Type VI secretion system effector proteins: Effective weapons for bacterial competitiveness
Funding information: Wellcome Trust, Grant/Award Number: 104556/Z/14/Z
Abstract
The Type VI secretion system (T6SS) is a protein translocation nanomachine widespread among Gram-negative bacteria and used as a means to deliver effectors directly into target bacterial or eukaryotic cells. These effectors have a wide variety of functions within target cells that ultimately help the secreting cell gain a competitive fitness advantage. Here, we discuss the different ways in which these effectors can be delivered by the T6SS and the diverse mechanisms by which they exert their noxious action upon recipient cells. We also highlight the existence of roles for T6SS effectors beyond simply the killing of neighbouring cells.
1 INTRODUCTION
Bacteria have evolved specialised protein secretion systems in order to deliver a wide variety of proteins into the extracellular space or even directly into other cells. Secretion systems allow bacteria to interact with their environment, a host organism or other bacteria. The Type VI secretion system (T6SS) is widespread in Gram-negative bacteria and can translocate toxic proteins, known as “effectors,” across the membrane of a neighbouring target cell. The primary role of the T6SS appears to be to act against competitor bacteria, with the potential to shape diverse polymicrobial communities, however, this versatile machinery can also be used against eukaryotic cells and to release metal-scavenging proteins extracellularly (Coulthurst, 2019).
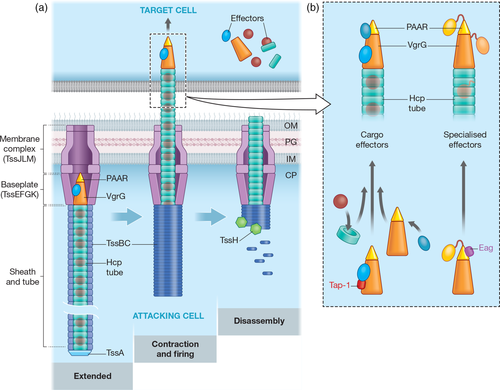
The T6SS is a large, contractile nanomachine, which expels a puncturing structure, made of a tube of Hcp (TssD) with a distal spike of VgrG (TssI) and PAAR proteins, decorated with effector proteins, out of the secreting cell (Figure 1a). As reviewed recently (Nguyen et al., 2018; Wang, Brodmann, & Basler, 2019), the T6SS operates via dynamic cycles of assembly, contraction and disassembly. A membrane complex (TssJLM) acts as a docking site for a cytoplasmic baseplate-like structure (TssEFGK), built around a central (VgrG)3-PAAR spike. The Hcp tube then assembles onto VgrG, surrounded by a contractile sheath made of TssBC, which polymerises in a high-energy extended conformation. Assembly is coordinated by TssA, which typically forms a cap at the distal end of the sheath and tube, although in some systems remains associated with the baseplate (Schneider et al., 2019). The sheath then contracts in a rapid firing step, propelling the Hcp–VgrG–PAAR puncturing structure out of the secreting cell and into the target cells, delivering the effector payload. Finally, the contracted sheath is disassembled by TssH (ClpV). T6SSs are encoded by large variable gene clusters, which contain genes encoding the essential core components (TssA-TssM and PAAR), and often also conserved accessory proteins involved in post-translational regulation or assembly of the machinery. T6SS-wielding bacteria typically also possess a number of species- or strain-specific T6SS genes, within and outside the main T6SS cluster, encoding regulators, effectors, immunity proteins and other effector-associated proteins (Coulthurst, 2019).
The T6SS can use its contraction-based mechanism to deliver a wide variety of effectors into target cells. Here, we examine recent advances in understanding T6SS-delivered effector proteins, considering their mode of delivery, action against different kingdoms and how the effector repertoire delivered by a particular T6SS may determine its role.
2 MODES OF EFFECTOR DELIVERY BY THE T6SS
In order to be secreted, effectors delivered by the T6SS associate with the expelled puncturing structure in two distinct ways: “Cargo” effectors interact non-covalently with one of the components of the puncturing structure (Hcp, VgrG or PAAR), whereas “specialised” effectors represent additional homologues of one of these components that carry an additional effector domain covalently fused to the core domain, almost always at the C-terminus (Figure 1b). Hcp-dependent cargo effectors sit within the lumen of the Hcp tube, which is formed of stacked hexamers of Hcp. This tube has an internal diameter of ~40 Å, and consequently Hcp-dependent effectors typically have a molecular weight under 20 kDa. Silverman et al. (2013) showed that Hcp1 from Pseudomonas aeruginosa could stabilise and protect its substrates, Tse1-3, from proteolysis, revealing that Hcp is not simply a passive conduit for T6SS effectors. Recently, specialised Hcps with C-terminal extension toxins (Hcp-ETs) have been reported to occur widely in the Enterobacteriaceae (J. Ma et al., 2017).
VgrG-dependent cargo effectors are believed to sit on the outside of the VgrG–PAAR spike and often have (phospho-)lipase activity, for example, Tle1 from Enteroaggregative E. coli (EAEC) (Flaugnatti et al., 2016). Many specialised VgrG proteins with C-terminal toxin domains have been identified, for example, the actin crosslinking domain of VgrG-3 from V. cholerae (Pukatzki, Ma, Revel, Sturtevant, & Mekalanos, 2007). Certain VgrGs also contain C-terminal “extensions,” which do not confer toxic functions per se, but specifically bind and recruit other effectors (Bondage, Lin, Ma, Kuo, & Lai, 2016; Flaugnatti et al., 2016). For example, the EAEC VgrG has a C-terminal extension comprising a DUF2345 domain, which extends the spike, and a TTR domain, which makes extensive contacts with Tle1 (Flaugnatti et al., 2020).
The first PAAR-dependent cargo effector, TseT from P. aeruginosa, was recently reported (Burkinshaw et al., 2018). In contrast, PAAR-containing specialised effectors are widespread, with a remarkable variety of anti-bacterial functions. They include Rhs proteins, a family of large polymorphic toxin proteins, which frequently possess C-terminal nuclease domains, and whose effector domains are predicted to be encased in a shell-like structure (Coulthurst, 2019; Koskiniemi et al., 2013). Indeed, it has been shown that the C-terminal domains of even very large specialised VgrG and PAAR proteins may fit within a variable cavity between the spike components and the remainder of the T6SS baseplate (Nazarov et al., 2018).
T6SSs typically contain multiple homologues of Hcp, VgrG and/or PAAR proteins and their genes are often linked with their cognate effectors, both within the main T6SS gene cluster and dispersed around the genome. It has been shown that different combinations of Hcp, VgrG and PAAR proteins can assemble functional T6SSs, particularly specific VgrG–PAAR spike combinations. These different assemblies then lead to the delivery of alternative sets of effectors during individual firing events (Cianfanelli et al., 2016; Wood, Howard, Wettstadt, et al., 2019).
2.1 Adaptors and secretion motifs of T6SS effectors
Some effectors require dedicated adaptor proteins for delivery by the T6SS. Several families of adaptor proteins necessary for stabilisation and/or loading of the cognate effectors onto the VgrG–PAAR spike have been characterised. The DUF4123 domain-containing family was identified in V. cholerae (TecL/Tap-1), Agrobacterium tumefaciens (Tap-1) and Aeromonas hydrophila (TecC) (Bondage et al., 2016; Liang et al., 2015; Unterweger et al., 2015). These adaptor proteins interact directly with their cognate effectors and the C-terminal extension of the correct VgrG, allowing VgrG-dependent cargo effectors to be loaded. A co-adaptor protein may also be needed to stabilise the adaptor protein, as reported for TecT and co-TecT in P. aeruginosa (Burkinshaw et al., 2018). Proteins containing DUF2169 domains are encoded upstream of some PAAR-containing specialised effectors and may also represent adaptors facilitating loading onto VgrG proteins (Bondage et al., 2016), while a DUF2875-containing adaptor, Tla3, is required to load a cargo effector, Tle3, onto a specialised VgrG, VgrG2b (Berni, Soscia, Djermoun, Ize, & Bleves, 2019). Adaptor proteins containing the DUF1795 domain represent a family of chaperone proteins required for the stability of PAAR-containing specialised effectors. These chaperones include EagR proteins, required for stabilisation and delivery of Rhs proteins in Serratia marcescens (Cianfanelli et al., 2016), and EagT6 of P. aeruginosa, which is required for the stability and loading of the PAAR-containing effector, Tse6, onto VgrG1 (Whitney et al., 2015). EagT6 binds to the PAAR-containing domain of Tse6 in the secreting cell and protects transmembrane helices that are ultimately used to generate a pore in the inner membrane of the target cell through which the effector domain translocates to the cytoplasm (Quentin et al., 2018). None of the above adaptor proteins are secreted with their cognate effectors and thus must be lost during the process of T6SS assembly and firing.
Recognition of effectors by the T6SS machinery is built-in for specialised effectors, but remains incompletely understood for cargo effectors. No universal secretion signals or motifs seem to exist, even within effectors using the same type of core component. Two domains found in certain subsets of T6SS effectors with diverse activities and which apparently represent effector recognition motifs have been identified: marker for type sIX effectors (MIX) (Salomon et al., 2014) and found in type sIX effectors (FIX) (Jana, Fridman, Bosis, & Salomon, 2019). Both MIX and FIX are usually found at the N-terminus of proteins also containing toxin/effector domains and likely represent intrinsic adaptor domains. In general, adaptor proteins have been suggested to allow a wider range of cargo effectors to interact with core components and to be co-acquired and co-evolve with new effectors (Unterweger et al., 2015). Overall, different combinations of core Hcp, VgrG and PAAR components, together with cognate adaptor proteins, allow one T6SS to deliver multiple, structurally diverse effector proteins.
3 ANTI-BACTERIAL EFFECTORS DELIVERED BY THE T6SS
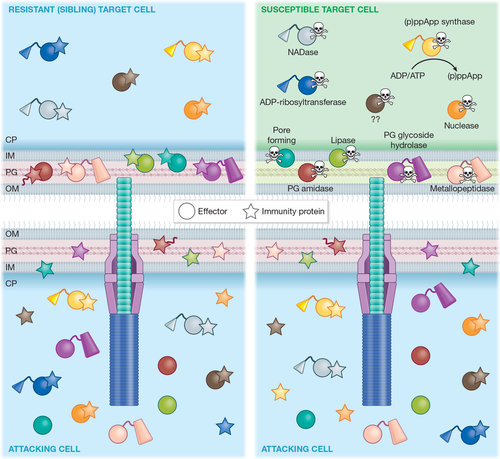
Anti-bacterial effectors represent the majority of identified and characterised effectors of the T6SS. As shown in Figure 2, T6SSs deliver a variety of anti-bacterial effector proteins into neighbouring bacterial cells, which act by different mechanisms to compromise conserved cellular structures and molecules, leading to lysis or growth inhibition of targeted cells.
3.1 Cell wall-disrupting effectors
The cell wall of Gram-negative bacteria, an essential structure needed for protection and stabilisation of the cell, is located within the periplasm and consists mainly of a thin layer of peptidoglycan (PG). PG comprises glycan strands linked by peptide cross-bridges and is a common target during interbacterial competition (Silhavy, Kahne, & Walker, 2010). Toxins with PG hydrolase activities represent a large and well-characterised class of T6SS effectors. T6SS PG amidase effectors, which cleave the peptide cross-bridges, were initially classified into four families named Type VI amidase effectors (Tae)1–4 (Russell et al., 2012). These families differ in their cleavage specificity, with Tae2 and Tae3 hydrolysing peptide cross-bridges between mDAP and d-Ala, whereas Tae1 and Tae4 cleave between d-Glu and mDAP. As more PG amidase effectors have been identified and studied, the diversity within this class has become further apparent. Two members of the Tae4 family in one strain of S. marcescens, Ssp1 and Ssp2, have distinct substrate specificities and unrelated immunity proteins (Srikannathasan et al., 2013), while an amidase effector recently identified in E. coli belongs to a distinct family of PG amidases, named TaeX, which cleave between N-acetylmuramoyl and l-Ala (J. Ma, Sun, Pan, Lu, & Yao, 2018). PG glycoside hydrolase effectors, which cleave the glycan backbone of PG, have been classified in three families, Type VI secretion glycoside hydrolase effectors (Tge)1–3. Tge1, and likely Tge3, show β-(1,4)-N-acetylmuramidase activity, while Tge2 is predicted, on the basis of structural data, to show N-acetylglucosaminidase activity (Whitney et al., 2013). In addition, specialised VgrG effectors that act upon the cell wall have been identified, including VgrG-3 of V. cholerae, which has a PG glycoside hydrolase domain (Brooks, Unterweger, Bachmann, Kostiuk, & Pukatzki, 2013), and VgrG2b of P. aeruginosa, which has a C-terminal zinc-metallopeptidase domain that has been suggested to act on proteins required for PG remodelling (Berni et al., 2019; Wood, Howard, Förster, et al., 2019).
3.2 Membrane-disrupting effectors
Many T6SS-delivered effectors disrupt the membranes of bacterial cells by degrading their lipid constituents. Russell et al. (2013) identified five families of predicted (phospho-)lipase effectors, named Type VI lipase effectors (Tle)1–5. They showed that Tle1 and Tle2 have phospholipase A2 (PLA2) and A1 (PLA1) activities, respectively, while Tle5 are phospholipase D enzymes. Subsequently, the metagenomic analysis indicated that such effectors are widespread, with considerable diversity in their structure and predicted activity (Egan, Reen, & O'Gara, 2015), while Tle1 from EAEC was shown to display both PLA1 and PLA2 activities consistent with diversity even within the designated Tle families (Flaugnatti et al., 2016).
T6SS effectors with membrane pore-forming activity have also been identified. VasX from V. cholerae was reported to share homology with pore-forming colicins and its action was shown to cause a loss of membrane potential and increase in cellular permeability (Miyata, Unterweger, Rudko, & Pukatzki, 2013). The impact of intoxication by Tse4 of P. aeruginosa indicates that this effector forms ion-selective pores in target cells, leading to dissipation of the membrane potential without causing permeability to larger molecules (LaCourse et al., 2018). Similarly, a distinct effector, Ssp6 of S. marcescens, causes inner membrane depolarisation in vivo and forms cation-selective pores in artificial lipid bilayers in vitro (Mariano et al., 2019). Interestingly, Ssp6 intoxication may also cause a loss of outer membrane (OM) integrity, although it is unclear if this is a direct or indirect effect.
3.3 Nucleic acid-targeting effectors
Toxins with nuclease activities also occur widely as T6SS-delivered effectors. Many T6SS-associated Rhs proteins (PAAR-containing specialised effectors), including Rhs1 and Rhs2 from Dickeya dadantii, have C-terminal endonuclease effector domains, which act to degrade target cell DNA (Koskiniemi et al., 2013). A. tumefaciens can deliver two DNase effectors, Tde1 and Tde2 (Type VI DNase effector). Both contain a toxin_43 nuclease domain, but Tde1 is a VgrG-dependent cargo effector and Tde2 a specialised PAAR effector (Bondage et al., 2016). Recently, Jana et al. (2019) identified an effector in V. parahaemolyticus that carries a novel but widely-occurring C-terminal DNase domain proposed to be part of the PD-(D/E)xK phosphodiesterase superfamily. More broadly, many other effectors, particularly PAAR-containing specialised effectors, are predicted to possess DNase, RNase or deaminase activity according to bioinformatic analyses (Coulthurst, 2019).
3.4 Effectors targeting other essential cytoplasmic molecules
Recently, the repertoire of characterised T6SS toxins has been expanded by effectors that disrupt other cellular molecules. The specialised PAAR-containing effector, Tre1 of S. proteamaculans, displays ADP-ribosyltranferase activity, adding ADP-ribose to FtsZ, a tubulin-like protein essential for bacterial cell division. ADP-ribosylation blocks polymerisation of FtsZ, inhibiting cell division and leading to the death of intoxicated cells (Ting et al., 2018). The essential cytoplasmic cofactors, NAD(P)+, are targeted by families of glycohydrolase effectors called Type VI secretion NADase effectors (Tne)1–2. Tse6 (Tne1) of P. aeruginosa and Tne2 of P. protegens both hydrolyse NAD+ and NADP+ in intoxicated cells, depleting the cofactor levels and leading to bacteriostasis (Tang, Bullen, Ahmad, & Whitney, 2018; Whitney et al., 2015). The energy balance of intoxicated cells can be disrupted directly by a new class of T6SS effector, exemplified by Tas1 from P. aeruginosa. Tas1 very rapidly synthesises (p)ppApp, depleting cellular ATP (and ADP), leading to irreversible disruption of essential metabolic pathways and cell death (Ahmad et al., 2019). Tas1 intoxication likely also accentuates the impact of other effectors by disabling ATP-dependent cellular repair pathways, and its product, (p)ppApp, also inhibits purine biosynthesis, further reducing the likelihood of cellular recovery.
3.5 Immunity proteins
In order to protect themselves from intoxication by their own effector proteins, or those injected by neighbouring sibling cells, secreting bacterial cells possess immunity proteins specific to each of their anti-bacterial effector proteins (Figure 2). These immunity proteins are encoded adjacent to the cognate effector and reside in the cellular compartment in which the effector acts. Immunity proteins bind tightly and specifically to the cognate effector to neutralise its action, typically occluding the active site of enzymatic effectors (Coulthurst, 2019). In the case of the ADP-ribosyltransferase effector, Tre1, the immunity protein, Tri1, can also act as an ADP-ribosylhydrolase. This allows Tri1 to remove ADP-ribose groups introduced by Tre1 and potentially also protect against other ADP-ribosyltransferase toxins (Ting et al., 2018). Mounting evidence suggests that bacteria may retain or actively accumulate “orphan” immunity proteins, those for effectors they do not (any longer) encode, as a means of protection against T6SS attacks by other bacteria in their niche (Ross et al., 2019).
4 ANTI-EUKARYOTIC EFFECTORS DELIVERED BY THE T6SS
The T6SS can also deliver effectors that target various cellular processes and structures in eukaryotic cells. These effectors may facilitate host invasion and pathogenesis, or, alternatively, be deployed to survive or compete against eukaryotic microbes.
4.1 Disruption of the actin cytoskeleton
Many anti-eukaryotic effectors target the actin cytoskeleton of host cells. For example, VgrG1 of A. hydrophila has actin ADP-ribosylase activity, which prevents actin polymerisation, and induces caspase 9-mediated apoptosis (Suarez et al., 2010), while VgrG-1 of V. cholerae has an actin crosslinking domain, which inhibits cytoskeleton rearrangement and phagocytosis, and promotes inflammatory diarrhoea in vivo (A. T. Ma & Mekalanos, 2010). TecA from Burkholderia cenocepacia can disrupt the actin cytoskeleton of macrophages by deamidating Rho GTPases, triggering inflammation through activation of the Pyrin inflammasome (Aubert et al., 2016), while the V. proteolyticus effector, Vpr01570, contains a CNF1-like deamidase domain that can induce cytoskeleton rearrangements in macrophages (Ray et al., 2017).
4.2 Cell invasion and colonisation
Other effectors can promote internalisation, intramacrophage growth or phagosomal escape. OpiAB and PdpCD, delivered by the T6SS of Francisella tularensis, contribute to intramacrophage growth. PdpC is required for phagosomal escape by the bacterium, and, in the absence of PdpC, OpiA promotes phagosomal escape through its PI(3)-kinase activity (Brodmann, Dreier, Broz, & Basler, 2017; Ledvina et al., 2018). The effector domain of VgrG2b can be delivered into the epithelial cells by the P. aeruginosa H2-T6SS, where it interacts with the microtubule network (specifically γ-tubulin ring complex) to allow normally non-phagocytic cells to internalise the bacterium (Sana et al., 2015). This function is in addition to the anti-bacterial activity of the domain (Berni et al., 2019; Wood, Howard, Förster, et al., 2019), making it an example of a “trans-kingdom” effector, acting on both prokaryotic and eukaryotic cells. Several P. aeruginosa phospholipase effectors also have trans-kingdom activity. TplE (Tle4 family) localises to the endoplasmic reticulum in eukaryotic cells, where it activates the unfolded protein response, leading to autophagy (Jiang et al., 2016), while PldA and PldB (Tle5 family) promote invasion of eukaryotic cells through the activation of the PI3K/Akt signalling pathway (Jiang, Waterfield, Yang, Yang, & Jin, 2014). EvpP from Edwardsiella tarda can also promote host-cell invasion, this time by inhibiting activation of the NLRP3 inflammasome, ultimately promoting bacterial colonisation in vivo (Chen et al., 2017). Finally, VgrG5 of B. pseudomallei is required for host cell fusion and cell-to-cell spread of bacteria, with its C-terminal domain proposed to insert into host cell membranes to mediate the fusion event (Toesca, French, & Miller, 2014).
4.3 Anti-amoebal and anti-fungal effectors
Three T6SS effectors contribute to protecting V. cholerae against Dictyostelium discoideum predation: VgrG-1 (actin crosslinking), TseL (lipase) and VasX (membrane-disrupting); with TseL and VasX being trans-kingdom effectors (Dong, Ho, Yoder-Himes, & Mekalanos, 2013). The T6SS has been shown to mediate resistance to amoebal predation in other organisms, for example, Xanthomonas citri (Bayer-Santos et al., 2018), but the effectors responsible are yet to be identified. The first two T6SS effectors specifically targeting fungal cells were recently identified in S. marcescens. Tfe1 intoxication causes plasma membrane depolarisation and can ultimately lead to fungal cell death, while Tfe2 causes disruption to nutrient transport and metabolism and induces autophagy, most likely as a starvation response (Trunk et al., 2018). Anti-fungal T6SS effectors are likely to be widespread, broadening the potential influence of the T6SS on many polymicrobial communities, which often contain fungal and bacterial cells.
5 T6SS EFFECTORS INVOLVED IN METAL UPTAKE
Recent studies have revealed that some effectors secreted by the T6SS do not need to be delivered into rival cells to perform their biological function, but instead play a role in metal uptake when secreted into the extracellular environment. During oxidative stress, the B. thailandensis T6SS-4 secretes TseM, which binds extracellular Mn2+ ions and subsequently interacts with a TonB-dependent OM transporter, MnoT, to allow active transport of manganese into the cell (Si, Zhao, et al., 2017). The same T6SS also secretes TseZ, a metallophore used for scavenging zinc and related to the first identified metal uptake effector, YezP of Yersinia pseudotuberculosis. During severe oxidative stress, the HmuR OM transporter switches from transporting heme to zinc, allowing Zn2+ bound by TseZ to be imported (Si, Wang, et al., 2017). In P. aeruginosa, the T6SS-secreted effector, TseF, participates in Fe3+ uptake in a distinct manner. Extracellular TseF interacts with Fe3+−bound PQS (a quinolone signalling molecule) present in OM vesicles. TseF then docks with the OM receptor, FptA, and porin OprF to facilitate transport of Fe3+ from the OM vesicles into the bacterium (Lin et al., 2017). In the same organism, the Azu effector is reported to scavenge Cu2+ and deliver it to the OM transporter, OprC (Han et al., 2019). T6SS-dependent secretion of metal uptake effectors may allow the secreting bacteria to efficiently scavenge scarce metal ions from the extracellular environment and thus outcompete bacterial competitors, and, in addition, make them better able to survive within host niches, which are typically metal-limited. Indeed, it appears that TseZ, TseM and TseF are able to confer such competitive advantages (Lin et al., 2017; Si, Wang, et al., 2017; Si, Zhao, et al., 2017).
6 PERSPECTIVES AND FUTURE DIRECTIONS
The T6SS is a highly versatile weapon for bacterial competitiveness, with mounting evidence that T6SSs play key roles in shaping the composition and dynamics of diverse microbial communities, including microbiota, reviewed by Coulthurst (2019) and Allsopp, Bernal, Nolan, and Filloux (2020). T6SS-mediated competition may also represent a key driver for bacterial evolution, with ongoing acquisition and diversification of new effector and immunity genes resulting in inter-species and inter-strain “arms races.” In addition, the action of many T6SS anti-bacterial effectors, such as cell wall hydrolases, can lead to lysis of targeted cells, providing the opportunity for naturally competent-attacking bacteria to acquire new genes, including antibiotic resistance determinants and new effector–immunity pairs (Coulthurst, 2019; Matthey et al., 2019). Thus, T6SS-dependent horizontal gene transfer provides an indirect competitive fitness advantage to go alongside the immediate and direct fitness advantage of killing or disabling rival bacterial cells in the same niche. Clearly, the large and diverse repertoire of anti-bacterial effectors, which can be deployed by the T6SS, is central to its effectiveness as a weapon for inter-bacterial competition. Effectors acting against eukaryotic microbes, while currently far less explored, should similarly promote bacterial survival in microbial communities, while those involved in metal scavenging represent another way to gain an edge in the fierce competition between microbes. The fact that T6SSs can also deploy anti-host effectors, and the existence of “trans-kingdom” effectors, highlights the impressive versatility of this system.
Looking forward, the mode of action of many anti-bacterial effectors remains unknown, and, given the diversity identified so far and evident from genomic surveys, we anticipate many more effectors remain to be discovered. Similarly, it is likely that we have only scratched the surface in terms of identifying anti-eukaryotic and contact-independent effectors. We also lack a full understanding of the mechanisms by which effectors are recognised and recruited by the T6SS. This includes how the need for specific recognition of effectors by the T6SS machinery may limit the horizontal acquisition of new effectors, and how strategies, such as modular adaptor proteins, may help to overcome this barrier. Importantly, evidence for synergistic action between different effectors has been reported (LaCourse et al., 2018). Defining such synergies will be critical for understanding the overall impact of the T6SS on targeted cells. Finally, further work is required to understand how and where effectors are released from T6SS components in target cells and how target cell functions may promote or protect against effector toxicity. Ultimately, deciphering the mechanisms and roles of T6SS effectors will allow us to understand how T6SSs influence microbial communities and provide new leads in the development of anti-microbial strategies, hopefully by allowing us to “learn from the experts” how microbes can so effectively kill each other.
ACKNOWLEDGMENTS
The authors are supported by the Wellcome Trust (104556/Z/14/Z). We apologise to colleagues whose contributions we were unable to cite due to space restrictions.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.