborative process involving distinct PRRs is required to induce a protective response. Remarkably, the administration of LPS to mice infected with F. pedrosoi substantially reduced the fungal burden. In addition, fungal burden decrease was also observed in mice infected subcutaneously with F. conidia and topically treated with Imiquimod, a synthetic agonist for TLR7. This imidazoquinolide derivative was successfully used in human chromoblastomycosis (de Sousa et al., 2014). The raft environment seems to be a distinct place where paired receptors could cooperate and amplify the cellular response. Indeed, the recruitment of TLRs to these domains after ligand engagement has been reported (Płóciennikowska, Hromada-Judycka, Borzęcka, & Kwiatkowska, 2015).
All TLRs, except TLR3, led to the recruitment of the adaptor molecule MyD88 (Kawasaki & Kawai, 2014). MyD88 contains a death domain at the N terminus that interacts with the kinase IRAK, forming the Myddosome complex, a platform that leads to transcriptional activation through NF-κB and AP-1. TLR4 clustering in lipid rafts produces a large oligomeric assembly of MyD88 with IRAK-1, which is associated with enhanced activation (Motshwene et al., 2009). The importance of rafts for MyD88-dependent TLR activation has been demonstrated in macrophages genetically modified expressing high lipid raft content (Zhu et al., 2010). These cells displayed a remarkable content of TLR4 in lipid raft regions and were more sensitive to TLR2, 7 and 9 but not to TLR3 stimulation. Other PRRs can collaborate with TLRs, including dectin-1, DC-SIGN and the scavenger receptor (CD36) (Gringhuis et al., 2007; Inoue et al., 2011; Inoue & Shinohara, 2014; Triantafilou et al., 2006). Their association could be linked with TLR recruitment to lipid rafts, amplifying the response. For instance, CD36 is a palmitoylated protein usually enriched in lipid rafts, functioning as a TLR2 collaborator within the domains (Triantafilou et al., 2006). Similarly, CD14 is a GPI-anchored protein present in the raft, and its association with LPS stimulates the translocation of TLR4 into lipid microdomains (Nakahira et al., 2006; Triantafilou, Miyake, Golenbock, & Triantafilou, 2002). Numerous studies have demonstrated that the association between TLR2, 4, 5, 7 or 9 and dectin-1 strengthens the immune response to achieve appropriate biological responses to pathogenic fungi (Dennehy et al., 2008; Ferwerda, Meyer-Wentrup, Kullberg, Netea, & Adema, 2008; Gantner, Simmons, Canavera, Akira, & Underhill, 2003; Inoue et al., 2011). Confirming that PRRs collaboration is important to an effective immune response Gantner and colleagues have shown that activation of TLR2 by zymosan particles is insufficient to induce high levels of reactive oxygen species production in macrophages, which requires collaboration with dectin-1 (Gantner et al., 2003). In contrast with TLRs, which is able to recognise soluble microbial components, dectin-1 becomes activated only with immobilised forms of β-(1,3)-glucan, leading to a “phagocytic synapse” and a robust antifungal response (Goodridge et al., 2011). The fact that dectin-1 translocates into lipid microdomains of mouse bone marrow-derived DC in response to zymosan stimuli suggests that clustering of these heterologous PRRs occurs in these platforms (Xu, Huo, Gunawan, Su, & Lam, 2009).
The cross-talk between PRRs expressed by DCs has additional importance for orchestrating the differentiation of T helper (Th) cells. Dectin-1 activation drives DCs maturation and priming of naive CD4 T cells towards Th1 and Th17 phenotypes, commonly involved with protection against pathogenic fungi (Agrawal, Gupta, & Agrawal, 2010). Translocation of dectin-1 into lipid microdomains is essential for Syk phosphorylation and consequently the signal transduction until the nuclear factors (Xu et al., 2009). Additional evidence shows that the enrichment of dectin-1 and Syk phosphorylation in zymosan contact sites require excluding the inhibitory signal of the phosphatases CD45 and CD148, which strongly reinforces the importance of the membrane domains architecture in the immune signalling pathway against fungi (Goodridge et al., 2011).
3 GLYCOSPHINGOLIPIDS-ENRICHED LIPID RAFTS AS A PLATFORM FOR FUNGAL RECOGNITION
Glycosphingolipids (GSLs) are a heterogeneous subclass of glycolipids (D'Angelo et al., 2013). Lactosylceramide (LacCer) is a remarkable GSL that, together with PRRs, mediate the immune response to pathogenic fungi. Yeasts species such as C. neoformans, Candida albicans, Saccharomyces cerevisiae, and the yeast-like Pneumocystis jirovecii as well as the dimorphic fungi H. capsulatum, Paracoccidioides brasiliensis and Sporothrix schenckii directly bind to this domain (Figure 1) (Jimenez-Lucho, Ginsburg, & Krivan, 1990). The presence of galactose as a terminal residue of LacCer is linked to β-glucan recognition (Goodridge, Wolf, & Underhill, 2009). LacCer is predominantly increased in human glioma brain cells and functions as binding sites for C. neoformans (Jimenez-Lucho et al., 1990). Furthermore, LacCer is enriched in lipid rafts of human neutrophils, and their association with β-glucan induces migration, phagocytosis and superoxide generation (Figure 1) (Iwabuchi et al., 2008; Iwabuchi et al., 2015; Nakayama, Iwahara, Takamori, Ogawa, & Iwabuchi, 2008). The presence of a β-1,6-long glucosyl side-chain in the β-glucan seems to be crucial for Lyn and PI3K activation and neutrophil migration (Sato et al., 2006).
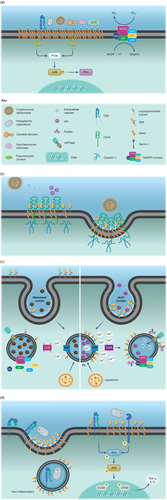
LacCer enriched domains could still interact with immune receptors, such as CR3, a complement receptor composed of integrins CD11b (Mac-1) and CD18 (Goodridge et al., 2009). CR3 co-localises with LacCer-enriched membrane microdomains when human neutrophils are activated with the CD11b monoclonal antibody VIM12 (Nakayama et al., 2008). The signalling transmitted by the stimulation is mediated through the interaction of LacCer and the C-terminal portion of CD18, which leads to Lyn phosphorylation (Figure 1).
4 ROLE OF LIPID RAFTS IN FUNGAL INFECTION
In this section, we will review studies that have focused on the contribution of lipid microdomains to host interaction with the following fungal pathogens: C. albicans, C. neoformans, P. jirovecii, Aspergillus fumigatus, P. brasiliensis and H. capsulatum (for a summary of molecular interactions involving host cell lipid rafts, see Table 1).
| Fungal pathogen | Host cell | Molecular interaction (pathogen/host) | Functional response | References |
|---|---|---|---|---|
| Candida albicans | Monocytes | β-(1,3)-glucan | Phagocytosis | de Turris et al. (2015) |
| Neutrophils | β-(1,6)-side chain-branched β-glucan | Chemotaxis | Sato et al. (2006) | |
| Cryptococcus neoformans | HBMEC | HA | Adhesion and brain invasion | Jong et al. (2008); Huang et al. (2011); Long, Huang, Wu, Shackleford, and Jong (2012) |
| EVs | Huang et al. (2012) | |||
| Pneumocystis jirovecii | AEC | β- (1,3)- glucan | MIP-2 synthesis | Evans, Kottom, Pagano, and Limper (2012); Hahn et al. (2003) |
| Human dendritic cells | β- (1,3)- glucan | Activation of the IL-23/IL-17 axis | Carmona et al. (2012) | |
| Aspergillus fumigatus | Macrophages | DHN melanin | Inhibition of acidification of PL | Schmidt et al. (2020) |
| Paracoccidioides brasiliensis | AEC | Not determined | IL-6 and IL-8 secretion | Maza, Straus, Toledo, Takahashi, and Suzuki (2008) |
| Histoplasma capsulatum | AEC | Not determined | IL-6 and IL-8 secretion | Maza and Suzuki (2016) |
| Macrophages | HSP60a and β- (1,3)- glucana | Phagocytosis and cytokine synthesis | Guimarães et al. (2018); Huang et al. (2015) |
- Abbreviations: AEC, alveolar epithelial cells; DHN, dihydroxynaphthalene; EVs, extracellular vesicles; HA, hyaluronic acid; HBMEC, human brain microvascular endothelial cells; HSP60, heat shock protein 60; MIP-2, macrophage inflammatory protein-2; PL, phagolysosome.
- a Possible ligands according to literature findings.
4.1 Candida albicans
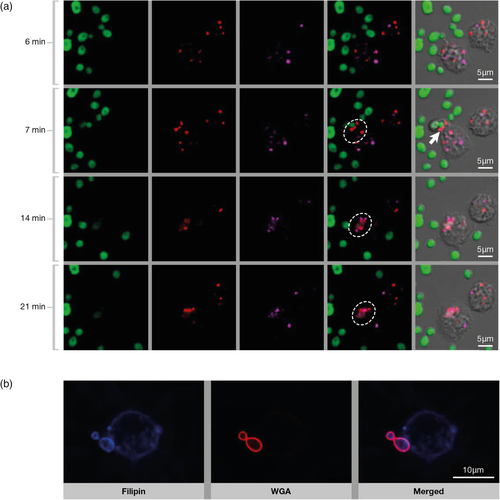
One of the common strategies to investigate the role of lipid rafts in PRRs crosstalk is the disruption of host cell lipid domains using methyl-β-cyclodextrin (m-β-CD) and Deoxycholate Amphotericin B (DAmb), drugs that extract and immobilise, respectively, sterols from the plasma membrane (Mahammad & Parmryd, 2015). Although DAmb and other compounds that immobilise membrane sterol in mammalian cells have been extensively used in lipid domains studies, we cannot rule out their steric interference that could impact fungal binding. Pre-treatment of monocytes with both drugs directly affected C. albicans engulfment, reducing lipid raft coalescence and dectin-1 recruitment to C. albicans binding sites (Figure 2) (de Turris et al., 2015). However, treatment of host cells with m-β−CD did not prevent the production of cytokines, indicating that the co-participation of non-raft PRRs could bypass the correct lipid raft assembly and trigger the stimulus required for cytokine synthesis. In addition, raft disruption on host cells drastically dampened Th17 and Th1 responses, suggesting that the preservation of dectin-1 dynamics in lipid-raft of antigen presenting cells is crucial for specific T-cell responses in this model (de Turris et al., 2015). Thus, although the recognition of C. albicans by human monocytes takes place independently of the integrity of lipid microdomains, a correct and balanced response by these leukocytes rely on the membrane organisation provided by the rafts. Further studies are necessary to investigate the impact of host lipid domains during interaction with C. albicans in other cells from the immune system.
4.2 Cryptococcus neoformans
A set of studies demonstrates that C. neoformans penetration into human brain microvascular endothelial cells (HBMEC) is a lipid raft-dependent process (Figure 1) (Huang, Long, et al., 2011; Jong et al., 2008; Long et al., 2012). Adhesion to HBMEC seems to be mediated by hyaluronic acid (HA), a fibrous structure outside the C. neoformans cell wall (Jong et al., 2007; Jong et al., 2012). Treatment with hyaluronidase and deletion of CPS1, the coding gene of hyaluronic synthase, significantly reduced the binding of C. neoformans to HBMEC (Jong, Wu, Chen, et al., 2007). In addition, C. neoformans HA is recognised by CD44 at the HBMEC surface, a step blocked by anti-CD44 antibodies and CD44 shRNA treatments (Jong et al., 2012). Association of C. neoformans with HBMEC induces the redistribution of CD44 to lipid rafts at the binding sites. Remarkably, treatment of host cells with filipin, a polyene antibiotic that immobilises and reduces sterol availability, impacting the lateral mobility of host cell membranes, significantly decreases yeast association with HBMEC, suggesting that lipid raft integrity is required.
The colocalisation of CD44 with caveolin-1 (Cav-1) also supports the role of these platforms during the internalisation of C. neoformans by HBMEC (Jong et al., 2008; Long et al., 2012). Cav-1 is the main component of caveolae, a subset of microdomains involved with signal transduction and endocytosis (Figure 1) (Kiss & Botos, 2009). The anchorage of caveolae on the plasma membrane is sustained by the connection of Cav-1 with the actin cytoskeleton (Mundy, 2002). Thus, caveolae-dependent endocytosis is regulated through reorganisation of the actin cytoskeleton and triggered by phosphorylation of Cav-1 (Zimnicka et al., 2016). When Cav-1 expression is down-regulated in HBMEC, several internalised yeasts is reduced, with a small impact on fungal adhesion, suggesting that Cav-1 is not involved with binding but has a strong impact on the fungus' internalisation (Long et al., 2012). The binding of C. neoformans and HA to HBMEC also induces Cav-1 phosphorylation, a change that has been linked to caveolae-mediated internalisation (Long et al., 2012). Cytoskeleton rearrangement was further investigated during C. neoformans internalisation. Inhibition of actin polymerisation and tyrosine kinase activity reduced the association and blood–brain barrier (BBB) transmigration of C. neoformans, confirming that both events are required for invasion (Kim, Crary, Chang, Kwon-Chung, & Kim, 2012). Actin rearrangement appears to be initiated by C. neoformans' binding to receptors at the endothelial plasma membrane, triggering the activation of Rho GTPases (RhoA, Rac1 and Cdc42) and phosphorylation of focal adhesion kinase (FAK), protein kinase C α (PKCα) and ezrin, promoting yeast transmigration across the BBB (Figure 1) (Kim et al., 2012).
A pre-adhesion step could be mediated by extracellular vesicles (EVs) released by C. neoformans (Figure 1) (Huang et al., 2012). EVs are lipid bilayer membrane compartments used by cells to export several molecules, including proteins, polysaccharides, pigments, lipids and nucleic acids (Rizzo, Rodrigues, & Janbon, 2020). Their participation during disease development has been suggested for different fungi. Treatment of HBMEC with C. neoformans-derived EVs induces membrane ruffling, a response usually caused by cytoskeletal rearrangement (Huang et al., 2012). These results were also observed when C. neoformans or HA were incubated with HBMEC (Jong et al., 2008). However, the response mediated by EVs is CD44-independent (Huang et al., 2012). Incubation with EVs promoted the redistribution of lipid raft components recruiting CD44, Cav-1 and up-shifted the intracellular distribution of β-actin. Changes induced by EVs could enhance C. neoformans recognition and transcytosis. Corroborating with the cellular changes, intravenous administration of EVs enhanced the ability of C. neoformans to reach the brain in a murine mice model of cryptococcosis.
Finally, the interplay between HIV infection and the invasion of C. neoformans into HBMECs seems to be mediated by lipid rafts (Huang et al., 2011; Jong et al., 2007). It has been reported that a specific domain of the glycoprotein 41 (gp41) induces the recruitment of CD44 to HBMECs lipid rafts and potentiate the C. neoformans invasion. The protein gp41 is a transmembrane subunit of the HIV envelope protein complex that mediates virus fusion (Pan, Liu, & Jiang, 2010). The recombinant peptide, encompassing the specific sequence of amino acids of gp41 (between 579 and 611), was able to stimulate C. neoformans invasion in vitro and its intravenous injection enhanced brain infection in mice by C. neoformans (Jong, Wu, Jiang, et al., 2007). In addition, treatment with gp41-I90 and C. neoformans synergistically enhanced the transmigration of monocytes in HBMEC and in vivo (He et al., 2016). These findings contribute novel perspectives about the role that lipid rafts play in comorbidity between HIV and fungal neuro-infection.
4.3 Pneumocystis jirovecii
A balanced inflammatory response in pneumocystosis (PcP) is crucial for a patient to resolve Pneumocystis infection (Limper, Offord, Smith, & Martin, 1989). Lipid rafts are involved in the response of alveolar epithelial cells (AEC) to the β-glucans of Pneumocystis (PCBG). LacCer was responsible for stimulating the release of MIP-2 from AEC in a process dependent on PKC (Evans et al., 2012; Hahn et al., 2003). Treatment of AECs with monoclonal antibodies against LacCer, but not GM1, impairs the internalisation of PCBG (Hahn et al., 2003). In DCs, PCBG stimulates IL-23 and IL-6 secretion, culminating in the activation of the IL-23/IL-17 axis (Carmona et al., 2012). This activation is lipid raft-mediated, and dectin-1 is the main receptor, leading to the downstream activation of Syk and NF-κB. However, there is evidence that suggests that LacCer works as a co-receptor in this process, as β-glucan stimulation acts to mobilise and concentrate LacCer within the lipid microdomains (Carmona et al., 2012). These findings are clinically relevant because these cytokines are crucial for a Th17 phenotype, which plays a major role in fungal clearance and defence (Ma et al., 2008; Milner et al., 2008).
Overall, the major problem in patients with PcP is the overstimulation of the inflammatory response, which is induced by the interaction of host cells and fungal β-glucans (Sokulska, Kicia, Wesołowska, & Hendrich, 2015). Interestingly, an array of studies show that this response is significantly dampened by the inhibition of host cells lipid rafts (Carmona et al., 2012; Kottom, Hebrink, Jenson, Gudmundsson, & Limper, 2015), not only evidencing their role in the pathology but also suggesting that they could be a novel therapeutic target to pharmacological agents such as nystatin, which is capable of inhibiting the assembly of glycosphingolipid-cholesterol rich microdomains in host cells, interrupting PCBG recognition and potentially impairing fungal internalisation (Evans et al., 2012).
4.4 Aspergillus fumigatus
For airborne pathogens, AECs and resident alveolar macrophages are the first cells involved with fungal recognition in the lung (Hartl et al., 2018). Recently, the ability of bronchial epithelial cells to attenuate the virulence of A. fumigatus conidia was also linked to a mechanism of internalisation involving lipid rafts (Clark, Powell, Simmons, Ayubi, & Kale, 2019). The presence of flotillin-2 and caveolin in a ring-like structure surrounding internalised conidia, along with other host cell proteins, were demonstrated. Similar to Cav-1, flotillin-2 is also a protein enriched in lipid rafts and associated with endocytosis (Clark et al., 2019). Remarkably, treatment of host cells with filipin reversed the ability of bronchial epithelial cells to decrease the conidia's virulence (Clark et al., 2019).
The pigment melanin is an important virulence factor that protects fungal cells against host oxidative responses (Smith & Casadevall, 2019). A recent study using melanised-wild type conidia and melanin-free conidia (pksP mutant) suggested that the presence of this pigment also helps A. fumigatus to control lipid rafts' composition at the initial steps of fungal internalisation and during the phagolysosomal maturation (Schmidt et al., 2020). Remarkably, GM1 and flotillin-1 were significatively reduced in the phagolysosomal membranes containing melanised conidia. Since the recruitment of the proton pump V-ATPase is dependent on the presence of flotillin-1, a substantial reduction in phagolysosome acidification was observed, allowing conidia germination (Dermine et al., 2001; Schmidt et al., 2020). Phagosome maturation involves gradual changes to membrane composition, including the assembly of subunits of V-ATPase, and NADPH oxidase (Pauwels, Trost, Beyaert, & Hoffmann, 2017). In line with that, NADPH oxidase complex assembly at flotillin-containing membrane microdomains was also compromised by melanin (Schmidt et al., 2020). The signalling pathway that triggers this cascade of events is not fully elucidated, but the same study shows that the formation of lipid rafts at the phagolysosomal membrane is dependent on calmodulin (CaM) activity. In this context, A. fumigatus' melanin mediates Ca2+ sequestration inside the phagosome lumen, which reduces the availability of these ions in the peri-phagosomal area where calmodulin activation takes place (Akoumianaki et al., 2016; Schmidt et al., 2020). Accordingly, treatment with calmodulin inhibitors significantly reduced the co-localisation of raft marker the ganglioside GM1 in phagolysosomes containing conidia (Schmidt et al., 2020).
The role of melanin is complex and can up or downregulate fungal phagocytosis depending on its structure (Smith & Casadevall, 2019). An anti-phagocytic role was observed for melanin in A. fumigatus conidia, which was correlated to the modulation of flotillin-associated microdomains (Schmidt et al., 2020). The pksP mutant conidia were more phagocytosed by macrophages, but in the absence of flotillin, the phagocytosis of this mutant was reduced. The presence of melanin and a hydrophobin rodlet at the conidia cell wall prevents immune recognition (Gow et al., 2017). However, the pksP conidia expose β-glucan, which activates LC3-associated phagocytosis (LAP), promoting fungal killing through NADPH oxidase activity in the phagosome (Akoumianaki et al., 2016). LAP activation was recently shown to enhance the antimicrobial activity in macrophages and DCs infected by C. albicans, A. fumigatus or H. capsulatum, but the association between LAP and membrane microdomains has not been evaluated (Akoumianaki et al., 2016; Huang et al., 2018). Purified melanin inhibits NADPH oxidase activity through the exclusion of its subunit p22phox from the phagosome membrane (Akoumianaki et al., 2016). Indeed, it is important to note that inhibition of Ca2+–CaM signalling by fungal melanin was also correlated with blocking LC3 recruitment to the phagosomal membrane of macrophages, which suggests a possible role of membrane microdomains in phagocytic routes involved with phagolysosomal activity (Kyrmizi et al., 2018).
4.5 Paracoccidioides brasiliensis
Paracoccidioides brasiliensis is a thermally dimorphic fungus, and the infection is initiated by the inhalation of conidia or arthroconidia that differentiate to yeasts in the host's lung (Martinez, 2017). Incubation of human AEC line A549 with yeasts of P. brasiliensis showed an enrichment of the raft marker GM1 at the fungal-host cell adhesion points. Binding was followed by rapid activation of SRC family kinase (SFK) and ERK1/2. Treatment of A549 cells with nystatin and m-β-CD resulted in reduced fungal association and impaired activation of AECs. The lipid raft composition analysis confirmed that the association of P. brasiliensis yeasts with A549 cells induced the phosphorylation of SFK in lipid rafts. Pre-treatment with m-β-CD also abolished activation of SFK. Further studies demonstrated that P. brasiliensis recognition by A549 also promotes upregulation of α3 and α5 integrins. Both integrins were recruited to caveolin-associated lipid rafts at the P. brasiliensis-A549 point of contact, suggesting the involvement of caveolae in fungal endocytosis (Maza et al., 2008). In fact, treatment of A549 cells with genistein, a tyrosine kinase inhibitor used to block caveolae-dependent endocytosis, inhibited the adhesion and invasion of the fungus, demonstrating the importance of caveolae domains on the internalisation of P. brasiliensis (Monteiro da Silva et al., 2007). The role of AEC in driving an inflammatory response to an invasive fungal infection has been addressed and linked to a correct raft assembly (Bigot et al., 2020; Wang et al., 2005). The recruitment of α3 and α5 integrins to these domains induces IL-6 and IL-8 secretion by A549 cells (Maza et al., 2008). IL-8 release by epithelial cells is essential for neutrophil recruitment and inflammatory response orchestration (Kunkel, Standiford, Kasahara, & Strieter, 1991).
The enrichment of GM1 at P. brasiliensis binding sites in A549 cells and the previous studies showing that other fungi recognise LacCer at the plasma membrane of neutrophils suggest that this pathogen could bind to other GSL at the host cell surface. In fact, GalCer, LacCer, CTH (Galα1-4Galα1-4Glcα1-1Cer), GD3, GM1 and GD1a supported P. brasiliensis adhesion (Ywazaki, Maza, Suzuki, Takahashi, & Straus, 2011). In addition, pre-incubation of P. brasiliensis yeast with GM1 made these cells reactive to the GM1-glycan ligand cholerae toxin subunit B (CTxB). Steric blocking of GM1 with CTxB, and GM3 with the antibody DH2, significantly reduced the adhesion of P. brasiliensis to human lung fibroblasts, confirming that these GSL could support fungal binding (Ywazaki et al., 2011).
4.6 Histoplasma capsulatum
Histoplasma capsulatum is another thermally dimorphic fungus that affects the lungs primarily. The mechanism of interaction of yeasts with AEC is similarly described for P. brasiliensis. Translocation of α3 and α5 integrins and signalling machinery to lipid domains are associated with the secretion of IL-6 and IL-8 by these cells. However, the role of AEC in fungal control remains elusive (Maza & Suzuki, 2016). On the other hand, infection of macrophages is known as a critical step during histoplasmosis development. In some virulent strains of H. capsulatum, yeasts have the ability to block macrophage activation by inhibiting the recognition of the β-(1,3)-glucan by dectin-1 (Brown, 2016).
The role of PRRs on the innate immune response to H. capsulatum yeast has been a focus of several studies (Chang et al., 2017; Lin, Huang, Hung, Wu, & Wu-Hsieh, 2010; Van Prooyen, Henderson, Hocking Murray, & Sil, 2016). H. capsulatum yeasts use the β2 integrin (CD18) of CR3 to mediate recognition by macrophages through the surface exposure of fungal 60 kDa heat shock protein (HSP60) (Long, Gomez, Morris, & Newman, 2003). CR3 might be the exclusive phagocytic receptor involved in the internalisation of this fungus (non-opsonised) by macrophages suggesting that a phagocytic synapse involving other PRRs at the lipid membrane domain is not required during host cell invasion (Lin et al., 2010). However, when sialic acids are removed from host cells by enzymatic treatment, impairment of macrophage association with H. capsulatum was observed. These results indicate that sialylated GSL could be involved in fungal interaction with immune cells (Lin et al., 2010). Results from our group suggest that GSLs are involved in lipid rafts organisation during macrophage infection by H. capsulatum (Figure 1) (Guimarães et al., 2018). We demonstrated the enrichment of cholesterol at the binding sites of H. capsulatum to macrophages (Figure 2). Depletion of cholesterol after treatment of macrophages with m-β-CD reduces H. capsulatum adhesion and internalisation. Since m-β-CD can also extract other host membrane components, it is important to confirm that the PRRs or their co-receptors are still at regular levels (Mahammad & Parmryd, 2015). In addition, peritoneal macrophages from mice deficient for complex gangliosides production, such as GM1 and GD1a (B4galnt1−/), have a reduced ability to recognise H. capsulatum (Guimarães et al., 2018). GM1-associated microdomains appear to be extremely important for H. capsulatum' adhesion to host cells, although not to opsonised yeast cells. Our data also suggest an interesting interplay between GM1 and the CD11b/CD18 integrin. We demonstrated that both GM1 and the CD18/CD11b integrin are recruited to the macrophage/H. capsulatum interaction site. Furthermore, CD18 recruitment to lipid microdomains fractions was reduced in macrophages from B4galnt1−/−, indicating that GM1 could be an accessory molecule that facilitates the recruitment of CR3 into macrophage lipid rafts (Guimarães et al., 2018). Remarkably, macrophage-H. capsulatum association induced recruitment of laterally organised lipid domains first at the site of fungus–phagocyte contact and then along the entire surface of the host cell.
As previously mentioned, the CR3 pathway involves lipid microdomains, and the signalling is mediated by LacCer-associated Lyn that activates cytoskeleton rearrangement (Nakayama, Ogawa, Takamori, & Iwabuchi, 2013). HSP60 is expressed on the fungal surface in clusters, which may facilitate the CR3 binding process (Long et al., 2003). However, CR3 contains multiple binding sites, including a lectin site at the C-terminal domain that recognises β-glucans (Ehlers, 2000). H. capsulatum lacking α-(1,3)-glucan induces activation of the dectin-1 signalling pathway in macrophages, and the colocalisation of dectin-1 and CR3 into GM1 microdomains was observed at the cell interface of interaction between host cells and heat-killed yeasts (Figure 1) (Huang et al., 2015). The collaborative work between dectin-1 and CR3 into lipid raft was crucial to trigger the cytokine response through downstream signalling of Syk, JNK, and transcriptional activity of AP-1 (Huang et al., 2015). These results collectively suggest that H. capsulatum yeasts can avoid the recruitment of specific receptors to the space of lipids rafts in the plasma membrane of macrophages to more effectively facilitate the yeasts' infection of macrophages.
5 CONCLUSION
Understanding lipid raft assembly during fungal-host cell interaction is an interesting paradigm that reveals other mechanisms by which each fungus can modify the host cell response and promote a variety of clinical conditions. It is a multistep process that impacts changes in the whole plasma membrane and, although the players are well-known, the dynamics behind the molecular events remain largely opaque. Because these pathways are tightly involved in fungal recognition and its intracellular fate and the elaboration of immune responses, they may pose as attractive targets for therapeutics and pharmacological management of mycoses.
ACKNOWLEDGEMENTS
This work was supported by grants from the Brazilian agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (311179/2017-7 and 408711/2017-7 to L.N.; 311470/2018-1 to AJG), FAPERJ (E-26/202.809/2018, E-26/202.696/2018 and E-26/202.760/2015). T.N.S. was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001). J.R. was supported by Pasteur-Roux-Cantarini fellowship from the Institut Pasteur. J.D.N. was supported in part by NIH R21 AI124797.
AUTHOR CONTRIBUTIONS
Writing—original draft preparation, Taiane N. Souza and Alessandro F. Valdez; writing—review and editing, Juliana Rizzo, Daniel Zamith-Miranda, Allan Jefferson Guimarães, Joshua D. Nosanchuk and Leonardo Nimrichter. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study (displayed in Figure 2) are openly available in https://doi.org/10.1371/journal.pone.0142531.g003 and at http://doi.org/10.1371/journal.pone.0142531 and http://doi.org/10.1111/cmi.12976 (de Turris et al., 2015; Guimarães et al., 2018).