The surreptitious survival of the emerging pathogen Staphylococcus lugdunensis within macrophages as an immune evasion strategy
Abstract
Staphylococcus lugdunensis is a commensal bacterium that can cause serious infection suggesting an ability to circumvent aspects of host immunity. We demonstrate here that macrophages fail to kill ingested S. lugdunensis and the bacteria persist for extended periods, without replicating, within mature LAMP-1-positive phagolysosomes. Phagocytosed S. lugdunensis also do not intoxicate host cells in contrast to Staphylococcus aureus. Optimal survival of S. lugdunensis requires O-acetylated peptidoglycan because an oatA mutant, which is more sensitive to killing by lysozyme than wild type, survived to a lesser extent in macrophages. In vitro models of macrophage infection reveal that viable intracellular S. lugdunensis bacteria can be made to grow by pharmacologic perturbation of phagosome function or by phagocyte intoxication by S. aureus toxins. Remarkably, replicating S. lugdunensis is not constrained by LAMP-1 and phosphatidylserine-positive endomembranes, which is distinct from S. aureus that replicates within phagolysosomes. In vivo, S. lugdunensis can also reside in the murine Kupffer cell where the bacteria persist without replicating and require oatA to resist killing in vivo. The intracellular environment of the macrophage represents a niche where S. lugdunensis can exist while protected from extracellular immune factors and may serve as a reservoir from which these bacteria could disseminate.
1 INTRODUCTION
Staphylococcus lugdunensis is a coagulase-negative staphylococcal species (CoNS) that may be found as a component of the commensal flora on human skin (Bieber & Kahlmeter, 2010; Frank, Del Pozo, & Patel, 2008). However, unlike other CoNS, S. lugdunensis has a heightened capacity to cause infection displaying virulence properties that, in some respects, resemble the prolific pathogen Staphyloccocus aureus (Frank et al., 2008; Thean, Siew, & He, 2008). Unfortunately, the true burden of S. lugdunensis infection remains unclear due to misidentification of clinical isolates as Staphyloccocus aureus (Argemi et al., 2015). Improved diagnostics through the implementation of mass spectrometry-based methods of bacterial identification has begun to enhance the detection of S. lugdunensis in clinical samples (Argemi et al., 2015; Elamin, Ball, & Millar, 2015; Marín, Arroyo, Espinosa-Martos, Fernández, & Rodríguez, 2017). Moreover, when identified, S. lugdunensis is often associated with significant disease (Argemi et al., 2015; Tan, Ng, & Ng, 2006). Akin to S. aureus, S. lugdunensis can cause infections that range from relatively minor skin and soft tissue infections (Arias et al., 2010; Bellamy & Barkham, 2002; Böcher, Tønning, Skov, & Prag, 2009) to more serious conditions such as bacteremia and infective endocarditis (Heilbronner et al., 2011; Liang, Mansell, Wade, Fisher, & Devlin, 2012; Non & Santos, 2016; Sotutu, Carapetis, Wilkinson, Davis, & Curtis, 2002). Additionally, infective endocarditis attributed to S. lugdunensis infection can be associated with significant tissue damage and elevated mortality indicating the potential significance of this bacterium (Anguera et al., 2005; Sabe, Shrestha, Gordon, & Menon, 2014). Moreover, drug-resistant S. lugdunensis strains with heightened resistance to beta-lactam antibiotics (e.g., oxacillin) have emerged, which could complicate treatment of infection (Pereira, Schuenck, Nouér, & dos Santos, 2011; Sampathkumar, Osmon, & Cockerill 3rd, 2000). Remarkably, few virulence factors have been identified in S. lugdunensis, and the interaction of this bacterium with immune cells such as macrophages has remained unexplored.
Macrophages are an important immune cell that participate in the eradication of bacteria due, in part, to their capacity to phagocytose (Flannagan, Cosío, & Grinstein, 2009; Flannagan, Heit, & Heinrichs, 2015a). Killing of bacteria during phagocytosis can be attributed to the concerted activity of immune effectors including NADPH oxidase activation and the production of reactive oxygen species (e.g., O2−; Canton, Khezri, Glogauer, & Grinstein, 2014; Laroux, Romero, Wetzler, Engel, & Terhorst, 2005), phagosome acidification (Huynh & Grinstein, 2007), lysozyme-mediated hydrolysis of peptidoglycan, and the activation of lysosomal proteases such as cathepsins (reviewed in Flannagan et al., 2009). Despite this, previous work has established that, both in vitro and in vivo, S. aureus can resist killing within the phagolysosomes of macrophages where the bacteria commence replicating (Flannagan et al., 2015b; Flannagan et al., 2018; Surewaard et al., 2016).
Here, we demonstrate for the first time that, akin to S. aureus, S. lugdunensis is trafficked into mature phagolysosomes within macrophages. However, unlike S. aureus, the bacteria reside within this niche without replicating. Moreover, we provide evidence that macrophages actively curtail the growth of intracellular S. lugdunensis through protease attack, because pharmacological inhibition of protease function or blockade of phagosome maturation altogether enables S. lugdunensis to proliferate intracellularly. Within the murine liver, S. lugdunensis resists rapid killing within Kupffer cells where the peptidoglycan O-acetyl-transferase (OatA), which confers lysozyme resistance upon S. lugdunensis, is required for maximal bacterial survival in vitro and in vivo. Failure of host macrophages to kill intracellular S. lugdunensis may represent an important shortcoming of host innate immunity that allows for intracellular reservoirs of viable S. lugdunensis to exist.
2 RESULTS
2.1 Staphylococcus lugdunensis persists within macrophages without replicating
As an unusually pathogenic CoNS, we reasoned S. lugdunensis might evade killing by macrophages. To explore the interaction of S. lugdunensis with macrophages, gentamicin protection assays were performed using the murine macrophage cell line RAW 264.7 (hereafter called RAW) and primary human M-CSF-derived macrophages. Macrophages were allowed to phagocytose S. lugdunensis HKU09-01, and at 1.5-hr postinfection (after gentamicin treatment), viable S. lugdunensis were recovered from infected RAW and primary human cells (Figure 1a–c). At 24-hr postinfection, viable S. lugdunensis was recovered from infected macrophages; however, it was evident that the burden of S. lugdunensis was unchanged over the 24-hr period (Figure 1a–c). To verify that this observation was unique to S. lugdunensis, parallel infections were performed using S. aureus USA300 and Micrococcus luteus, representative bacteria previously established to overcome macrophage restriction or to be killed, respectively (Flannagan et al., 2015b). Consistent with previous observations, M. luteus, although recovered at 1.5-hr postinfection, is eradicated by 24 hr (Figure 1a–b). In contrast to S. lugdunensis and M. luteus, S. aureus USA300 grew over the same 24-hr period indicating that the macrophages failed to restrict S. aureus (Figure 1a–b). To determine whether S. lugdunensis could persist intracellularly for an extended period of time, infected primary human macrophages were monitored over 72 hr (Figure 1c). This analysis demonstrated that at 48- and 72-hr postinfection, viable S. lugdunensis could be recovered from macrophages, although by 72 hr, a modest yet statistically significant decrease in bacterial viability was observed (Figure 1c). Although the preceding experiments indicated that macrophages, although not microbicidal towards S. lugdunensis, do restrict S. lugdunensis growth, we sought to establish that this phenotype was not strain dependent. Therefore, we infected macrophages with S. lugdunensis M23590 and two randomly selected S. lugdunensis clinical isolates recovered from infections at a local hospital. Importantly, each of these isolates behaved identically to S. lugdunensis HKU09-01 in macrophages (data not shown), indicating that impaired growth within the macrophage is a general trait of S. lugdunensis. Finally, the inability of S. lugdunensis to proliferate within macrophages is due to active macrophage functions as S. lugdunensis proliferates in tissue culture medium (Roswell Park Memorial Institute [RPMI] with 10% [vol/vol] fetal bovine serum [FBS]) in the absence of macrophages (data not shown).
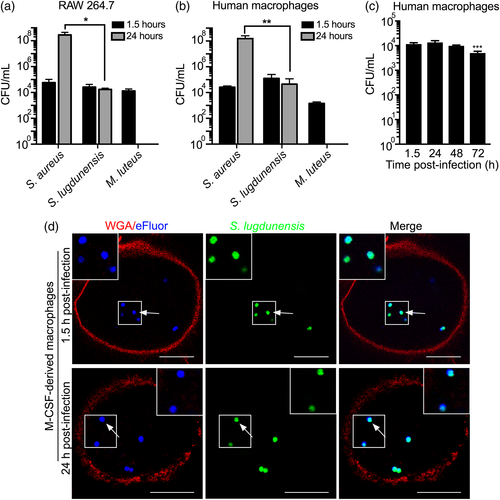
To confirm our finding that S. lugdunensis fails to replicate within macrophages, green fluorescent protein (GFP)-expressing S. lugdunensis was labelled with the far-red proliferation dye eFluor670 such that at the outset of the experiment, all phagocytosed bacteria were GFP- and eFluor670-positive. Primary human M-CSF-derived macrophages, at 1.5-hr postinfection and, remarkably, at 24-hr postinfection, contained GFP and eFluor-positive S. lugdunensis, indicating that the bacteria had not replicated (Figure 1d; bacteria having replicated would have lost the eFluor label). Similar experiments performed in RAW cells yielded the same result (data not shown). Taken together, these data indicate that S. lugdunensis demonstrates a unique intracellular fate as compared with S. aureus and M. luteus wherein the bacteria remain viable within macrophages for extended periods of time in the absence of replication.
2.2 Phagocytosed Staphylococcus lugdunensis is trafficked to mature phagolysosomes in macrophages
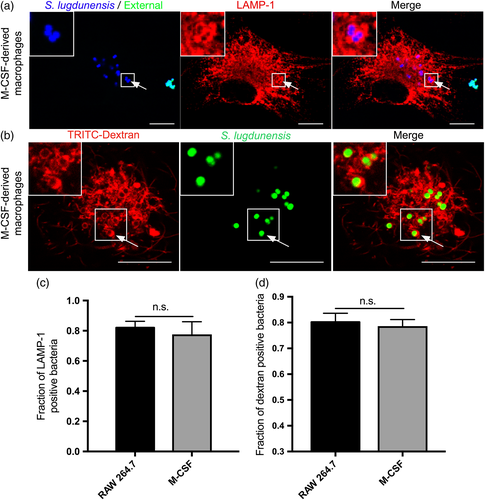
We next sought to identify the subcellular niche occupied by S. lugdunensis. Particulates ingested by macrophages into phagosomes are rapidly trafficked into lysosome-associated membrane protein 1 (LAMP-1)-positive phagolysosomes (Huynh et al., 2007; Pitt, Mayorga, Stahl, & Schwartz, 1992). To determine whether phagocytosed S. lugdunensis is contained within phagolysosomes demarcated by LAMP-1, infected primary human M-CSF-derived and RAW macrophages were immunostained using anti-LAMP-1 antibody and visualised by microscopy. This analysis revealed that at 1.5-hr postinfection, the majority (~80%) of intracellular S. lugdunensis co-localise with the LAMP-1 protein as indicated by the accumulation of anti-LAMP-1 fluorescence around intracellular cocci (Figure 2a,c and data not shown). To confirm that ingested S. lugdunensis reside in mature phagolysosomes, we also performed dextran pulse-chase experiments to fluorescently label lysosomes, which fuse with phagosomes during infection. This analysis revealed that in both primary cells and RAW macrophages, ~80% of intracellular S. lugdunensis also co-localise with lysosomal dextran establishing that most intracellular S. lugdunensis bacteria reside within mature phagolysosomes by 1.5-hr postinfection (Figure 2b,d and data not shown).
2.3 Perturbation of phagolysosome function enables Staphylococcus lugdunensis to proliferate intracellularly
Ostensibly, restriction of S. lugdunensis by macrophages can be attributed to the antimicrobial functions of the phagolysosome. To determine whether this is indeed the case, we utilised pharmacologic inhibitors known to perturb effectors that operate during phagocytosis and phagosome maturation. As such, macrophages were treated with either the vacuolar-ATPase inhibitor concanamycin A (ConA; Dröse et al., 1993), the protease inhibitors Pepstatin A and Antipain (Tranchemontagne, Camire, O'Donnell, Baugh, & Burkholder, 2015), the NADPH oxidase inhibitor diphenyleneiodonium (DPI; Lam et al., 2011), or a combination thereof. Bacterial proliferation assays were performed on infected macrophages that were pretreated with each inhibitor alone or in combination. Microscopy revealed that at 1.5-hr postinfection, inhibitor treatment alone or in combination did not impair the phagocytosis of S. lugdunensis as equal numbers of intracellular bacteria were observed irrespective of treatment (data not shown). Moreover, it was evident that bacterial replication had not occurred under any condition as all bacteria remained GFP and eFluor670-positive. In contrast, by 24-hr postinfection, inhibitor treatment did promote the proliferation of S. lugdunensis within RAW macrophages as intracellular GFP-positive, yet eFluor670-negative S. lugdunensis could be visualised (Figure 3a, right panels, and Figure 3b). In contrast, in untreated control macrophages, all intracellular bacteria, even at 24-hr postinfection, remained eFluor670-positive (Figure 3a, left panels, and Figure 3b). Quantitation of the fraction of macrophages containing replicating bacteria revealed that treatment of macrophages with ConA or protease inhibitors alone permitted replication within approximately 8% to 11% of infected macrophages, and the simultaneous treatment of macrophages with ConA and protease inhibitors did not have any significant additive effect (Figure 3b). Notably, this combined treatment with the addition of DPI increased the fraction of infected cells containing replicating S. lugdunensis to ~24% despite the observation that DPI alone was without effect (Figure 3b). Importantly, control experiments using M. luteus in the presence of the same cocktail of inhibitors did not rescue M. luteus from being killed (data not shown). Given that a fraction of macrophages could support the intracellular growth of S. lugdunensis upon pharmacological treatment, we next determined whether the intracellularly replicating S. lugdunensis were toxic to the host cells. To this end, macrophages were treated with vehicle control or the cocktail of inhibitors that enabled S. lugdunensis growth and were stained with the viability dye propidium iodide (PI) to assess integrity of the macrophage plasmalemma and phagocyte viability (Figure S1a,b). In these experiments, macrophages were rarely PI-positive (3% to 6%), irrespective of pharmacological treatment or infection with S. lugdunensis (Figure S1a,b). Given infected macrophages remain refractory to PI, we next determined whether infected macrophages are capable of phagocytosis. Macrophages were treated with and without inhibitors and in the presence and absence of S. lugdunensis infection. After 24-hr, immunoglobulin G (IgG)-opsonised latex beads were incubated with control and infected macrophages, and we observed that control and infected macrophages, including those containing replicating bacteria, retained their ability to ingest IgG-opsonised phagocytic targets (Figure S1c,d). These data indicated that in RAW macrophages S. lugdunensis, even when replicating, do not compromise basic macrophage functions.
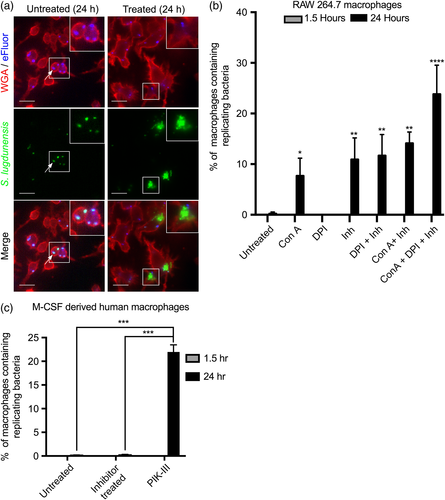
Conceivably, RAW macrophages are more permissive than primary human macrophages and, consistent with this notion, we found that in similar experiments, pretreatment of M-CSF-derived human macrophages with a combination of DPI, ConA, Antipain, and Pepstatin A did not permit intracellular S. lugdunensis growth (Figure 3c). As these inhibitors enhanced growth of S. lugdunensis in RAW macrophages, we reasoned that lack of growth could not be due to antimicrobial effects of these compounds. Therefore, as an alternate means to perturb phagosome function in human macrophages, we employed the PI3-kinase inhibitor PIK-III to disrupt Vps34 activity, which modulates phagosome maturation and its antimicrobial function (Berger et al., 2010; Vieira et al., 2001). As previously observed, control macrophages restricted S. lugdunensis proliferation entirely; however, in the presence of PIK-III, GFP-positive yet eFluor-negative S. lugdunensis could be found within ~22% of the infected macrophage population indicating S. lugdunensis under these conditions grew intracellularly (Figure 3c). In the preceding experiments, macrophages were pretreated with inhibitors to protect S. lugdunensis from experiencing antimicrobial aspects of the phagolysosome; however, we sought to determine whether the timing of phagosome perturbation influenced the ability of S. lugdunensis to grow. Treatment of RAW or human macrophages with either PIK-III or the cocktail of inhibitors (for RAW cells) after the bacteria had been phagocytosed and allowed to traffic to the phagolysosome (i.e., at 1.5-hr postinfection) was still sufficient to promote intracellular S. lugdunensis growth mimicking entirely the observations presented in Figure 3 (data not shown). Taken together, these data indicate that phagocytosed S. lugdunensis can, in principle, replicate within the macrophage without being overtly toxic, and ongoing processes in the macrophage phagolysosome must restrict S. lugdunensis growth.
2.4 Proliferating Staphylococcus lugdunensis escape from the macrophage phagolysosome
The data presented thus far have shown that we can manipulate the macrophage pharmacologically to permit intracellular replication of S. lugdunensis. We next sought to determine whether replicating bacteria remain within the phagolysosome. LAMP-1 immunofluorescence analyses, performed as above, showed that at 24-hr postinfection, using either RAW or primary human macrophages, non-replicating bacteria remain within LAMP-1-positive compartments (Figure 4a, top panels, and Figure 4b). Remarkably, however, and in contrast to what we have previously found for S. aureus (Flannagan et al., 2015b), intracellularly replicating S. lugdunensis HKU09-01 (i.e., that are eFluor-negative) are not demarcated with LAMP-1 fluorescence (Figure 4a, bottom panels, and Figure 4c). The absence of LAMP-1 fluorescence from replicating S. lugdunensis bacteria was also observed for S. lugdunensis N920134, confirming other strains can be made to replicate intracellularly with similar consequences (Figure S4). Conceivably, replicating bacteria are no longer constrained by a vacuole, and so, to analyse this scenario, RAW macrophages, prior to infection, were transfected with a plasmid encoding LactC2-RFP, a biosensor for phosphatidylserine (PS) (Yeung et al., 2008). Importantly, because PS is found on the cytoplasmic face of compartments comprising the endo/lysosomal network (Fairn et al., 2011), we reasoned that if replicating S. lugdunensis is indeed confined to a vacuole, the LactC2-RFP probe should accumulate around these bacteria. At 24-hr post-phagocytosis, analysis of the distribution of LactC2-RFP showed that replicating S. lugdunensis was not demarcated by the LactC2-RFP lipid biosensor and are thus not in a PS-positive vacuole (Figure 4d). In contrast, non-replicating S. lugdunensis and phagocytosed IgG-coated beads remained LactC2-RFP positive, even within the same cell where we could observe replicating bacteria having escaped a vacuole. These latter observations confirm that the exclusion of LactC2-RFP from around replicating S. lugdunensis is not due to a global disturbance in PS localisation. To confirm these observations, macrophages infected with S. lugdunensis were also visualised by transmission electron microscopy at 24-hr postinfection in the presence and absence of inhibitor treatment. This analysis confirmed that non-replicating S. lugdunensis remains confined to small vacuoles demarcated by continuous electron dense membrane (Figures 4e and S2). In contrast, replicating S. lugdunensis resides in large vacuolar structures containing numerous bacteria and the limiting membrane of these structures appears discontinuous or is altogether absent (Figures 4e and S2).
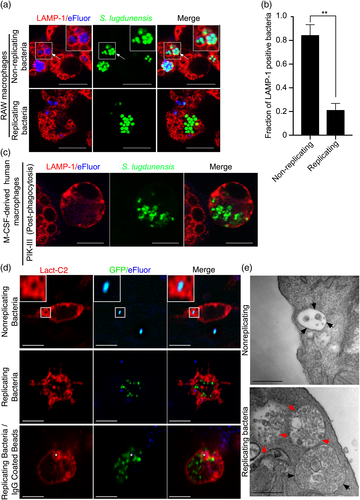
2.5 Staphylococcus lugdunensis counteracts macrophage killing inside the phagolysosome
Because S. lugdunensis remains viable within the phagolysosome, we sought to determine whether the bacteria could be sensitised to intracellular killing. To this end, we constructed an oatA deletion mutant of S. lugdunensis that will lack acetylated peptidoglycan which confers lysozyme resistance (Bera, Biswas, Herbert, & Götz, 2006; Herbert et al., 2007). In vitro growth experiments demonstrated that oatA-deficient S. lugdunensis was incapable of growth at lysozyme concentrations in excess of 200 μg ml−1, whereas wild-type S. lugdunensis grew at lysozyme concentrations ≥3,200 μg ml−1 (Figure S3a). In contrast, in tryptic soy broth (TSB) without lysozyme, wild-type and oatA-deficient S. lugdunensis grew indistinguishably (Figure S3a and data not shown). We next assessed the ability of the oatA mutant to survive within RAW macrophages. This analysis revealed that at 24-hr postinfection, there was a significant (approximately sevenfold) decrease in colony-forming unit per millilitre for the oatA mutant as compared with wild-type bacteria (Figure S3b). The significant intracellular growth defect for the oatA-deficient S. lugdunensis uncovers a key resistance gene in this species.
2.6 Staphylococcus aureus toxins promote Staphylococcus lugdunensis replication from within macrophages
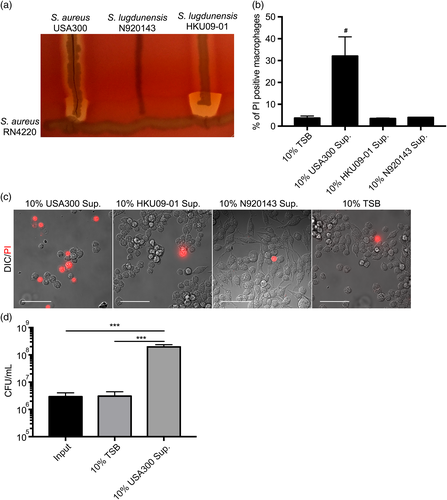
We next wished to determine whether S. lugdunensis could be coaxed to grow within macrophages that were exposed to biological toxins. We began by exposing macrophages to culture supernatant from either S. aureus USA300 or that of S. lugdunensis strains HKU09-01 or N920143. We chose these particular strains because S. aureus USA300 on blood agar is haemolytic and its toxins synergise with β-toxin produced by S. aureus RN4220 (Figure 5a). S. lugdunensis N920143 is non-haemolytic even when in proximity to β-toxin producing S. aureus (Figure 5a), and S. lugdunensis HKU09-01, which demonstrates α-haemolysis, potently causes complete red cell lysis when growing in proximity to β-toxin producing RN4220 (Figure 5a). When culture supernatants from these strains were added to RAW macrophages, it was evident that S. aureus USA300 is highly toxic as indicated by massive destruction of host cell integrity and PI staining of macrophages that remained on the coverslip (Figure 5b,c). In contrast, supernatants from S. lugdunensis HKU09-01 and N920134 were both non-toxic, causing macrophage PI staining that was similar to a TSB control (Figure 5b,c). When supernatant from S. aureus USA300 was added to macrophages 1.5 hr after infection with S. lugdunensis HKU09-01, an ~100-fold increase in S. lugdunensis bacteria over control or S. lugdunensis HKU09-01 supernatant treated macrophages is observed by 24-hr postinfection (Figure 5d and data not shown). These data reveal that toxins produced by extracellular S. aureus could exacerbate S. lugdunensis infection by intoxicating infected macrophages and allowing release of phagocytosed S. lugdunensis.
2.7 Staphylococcus lugdunensis resides within Kupffer cells in the murine liver
To establish that S. lugdunensis can persist inside macrophages in vivo, we performed infections using a systemic murine model of infection. Previously, it has been established that in this model, S. aureus are phagocytosed by, and reside within, liver resident Kupffer cells for at least 8- to 10-hr postinfection (Surewaard et al., 2016; Zeng et al., 2016). Therefore, live GFP-expressing S. lugdunensis were injected intravenously into anaesthetised animals, and bacterial localisation in the liver was monitored over time by intravital microscopy. By 15 min post-injection, live GFP-expressing S. lugdunensis was visualised in the liver where they co-localised with F/480+ Kupffer cells (Figure 6a,b). Importantly, GFP-positive S. lugdunensis bacteria remained in association with F/480+ Kupffer cells for the duration of the infection (up to 16-hr postinfection; Figure 6b). Notably, there was no evidence that the bacteria were replicating, indicated by the absence of any increase in GFP fluorescence (Figure 6b). To verify that the bacteria were viable, infections were repeated with heat-inactivated GFP-positive S. lugdunensis. Killed S. lugdunensis were rapidly captured by F/480+ Kupffer cells; however, unlike live S. lugdunensis, killed S. lugdunensis were degraded by 4-hr postinfection as indicated by the absence of detectable GFP fluorescence (Figure 6a,b). Together, these data revealed that S. lugdunensis is captured by Kupffer cells in vivo and that these bacteria can remain viable within these cells long after initial infection.

To establish that although in the Kupffer cell, S. lugdunensis resists macrophage killing, mice were infected systemically with wild-type S. lugdunensis and the oatA mutant, and the bacterial burden in livers was determined at 8-hr postinfection (Figure 6c). In agreement with our intravital imaging data, we found that live wild-type S. lugdunensis bacteria can readily be recovered from livers at 8-hr postinfection (Figure 6c). Moreover, we found that significantly fewer oatA-deficient S. lugdunensis bacteria are recovered at the same time point (Figure 6c). These in vivo data are in agreement with our aforementioned cell culture data demonstrating the importance of OatA to survival of S. lugdunensis within the macrophage (see Figure S3). These data also indicate that, in vivo, S. lugdunensis evades rapid killing within Kupffer cells, in part through expression of oatA, where the bacteria otherwise remain viable without replication. Taken together, these data are consistent with the notion that S. lugdunensis does not passively exist inside macrophages, and ongoing bacterial processes are needed for intracellular survival in vitro and in vivo.
3 DISCUSSION
Staphylococcus lugdunensis is part of the normal skin flora (Bieber & Kahlmeter, 2010; Herchline & Ayers, 1991); however, it is clear that this bacterium is unique among CoNS in its ability to cause infection (Choi et al., 2010; Frank et al., 2008; Heldt Manica & Cohen, 2017; Liang et al., 2012; Sabe et al., 2014; Seng et al., 2017). Despite this, relatively few virulence factors have been identified, and investigation into the pathogenic mechanisms of S. lugdunensis has been limited (Bourgeois et al., 2009; Donvito et al., 1997; Heilbronner, Hanses, Monk, Speziale, & Foster, 2013; Mitchell, Tristan, & Foster, 2004; Nilsson et al., 2004). Here, we report that S. lugdunensis can evade macrophage killing while persisting in the phagolysosome for an extended period of time. Conceivably, the ability of S. lugdunensis to persist within professional phagocytes without replication or being overtly toxic contributes to the apparent reduced virulence of this CoNS species as compared with S. aureus strains, which can replicate within macrophages (Flannagan et al., 2015b; Surewaard et al., 2016). The seemingly tranquil intracellular persistence of S. lugdunensis may represent a survival strategy where it is advantageous not to intoxicate the host cell. Conceivably, this would allow intracellular S. lugdunensis to avoid extracellular immune factors that in the absence of additional evasion mechanisms would lead to bacterial killing.
Staphylococcus lugdunensis is clearly not equipped with the same repertoire of virulence factors that other successful pathogens such as S. aureus (Flannagan et al., 2015a) or Salmonella enterica serovar Typhimurium (Jennings, Thurston, & Holden, 2017) are; however, the ability to persist intracellularly for extended duration is in agreement with what constitutes a pathogen under the “macrophage paradox” described by Price and Vance (2014). Indeed, the ability of macrophages to restrict intracellular growth of S. lugdunensis undoubtedly represents an anti-bacterial phenotype; however, the inability to eradicate ingested cocci represents a shortcoming that could enable S. lugdunensis to persist inside the body with macrophages acting as bacterial reservoirs. Our data showing that macrophage intoxication by secreted extracellular S. aureus toxins (e.g., LukAB, HlgAB, and HlgCB) increases growth of S. lugdunensis supports this notion. The ability of S. lugdunensis to survive within macrophages could be important in the context of systemic infections where Kupffer cells clear the staphylococci from circulation (Surewaard et al., 2016; Zeng et al., 2016). Kupffer cells rapidly remove S. aureus from circulation and, due to their inability to eradicate phagocytosed S. aureus, provide these bacteria with a protected intracellular niche where the bacteria are able to proliferate (Lehar et al., 2015; Surewaard et al., 2016). Through intravital microscopy and determination of bacterial burdens, we have also now established that in the liver of infected animals, S. lugdunensis can reside within Kupffer cells while remaining viable. Moreover, S. lugdunensis within Kupffer cells in vivo fails to replicate for at least 16-hr postinfection in agreement with our in vitro macrophage data.
The inability of S. lugdunensis to proliferate within macrophages differs significantly from S. aureus (Flannagan et al., 2015b; Surewaard et al., 2016), and this may, in part, be attributable to differences in nutrient acquisition. S. lugdunensis is unable to synthesise either staphylococcal siderophore, staphyloferrin A and B, despite encoding and expressing the receptors HtsA and SirA that allow for staphyloferrin A and B utilisation (Brozyna, Sheldon, & Heinrichs, 2014). This would imply that S. lugdunensis, in the absence of S. aureus co-infection, could exist in an iron-starved state even if iron is available. Despite these differences in iron acquisition systems, the inability of S. lugdunensis to proliferate within the macrophage is unlikely to be due solely to their inability to acquire nutrients. This assertion is supported by our observation that phagocytosed S. lugdunensis can grow in the presence of protease inhibitors indicating that under these conditions, the nutrients needed for intracellular growth are available. The fact that Antipain and Pepstatin A promote intracellular growth of S. lugdunensis suggests that a yet unidentified protease contributes to growth restriction in macrophages. Presumably, because ConA also promotes proliferation of S. lugdunensis, the protease in question also acts optimally at acidic pH akin to some cathepsins (Turk, Dolenc, Turk, & Bieth, 1993; Wang et al., 1998). The observation that protease inhibition in primary human macrophages did not promote S. lugdunensis growth, but Vps34 inhibition does, emphasises that there are indeed functional differences between RAW and primary human macrophages. Moreover, this observation highlights the importance of employing primary cells in cellular models of infection.
In contrast to other intracellular pathogens such as Listeria monocytogenes and Legionella pneumophila (Brüggemann, Cazalet, & Buchrieser, 2006; Shaughnessy, Hoppe, Christensen, & Swanson, 2006), we find that S. lugdunensis does not perturb phagosome maturation. This observation parallels S. aureus USA300, which also resides in phagolysosomes where these bacteria commence replicating without intervention (Flannagan et al., 2015b; Surewaard et al., 2016). Although macrophages normally restrict S. lugdunensis, it is of interest that when replication does occur, the bacteria are no longer constrained by LAMP-1 or PS-positive membrane. This contrasts non-replicating S. lugdunensis and represents a significant difference that distinguishes S. lugdunensis from S. aureus, the latter of which replicates in PS and LAMP-1-positive vacuoles (Flannagan et al., 2015b; Surewaard et al., 2016). At present, whether other CoNS species demonstrate similar intracellular behaviours has not been explored. S. lugdunensis HKU09-01 produces cytolytic proteins (see Figure 5a and Donvito et al., 1997) that could contribute to this effect; however, the apparent production of such haemolytic proteins does not correlate well with the ability to escape the phagosome upon intracellular replication. This is evidenced by S. lugdunensis N920134 which can be made to replicate without being confined to LAMP-1 positive membranes while not displaying haemolytic activity (see Figures S4 and 5a). Ostensibly, replicating S. lugdunensis bacteria have direct access to the host cell cytosol, a notion supported by our transmission electron microscopy (TEM) data; however, in this instance, pyroptotic death of the infected macrophage would be expected (Miao et al., 2010). We find no evidence of pyroptosis, even after 24 hr, as macrophages harbouring replicating S. lugdunensis are refractory to PI staining (see Figure S1). It is tantalising to speculate that phagocytosed S. lugdunensis may actively interfere with inflammasome activation as is the case for other bacterial pathogens (Stewart & Cookson, 2016). Addressing this question and determining how S. lugdunensis escape the phagosome when replicating are an active area of investigation in our laboratory.
Regardless of where S. lugdunensis is replicating inside the macrophage, it is clear that both murine and human macrophages fail to kill phagocytosed S. lugdunensis indicating that the bacteria are able, in some capacity, to circumvent antimicrobial aspects of the phagolysosome. This is supported by our observation that inactivation of oatA renders S. lugdunensis more susceptible to macrophage killing in vitro and in vivo. Interestingly, oatA-deficient bacteria are not entirely eradicated, indicating that other factors must also contribute to intracellular survival of S. lugdunensis.
Although S. lugdunensis fails to replicate inside macrophages, we have demonstrated that this bacterium is not benign, and presumably, its prolonged survival could have profound consequences. For instance, in the event of an S. aureus infection, could S. lugdunensis within macrophages be liberated and disseminate to cause infection? Previous work has elegantly demonstrated, using intravital imaging, that S. aureus is rapidly captured by Kupffer cells in the murine liver after tail vein injection (Surewaard et al., 2016; Zeng et al., 2016). Here, we show that S. lugdunensis is also removed from circulation by Kupffer cells in the murine liver where S. lugdunensis persists for at least 16-hr postinfection. Importantly, persistence requires ongoing bacterial processes as indicated by our data that showed that heat-killed S. lugdunensis are rapidly degraded, and oatA-deficient bacteria are less able to survive at a time (8-hr postinfection) when wild-type live bacteria are still contained by Kupffer cells.
In summary, S. lugdunensis is an important infectious CoNS species that is not killed by macrophages but rather persists intracellularly without overtly intoxicating infected cells. This surreptitious survival may enable S. lugdunensis to evade immune killing and likely represents bacterial reservoirs that can emerge to cause subsequent infection.
4 EXPERIMENTAL PROCEDURES
4.1 Reagents
The Cell Proliferation Dye eFluor® 670 was from eBiosciences. Fluorescent tetramethylrhodamine-conjugated wheat germ agglutinin and FITC-Avidin 488 were from Molecular probes, ThermoFisher Scientific. The rat anti-mouse LAMP-1 antibody (clone 1D4B) and the mouse anti-human LAMP-1 antibody (clone H4A3) were obtained from the Developmental Studies Hybridoma Bank at the University of Iowa. Goat anti-rat Alexa Fluor® 488 IgG, goat-anti rat, and goat anti-human Cy3 conjugated IgG were all purchased from Jackson Immunoresearch. For cell culture, recombinant human M-CSF, IFN-γ, and GM-CSF were purchased from Peprotech, and Lipopolysaccharide was from Sigma-Aldrich. ConA, Pepstatin A, and Antipain were purchased from Santa Cruz Biotechnology. DPI was purchased from Tocris Bioscience.
4.2 Bacterial strains and culture conditions
Staphylococcus aureus strain USA300 (plasmid cured; Arsic, Zhu, Heinrichs, & McGavin, 2012), M. luteus (Flannagan et al., 2015a, 2015b), S. lugdunensis HKU09-01 (Tse et al., 2010), S. lugdunensis M23590 American Type Culture Collection (ATCC), S. lugdunensis N920143 (Heilbronner et al., 2011), S. lugdunensis ΔoatA mutant derived from HKU09-01 (this study), and two clinical isolates from the Heinrichs laboratory strain collection were used in this study. Escherichia coli DH5α was used for all cloning experiments and E. coli SL01 was used to modify plasmids to be introduced into S. lugdunensis. Clinical isolates were a kind gift from Dr. Yohan Delport, London Health Sciences Centre, The University of Western Ontario. Bacteria were routinely cultured at 37°C in TSB (Difco) with shaking or on TSB agar (1.5% wt/vol). When needed S. lugdunensis carrying the constitutive GFP expression vector pRSA-GFP (Sayedyahossein et al., 2015) was cultured in the presence of erythromycin (3 μg ml−1) and lincomycin (20 μg ml−1). When necessary, the staphylococcal selective medium mannitol salt agar was also used, and bacteria were cultured on mannitol salt agar at 37°C.
4.3 Mammalian cell culture
RAW 264.7 macrophages were from the American Type Culture Collection. RAW 264.7 macrophages were routinely cultured in RPMI 1640 (Wisent) buffered with sodium bicarbonate and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) supplemented with 5% (vol/vol) non-inactivated FBS (Wisent). Macrophages were passaged by cell scraping and grown at 37°C in the presence of 5% CO2. RAW macrophages were passaged for no longer than 6 weeks prior at which time new cells were revived from liquid nitrogen.
Primary human macrophages were derived from peripheral blood monocytes isolated from healthy volunteer blood samples. Monocytes were isolated as previously described (Flannagan et al., 2015a, 2015b). In brief, monocytes were enriched for by virtue of their rapid adhesion to glass coverslips. Differentiation of adhered monocytes was accomplished by culturing cells as described above in RPMI supplemented with 10% (vol/vol) non-inactivated FBS and antibiotics. For the differentiation of M0 macrophages, adhered monocytes were cultured in the presence of recombinant human M-CSF (10 ng ml−1) for 5 days followed by RPMI containing 10% (vol/vol) FBS and M-CSF without antibiotics for an additional 2 days. Macrophages were used from Days 7 to 10. To generate primary M1 polarised human macrophages, adhered monocytes were cultured as above; however, recombinant human GM-CSF (20 ng ml−1) was used. At Day 5 post-isolation, the medium was replaced with RPMI containing 10% (vol/vol) FBS without antibiotics but containing GM-CSF (20 ng ml−1), recombinant human IFN-γ (10 ng ml−1), and lipopolysaccharide (250 ng ml−1). Cells were used from Days 7 to 10.
4.4 Infection assays and enumeration of gentamicin protected bacteria
All macrophages adhered to glass coverslips were infected with bacteria at an multiplicity of infection (MOI) of 10 unless otherwise stated. Bacteria were grown overnight in TSB then pelleted and resuspended in serum-free RPMI and diluted to the appropriate cell density. Bacteria were then added to macrophages that had been cultured overnight in individual wells of 12-well tissue culture plate. To synchronise phagocytosis, tissue culture plates were centrifuged at 277 × g for 2 min and then incubated at 37°C with 5% CO2 for 30 min. Next, cells were washed with PBS and the medium was replaced with serum-free RPMI containing gentamicin at 100 μg ml−1 for 60 min. For some infection experiments either 0.02% or 0.002%, Triton X-100 was added to the macrophages after treatment with gentamicin to release the bacteria from the macrophages.
To enumerate gentamicin protected cells at 1.5-hr postinfection, the macrophages were washed with PBS after gentamicin treatment and then lysed with sterile water and 0.01% (vol/vol) Triton X-100 in PBS. For infections longer than 1.5 hr, infected cells were washed with sterile PBS and then incubated in RPMI containing 5% (vol/vol) FBS without gentamicin. At 24-hr postinfection or longer as necessary, the culture medium was collected and centrifuged to collect any bacteria having escaped from macrophages. Adherent macrophages were lysed as described above, and the cell pellet from the culture medium was resuspended in the Triton solution containing lysed macrophages and gentamicin protected bacteria. The samples were serially diluted and plated to determine the number of colony-forming units.
In some instances, mock infections were performed as controls. The input bacterial samples were prepared identically to those above but were added to individual wells of a tissue culture plate without macrophages. The samples were grown in RPMI with 5% (vol/vol) FBS at 37°C with 5% CO2. These cells were not treated with gentamicin but were collected at 1.5- and 24-hr post-inoculation and were diluted and plated as above.
4.5 Biotinylation of bacteria
Bacteria were surface biotinylated as previously described (Sarantis et al., 2012). In brief, bacteria from an overnight culture were pelleted and washed in PBS pH 8.0. Next, the bacterial pellet was resuspended in 1 ml of PBS pH 8.0 containing 0.1 mg EZ-Link-NHS-LC Biotin (Thermo Scientific) and incubated at room temperature for 10 min. Bacteria were then pelleted and incubated for 2 min with 1 ml of TSB to quench any unreacted biotin. After washing with PBS, bacteria were resuspended in serum-free RPMI and used for infections as described above. Biotinylated bacteria remaining outside macrophage were detected by adding eBioscience™ streptavidin-FITC (Thermo Scientific).
4.6 Construction of a Staphylococcus lugdunensis oatA mutant
Allelic replacement was performed using the pKOR1 plasmid as previously described (Bae & Schneewind, 2006). In brief, the OatA-Attb1/OatA5′R (GGGGACAAGTTTGTACAAAAAAGCAGGCTCAACAAATTTTTGGAGG/GGACCTCCGCGGCTTAACTTGTAGTTCCG) and the OatA3′F/OatAAttb2 (GGACCTCCGCGGCCATTGAACAAACAGAAAAAG/GGGGACCACTTTGTACAAGAAAGCTGGGTCCAGGATTTACAACACATG) primer pairs were used to PCR amplify 900–1,100 base pair regions upstream and downstream of the oatA gene. The fragments were cloned into the pKOR1 plasmid to generate the pKOR1ΔoatA plasmid. This plasmid was obtained in E. coli DH5α and passaged through E. coli SL01 to allow it to be transformed into S. lugdunensis HKU09-01. Chromosomal recombination then resulted in the deletion of the oatA gene from S. lugdunensis. Chromosomal deletions were confirmed by PCR amplification using the forward and reverse primers OatA-Check-F/R (CAAATTTTATGTTATAGTTTCTAG/GTTCTAAATTTAATGGAACGGGG) that anneal to the chromosome flanking the entire the regions of homology.
4.7 Lysozyme minimum inhibitory concentration
Wild-type and oatA-deficient S. lugdunensis bacteria were streaked onto TSA plates and grown overnight at 37°C. Isolated colonies were inoculated into individual glass test tubes containing 5 ml TSB, in triplicate for each strain, and grown overnight with shaking. The next day, the bacteria were pelleted and washed with sterile 1× PBS and diluted to an OD600nm of 0.5. Next, a 1 in 100 dilutions of these bacterial suspensions (final OD600nm of 0.005) was used to inoculate RPMI supplemented with 0.5% (wt/vol) casamino acids in the presence of lysozyme. Twofold dilutions of lysozyme were used starting with 3,600 μg ml−1 of lysozyme. Growth analysis was performed in 1-ml culture volumes in 13-ml polypropylene round bottom sterile tubes, and the OD600nm was determined at 20-hr post-inoculation allowing for minimum inhibitory concentration determination.
4.8 Immunofluorescence staining of LAMP-1
Macrophages were infected as above, and at the desired time postinfection, macrophages were fixed with 4% (vol/vol) PFA for 20 min at room temperature. The cells were then permeabilised with ice-cold methanol for 2 min and then blocked with 1 ml 5% (wt/vol) skim milk in sterile 1× PBS for at least 1 hr. Macrophages were then incubated for 1 hr at room temperature with either mouse anti-human LAMP-1 antibody (clone H4A3) or with rat anti-mouse LAMP-1 antibody (clone 1D4B) diluted 1:100 in blocking solution as appropriate. Cells were then rinsed and subsequently incubated for 1 hr with 0.75 μg ml−1 of the appropriate fluorophore conjugated secondary antibody diluted in blocking solution and then rinsed. LAMP-1 immunostained cells were analysed by confocal or wide-field fluorescence microscopy.
4.9 Dextran loading of macrophages
Macrophages adhered to coverslips were incubated overnight with 10 kDa dextran conjugated with fluorescein isothiocyanate (FITC) or tetramethylrhodamine (TRITC) at 100 μg ml−1 (Molecular Probes®, Thermo Fisher Scientific) in complete tissue culture medium. Prior to infection, dextran loaded macrophages were washed with PBS, and the medium was replaced with serum-free RPMI without dextran for at least 1 hr to allow for labelling of lysosomal compartments. After this, infections were carried out as described above, and at 1.5-hr postinfection, the cells were imaged live on a Leica TCS SP5 laser scanning confocal microscope.
4.10 eFluor proliferation assays
Staphylococcus lugdunensis containing the pRSA-GFP plasmid were labelled with 5 μg ml−1 eBioscience™Cell Proliferation Dye eFluor™ 670 in 0.9% (wt/vol) saline for 5 min. The bacteria were then washed with Luria–Bertani to quench the unreacted eFluor dye and were then resuspended in serum-free RPMI to carry out infections as described above. At 1.5- and 24-hr postinfection, the samples were fixed and imaged by wide field fluorescence microscopy. In some instances, macrophages were either pretreated or treated after gentamicin exposure (i.e., 1.5-hr postinfection) with ConA (0.5 μM), DPI (10 μM), Pepstatin A (100 μM), and Antipain (10 μg ml−1) alone or in combination. Once added, pharmacological agents were maintained throughout the entire experiment. In some instances, PI was added to cells (1 μg ml−1), which were then imaged live by fluorescence microscopy.
4.11 IgG opsonisation and phagocytosis of beads
Silica beads (3.14 μm, Bangs Laboratories) were incubated for 1 hr with 0.8 mg ml−1 human IgG prior to washing. Next, beads were added to macrophages and centrifuged at 277 × g to synchronise binding of IgG-coated beads to macrophages. After a 30-min incubation at 37°C, an anti-human IgG conjugated to AlexaFluor-647 (0.75 μg ml−1) was added to mark extracellular beads prior to fixation with 4% (vol/vol) paraformaldehyde. Macrophages were then imaged by fluorescence microscopy, and the phagocytic index was determined by counting the number of internalised beads (i.e., AlexaFluor-647 negative) divided by the total number of macrophages counted. Macrophages without beads, either internalized or adhered to the cell surface, were not included in the analysis.
4.12 Fluorescence microscopy
Laser scanning confocal microscopy was performed using a Leica TC5 SP5 microscope composed of a DMI6000 CS inverted microscope. The microscope contains an argon/2 (458, 476, 488, and 514 nm), HeNe1 (543 nm), and HeNe2 (633 nm) lasers and a PL-Apo 63× oil immersion objective lens. The microscope also contains a triple dichroic (488/543/633), a double dichroic (488/543), and a tunable Acousto-Optical Beam Splitter for all laser lines. All confocal Imaging was done at the Robarts Research Institute Imaging Facility (The University of Western Ontario).
Wide-field fluorescence and differential interference contrast microscopy was performed on a Leica DMI6000 B inverted microscope. The microscope is equipped with 40× (NA 1.3), 63× (NA 1.4), and 100× (NA 1.4) oil immersion PL-Apo objectives, a Leica 100 W Hg high-pressure light source, and a Photometrics Evolve 512 Delta EM-CCD camera. Deconvolution of wide-field images acquired as z-stacks was done using the Leica LAS X software.
For acquisition of S. lugdunensis in eFluor-670 proliferation assays, acquisition parameters for far-red fluorescence within each experiment were established on 1.5 hr samples where all bacteria were eFluor-positive. EM gain (~100), excitation intensity (100%), and exposure time (~200 ms) were set to obtain maximal fluorescence without achieving saturation of the far-red eFluor-670 signal at the 1.5 hr time point where the signal would be greatest. The acquisition parameters established for each 1.5 hr sample were applied to the 24 hr samples to identify bacteria that had become eFluor-negative due to dilution of the dye as a consequence of bacterial replication. Images were acquired using either the 63× or 100× objectives, and images were processed using Image J software (Schneider, Rasband, & Eliceiri, 2012) for contrast enhancement always with a gamma setting of 1. Images were cropped as necessary using Image J after contrast enhancement, and finally, scale bars were applied to each image series. The percentage of macrophages containing replicating bacteria was determined by counting at least 300 infected macrophages from at least 20 randomly acquired images per condition for each experiment. Those macrophages containing GFP-positive yet eFluor-negative cocci were counted as macrophages containing replicating bacteria. The number of macrophages containing GFP-positive yet eFluor-negative bacteria divided by the total number of infected macrophages was used to determine the percentage of cells with replicating bacteria.
4.13 Transmission electron microscopy
Macrophages infected with S. lugdunensis were fixed with 2% (vol/vol) glutaraldehyde in 0.2 M Sorenson's phosphate buffer (pH 7.2) for 5 min after which the cells were scraped off the coverslip, pelleted by centrifugation, and resuspended in fixative for an additional 2 hr. Fixed macrophages were subsequently embedded in agarose and post-fixed with 0.5% (wt/vol) osmium tetroxide for 2 hr at room temperature. Next, cells were stained en bloc for 1 hr with 1% (wt/vol) uranyl acetate in dH2O. Samples were then progressively dehydrated with increasing concentrations of acetone and then embedded in Epon-Araldite resin. Eighty-nanometre-thick sections were cut and placed on copper grids to allow viewing with a Philips CM10 transmission electron microscope. Images were taken using a Hamamatsu Orca 2 MPx HRL camera.
4.14 Murine model of systemic infection
All protocols for murine infection were reviewed and approved by the University of Western Ontario's Animal Use Subcommittee, a subcommittee of the University Council on Animal Care. Six-week-old, female, BALB/c mice were obtained from Charles River Laboratories and housed in microisolator cages. S. lugdunensis strains were grown to exponential phase (OD600 2-4) in TSB, washed twice with PBS, and resuspended in PBS to an OD600 of 0.50. In instances where S. lugdunensis was heat-inactivated, the bacterial suspension at an OD600 of 0.5 were heated at 80°C for 30 min and plated on TSA to confirm loss bacterial killing. For injections, 100 μl of the bacterial suspension, equivalent to ~2–3 × 107 cfu, was injected into each mouse via tail vein. After 8 hr of infection, mice were anaesthetised and euthanised via cervical dislocation. Livers were aseptically harvested into ice-cold PBS with 0.1% (vol/vol) Triton X-100, homogenised, serially diluted, and plated onto TSA to enumerate bacterial burden. Bacterial burden from liver is presented as log10 colony-forming unit per liver.
4.15 Intravital spinning disk confocal microscopy
The protocols employed here have been established by Surewaard and Kubes (2017). For whole animal imaging studies, experiments were performed with 6- to 10-week-old adult male mice, following protocols approved by the University of Calgary Animal Care Committee and in compliance with the Canadian Council for Animal Care Guidelines. All animals were maintained in a specific pathogen-free environment at the University of Calgary Animal Resource Centre, and mice were housed under standardised conditions for temperature (21–22°C) and illumination (12 hr light/12 hr darkness) with constant access to tap water and food.
For injections, a tail vein catheter was inserted into mice anaesthetised with 200 mg kg−1 ketamine (Bayer Animal Health) and 10 mg kg−1 xylazine (Bimeda-MTC). Surgical preparation of the liver for intravital imaging was performed as described by Wong et al. (Wong, Jenne, Lee, Léger, & Kubes, 2011). Mouse body temperature was maintained at 37°C with a heated stage, and image acquisition was performed using Olympus IX81 inverted microscope, equipped with an Olympus focus drive and a motorised stage (Applied Scientific Instrumentation). Objectives position was controlled with a motorised objective turret equipped with 4×/0.16 UPLANSAPO, 10×/0.40 UPLANSAPO, and 20×/0.70 UPLANSAPO objective lenses and coupled to a confocal light path (WaveFx; Quorum Technologies) based on a modified Yokogawa CSU-10 head (Yokogawa Electric Corporation). Kupffer cells were visualised in vivo by intravenous injection of 2.5 μg fluorescently conjugated anti-F4-80 mAb (clone BM8). S. lugdunensis bacteria were visualised through constitutive expression of GFP, and 1.0 × 107 bacteria were injected per mouse. Laser excitation wavelengths 491, 561, 642, and 730 nm (Cobolt) were used in rapid succession, together with the appropriate band-pass filters (Semrock). A back-thinned EMCCD 512 × 512 pixel camera was used for fluorescence detection (Hamamatsu). Volocity software (Perkin Elmer) was used to drive the confocal microscope and for image acquisition and analysis. The find objects function in Volocity software was used to identify individual bacteria that were captured by KCs (F4/80+ cells in liver) and to determine the fluorescence intensity per particle.
4.16 Statistical analysis
Data are presented as the mean ± SD. All statistical analyses and graph production were done through GraphPad Prism (GraphPad Software, La Jolla, CA).
ETHICS STATEMENT
Human blood was obtained, with written permission, from healthy adult volunteers in compliance with Protocol #109059 approved by the Office of Research Ethics at The University of Western Ontario.
ACKNOWLEDGEMENTS
This work was supported by grants, to DEH and to PK, from the Canadian Institutes of Health Research (http://www.cihr-irsc.gc.ca; the Heart and Stroke Foundation of Canada (www.heartandstroke.ca; to P. K.), and the Canada Research Chairs program (http://www.chairs-chaires.gc.ca; to P. K.). B. G. J. S. is supported by a postdoctoral fellowship from CIHR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors wish to thank Professor T.J. Foster of Trinity College Dublin for the Escherichia coli strain SL01. The authors wish to thank Dr. Arnold S. Bayer (LA Biomed) for constructive comments on the manuscript. We would also like to acknowledge members of the Heinrichs laboratory for their helpful discussions.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.