Extracellular HtrA serine proteases: An emerging new strategy in bacterial pathogenesis
Abstract
The HtrA family of chaperones and serine proteases is important for regulating stress responses and controlling protein quality in the periplasm of bacteria. HtrA is also associated with infectious diseases since inactivation of htrA genes results in significantly reduced virulence properties by various bacterial pathogens. These virulence features of HtrA can be attributed to reduced fitness of the bacteria, higher susceptibility to environmental stress and/or diminished secretion of virulence factors. In some Gram-negative and Gram-positive pathogens, HtrA itself can be exposed to the extracellular environment promoting bacterial colonisation and invasion of host tissues. Most of our knowledge on the function of exported HtrAs stems from research on Helicobacter pylori, Campylobacter jejuni, Borrelia burgdorferi, Bacillus anthracis, and Chlamydia species. Here, we discuss recent progress showing that extracellular HtrAs are able to cleave cell-to-cell junction factors including E-cadherin, occludin, and claudin-8, as well as extracellular matrix proteins such as fibronectin, aggrecan, and proteoglycans, disrupting the epithelial barrier and producing substantial host cell damage. We propose that the export of HtrAs is a newly discovered strategy, also applied by additional bacterial pathogens. Consequently, exported HtrA proteases represent highly attractive targets for antibacterial treatment by inhibiting their proteolytic activity or application in vaccine development.
1 INTRODUCTION: THE STRUCTURE AND FUNCTIONS OF THE SERINE PROTEASE AND CHAPERONE HTRA
Proteases of the high temperature requirement A (HtrA) family are widely expressed by microbes including archaea and Gram-negative and Gram-positive bacteria. The important roles of HtrA in the protein quality control through the prevention of severe cellular malfunctions due to accumulation of fragmented, mislocalized, or misfolded proteins under physiological and stress conditions have been summarised in several excellent reviews (Clausen, Southan, & Ehrmann, 2002; Kim & Kim, 2005; Krojer et al., 2008; Hansen & Hilgenfeld, 2013; Skorko-Glonek et al., 2013). Although the amino acid sequence of HtrA family members is highly conserved, the domain architecture can vary considerably (Figure 1A). Three homologous HtrA proteins (DegP, DegQ, and DegS) in Escherichia coli serve as paradigms for HtrA function and regulation in Gram-negative bacteria (Waller & Sauer, 1996). DegP and DegQ proteases contain an N-terminal signal peptide, which is important for periplasmic localization, followed by a conserved chymotrypsin-like serine protease domain with the catalytic triad composed of histidine, aspartate, and serine residues. At the C-terminus, two PDZ (post synaptic density of 95 kDa, discs large, and Zonula Occludens 1) domains are located that are involved in substrate recognition, binding, and in HtrA oligomerization (Figure 1a,b). This compares with DegS protease that harbours an N-terminally located transmembrane segment instead of a signal peptide and only one PDZ domain. A trimeric DegS structure is formed in the periplasmic space. Similar to DegS, many Gram-positive bacteria express HtrA proteins, which also contain an N-terminal transmembrane domain (Kim & Kim, 2005).
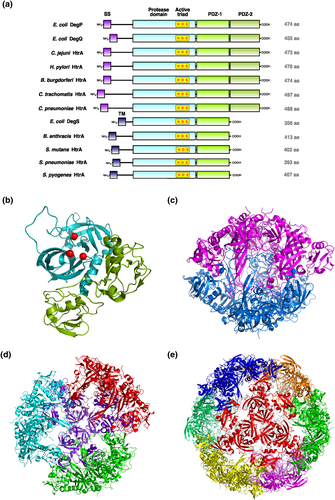
The catalytic activity of E. coli DegP can be reversibly switched on and off dependent on the formation of oligomeric structures upon substrate binding (Kim, Grant, & Sauer, 2011; Krojer, Sawa, Huber, & Clausen, 2010). Conformational rearrangements lead to dissociation of the resting state hexamer (Figure 1c) and subsequent assembly of higher order oligomers consisting of active 12 or 24 DegP monomers (Figure 1d,e; Jiang et al., 2008; Krojer et al., 2008; Shen et al., 2009), but also the conversion from inactive trimers into active trimers upon substrate binding was described (Kim & Sauer, 2012). Several substrates for DegP were identified, including misfolded proteins (e.g., maltose binding protein, alkaline phosphatase, α-amylase, etc.) and native proteins (e.g., acylated precursor of colicin A lysis protein, CpxP). Periplasmic DegS is considered as a regulatory protease targeting the antisigma factor RseA, which is involved in sensing protein folding stress. DegS processes RseA, which represents a rate-limiting step in the sigma E pathway through downregulation of outer membrane proteins (Hansen & Hilgenfeld, 2013). In addition, numerous bacteria have been convincingly reported to deliver HtrA directly into the extracellular space including Gram-negative and Gram-positive pathogens such as Helicobacter pylori, Campylobacter jejuni, Borrelia burgdorferi, Bacillus anthracis, and Chlamydia species. (Boehm et al., 2013; Lower et al., 2008; Wu et al., 2011). Thus, these HtrAs can directly interfere with host cells and cause tissue damage during infection. Here, we review the role and function of these exported HtrAs as an emerging new strategy in bacterial pathogenesis.
2 THE SECRETION ROUTE OF HTRA BY BACTERIAL PATHOGENS
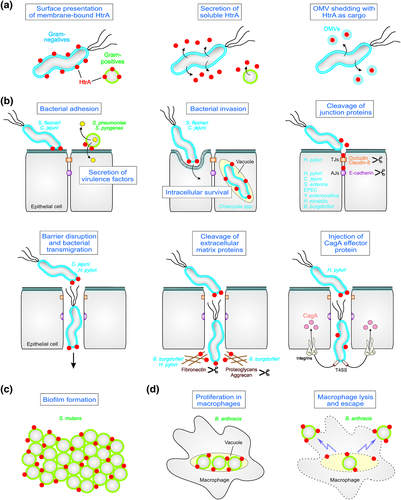
For a long time, bacterial HtrA function in virulence has been primarily attributed to increased fitness of pathogens due to resistance against stress conditions during infection. However, in recent years, it became apparent that HtrAs are also directly implicated in multiple pathogen-host interactions because considerable amounts of the protease can be released into the environment (Table 1). Altogether, three HtrA export pathways have been reported: (a) presentation of membrane-anchored HtrA at the bacterial surface, (b) secretion of soluble proteins into the supernatant, and (c) delivery of HtrA as cargo inside of shedded outer-membrane vesicles (OMVs; Figure 2a). HtrA of various Gram-positive pathogens such as B. anthracis is anchored in the phospholipid bilayer of the cell through a transmembrane domain with the C-terminal enzymatically active portion exposed to the environment (Sela-Abramovich et al., 2009). It has been proposed that membrane-bound HtrA can be also released in the extracellular space by autocleavage. Thus, B. anthracis HtrA has been detected in serum samples from infected rabbits. Interestingly, HtrA was not observed in the secretome of the related pathogens Bacillus cereus and Bacillus thuringiensis, suggesting a species-specific release mechanism (Sela-Abramovich et al., 2009). The extracellular presence of HtrAs was approved experimentally by fractionation and immunogold labelling using anti-HtrA antibodies combined with electron microscopy of in vitro cultured human cells infected with Chlamydia muridarum, H. pylori, and C. jejuni (Bartolini et al., 2013; Boehm et al., 2012, 2013; Hoy et al., 2010; Tegtmeyer et al., 2017) and of biopsy samples from H. pylori-infected patients in vivo (Tegtmeyer et al., 2017). In addition, extracellular HtrA was identified by shedding of outer cell surface proteins in B. burgdorferi and subsequent mass spectrometry and immunodetection of released HtrA (Coleman, Crowley, Toledo, & Benach, 2013; Gesslbauer et al., 2012; Russell, Delorey, & Johnson, 2013). Although the presence of an extracellular fraction of HtrA was convincingly demonstrated, the molecular mechanism of protein export by Gram-negative bacteria remains puzzling. Many Gram-negative HtrAs contain a signal sequence for Sec-dependent processing and delivery of the protease over the inner bacterial membrane into the periplasm (Boehm et al., 2012; Lower et al., 2008). However, how these HtrAs can travel across the bacterial outer membrane is not yet clear. Commonly, HtrAs do not exhibit homology to typical autotransporters, which process themselves by autocleavage. This idea is supported by the observation that C. jejuni HtrA protease activity was not required for secretion (Boehm et al., 2013). In addition, they do not appear to be secreted by Type I–VII transporters (Boehm et al., 2013; Coleman et al., 2013; Lower et al., 2008). Another possibility is the extracellular passage of HtrA within OMVs, which are continuously shed by Gram-negative bacteria. Indeed, HtrA was found in OMVs isolated from H. pylori, C. muridarum, and C. jejuni (Bartolini et al., 2013; Elmi et al., 2016; Olofsson et al., 2010). However, how HtrA can be released from the OMVs is yet unknown. Future studies should investigate more systematically the secretion route of HtrA by Gram-negative bacteria.
| Bacterial pathogen | Associated disease | Pathogenic process | Proposed HtrA function | References |
|---|---|---|---|---|
| Bacillus anthracis | Anthrax disease | Respiratory, gastrointestinal, and cutaneous entry | Proliferation in macrophages and lysis of macrophages | Sela-Abramovich et al., 2009; Chitlaru et al., 2011 |
| Borrelia burgdorferi | Lyme disease | Epithelial cell invasion | Cleavage of E-cadherin, aggrecan, fibronectin, and proteoglycans | Russell et al., 2013a/b |
| Campylobacter jejuni | Campylobacteriosis, Guillian Barré syndrome | Bacterial adhesion, transmigration, and invasion | Adhesion and invasion of cells, cleavage of E-cadherin, apoptosis, and immune responses | Boehm et al., 2012; Boehm et al., 2013; Heimesaat, Alutis et al., 2014; Heimesaat, Fischer et al., 2014 |
| Chlamydia pneumoniae | Infectious asthma | Intracellular survival and spread | Intracellular survival of the bacteria | Mukhopadhyay et al., 2006; Wu et al., 2011 |
| Chlamydia trachomatis | Trachoma, sexually transmitted diseases | Intracellular survival and spread | Intracellular survival of the bacteria | Patel et al., 2014; Wu et al., 2011; Bartolini et al., 2013 |
| Enteropathogenic Escherichia coli | Diarrhoea | Epithelial cell adhesion | Cleavage of E-cadherin | Hoy et al., 2012 |
| Helicobacter pylori | Gastritis, ulcers, gastric carcinoma | Bacterial transmigration, activation of Type IV secretion | Cleavage of occludin, claudin-8, E-cadherin, and fibronectin | Hoy et al., 2010; Schmidt, Perna, et al., 2016; Tegtmeyer et al., 2017 |
| Proteus mirabilis | Sepsis, pneumonia | Urinary tract and wound infections | Cleavage of E-cadherin | Abfalter et al., 2016 |
| Salmonella enterica | Salmonellosis | Epithelial cell invasion | Cleavage of E-cadherin | Hoy et al., 2012; Abfalter et al., 2016 |
| Shigella flexneri | Shigellosis | Epithelial cell invasion | Cleavage of E-cadherin | Hoy et al., 2012 |
| Streptococcus mutans | Dental caries | Colonisation of the oral cavity | Biofilm formation | Biswas and Biswas, 2005 |
| Streptococcus pneumoniae | Community-acquired pneumonia | Chronic airway infections | Quality control of secreted proteins | Ibrahim et al., 2004; Cassone et al., 2012; Kochan and Dawid, 2013; de Stoppelaar et al., 2013 |
| Streptococcus pyogenes | Purulent diseases of the pharynx and skin | Tissue invasion and hemolysis | Processing and secretion of virulence factors | Lyon and Caparon, 2004; Cole et al., 2007 |
| Yersinia enterocolitica | Gastrointestinal diseases | Epithelial cell invasion | Cleavage of E-cadherin | Abfalter et al., 2016 |
3 SECRETED HTRAS TARGET HOST CELLS DURING BACTERIAL INFECTION
In the last years, it became evident that extracellular HtrAs can directly target surface proteins of bacterial and host cells to promote virulence of a number of pathogens, which represent a striking new mechanism in pathogenesis (Figure 2b-d). In this context, it was indicated that HtrA can process microbial outer membrane proteins, such as adhesins and virulence factors (Frees, Brondsted, & Ingmer, 2013; Skorko-Glonek et al., 2013). For example, DegP of Shigella flexneri might play a role in surface presentation of the adhesin IcsA (Purdy, Fisher, & Payne, 2007). Furthermore, reduced adherence to host cells by a C. jejuni ΔhtrA mutant was observed in vitro, suggesting that adhesion factors are ineffective (Brondsted, Andersen, Parker, Jorgensen, & Ingmer, 2005). However, in a new in vivo animal model for C. jejuni, HtrA expression did not alter colonisation rates but exhibited significant effects on host cell apoptosis and the intestinal and extraintestinal proinflammatory immune responses (Heimesaat et al., 2014; Heimesaat et al., 2014). These data might indicate that extracellularly located HtrA is implicated in direct host-pathogen interactions. In addition, chlamydial strains can secrete HtrA into vesicles present in the cytosol of infected host cells (Wu et al., 2011). HtrA exhibits a proposed role in the replicative cycle of Chlamydia and penicillin resistance; however, host-derived substrates were not yet identified (Marsh et al., 2015; Mukhopadhyay et al., 2006; Ong et al., 2013; Patel, De Boer, Timms, & Huston, 2014). As pointed out above, B. burgdorferi exhibited HtrA proteins both in membrane-anchored and soluble forms. HtrA-negative bacteria were unable to colonise mice, and this phenotype was explained by processing of the BB0323 protein, implicated in bacterial survival (Ye et al., 2016). Besides several endogenous substrates, surface-exposed HtrA can degrade the extracellular matrix components aggrecan, fibronectin, and numerous proteoglycans to promote invasiveness, suggesting important consequences of secreted HtrA for the infection process (Russell et al., 2013; Russell & Johnson, 2013).
Detailed information on secreted HtrAs is available for a number of gastrointestinal pathogens. HtrA exported by H. pylori serves as a paragon for the direct involvement in pathogen-host interactions. H. pylori delivers HtrA as an active soluble serine protease into its environment (Bumann et al., 2002; Lower et al., 2008). Importantly, extracellular HtrA cleaves E-cadherin on gastric epithelial cells, which was identified as the first biologically significant substrate (Hoy et al., 2010). E-cadherin is an important adherens junction (AJ) protein and tumour suppressor. Dysfunction of E-cadherin has been strongly connected to gastric tumour progression and metastasis in humans (Chan, 2006). Generally, ectodomain shedding of E-cadherin leads to loss of intercellular adhesion, and disintegration of the intracellular AJ complex is involved in cancer-associated signal transduction, which has been summarised in several excellent review articles (Chan, 2006; Repetto, De Paoli, De Re, Canzonieri, & Cannizzaro, 2014). The finding that human E-cadherin is a heterologous substrate for HtrA uncovered a fascinating novel mechanism in the multistep process of H. pylori-mediated disruption of the epithelial barrier allowing the pathogen to open AJs and transmigration across the epithelium (Hoy et al., 2010; Tegtmeyer et al., 2017). At the molecular level, H. pylori HtrA targets the calcium binding sites between the individual repeats in the extracellular E-cadherin domain containing the conserved signature sequence [VITA]-[VITA]-x-x-D-[DN] (Schmidt, Goetz, Huemer, Schneider, & Wessler, 2016; Schmidt, Perna, et al., 2016). The interference of HtrA with gastric epithelial cells became more complex by the finding of additional HtrA substrates exposed on gastric epithelial cells. H. pylori HtrA also cleaves fibronectin, claudin-8, and occludin (Hoy et al., 2010; Tegtmeyer et al., 2017). Claudin-8 and occludin are structural components of tight junctions, which are necessary for forming an impermeable epithelial barrier. Both HtrA-mediated disruption of tight junctions and AJs drastically increased bacterial transmigration via the paracellular route and subsequently enhanced Type IV secretion-mediated delivery of the effector protein CagA across the basolateral receptor integrin-α5β1 (Harrer, Boehm, Backert, & Tegtmeyer, 2017; Tegtmeyer et al., 2017). These findings filled a gap in our understanding how H. pylori pathogenesis is accomplished, and which molecular mechanisms are necessary to connect early and late events in infections of a polarised epithelium.
HtrA-mediated cleavage of E-cadherin has been also observed for a number of additional gastrointestinal and urogenital Gram-negative pathogens including C. jejuni, S. flexneri, enteropathogenic E. coli, Yersinia enterocolitica, Salmonella enterica, and Proteus mirabilis (Abfalter et al., 2016; Boehm et al., 2012; Elmi et al., 2016; Hoy et al., 2012). Among those bacteria, C. jejuni attracted special attention, since HtrA is secreted and responsible for E-cadherin shedding in cell culture infection models (Boehm et al., 2013; Hoy et al., 2012). HtrA activity in C. jejuni is important not only for heat tolerance and oxygen resistance but also for bacterial invasion and transmigration, which finally contributes to in vivo pathogenesis (Boehm, Lind, Backert, & Tegtmeyer, 2015; Heimesaat, Alutis, et al., 2014; Heimesaat, Fischer, et al., 2014). In contrast to H. pylori, C. jejuni HtrA does not cleave fibronectin, which is a receptor for CadF-mediated invasion of host cells (Boehm et al., 2012), suggesting that individual HtrAs differ in activity and substrate selectivity. This was recently confirmed in an in vitro study analysing different HtrA homologues from different pathogens. Since many pathogens express different numbers and combinations of DegP, DegQ, and DegS, it was investigated whether HtrA variants show overlaps in in vitro substrate cleavage. In fact, DegP and DegQ variants targeted E-cadherin but not DegS, indicating possible redundancy of DegP and DegQ as secreted HtrA proteases (Abfalter et al., 2016). However, whether substrate selectivity has consequences in vivo is not yet clarified.
The role of secreted HtrAs has been also investigated in a few Gram-positive pathogens. B. anthracis represents the etiological agent of anthrax disease and forms spores. HtrA is exposed at the bacterial membrane (Chitlaru et al., 2011) and secreted HtrA was abundantly detected in bacteremia blood (Sela-Abramovich et al., 2009). Mutation of HtrA significantly reduced bacterial survival in the host (Sela-Abramovich et al., 2009) and altered the secretion of several toxins and virulence proteins, including the starvation-associated neutral protease NprA (Chitlaru et al., 2011). Bacillus anthracis ΔhtrA mutants exhibit impaired proliferation in macrophages, and the lysis of macrophages was delayed about 3-fold. In addition, ΔhtrA mutants revealed a decrease of over six orders of magnitude in virulence in the guinea pig model of anthrax (Chitlaru et al., 2011). The role of secreted HtrA has been also investigated in streptococci. Streptococcus pyogenes HtrA is recruited to the ExPortal secretory microdomain at the bacterial cell surface, revealing a critical function in controlling various secreted proteins (Cole et al., 2007; Lyon & Caparon, 2004). For example, HtrA is utilised for maturation and processing of several virulence factors such as the hemolysin streptolysin S and cysteine protease SpeB. For SpeB, inactivation of HtrA resulted in a failure to proteolytically process the zymogen to an active protease (Lyon & Caparon, 2004). For streptolysin S, depletion of HtrA leads to a dramatic increase in hemolytic activity. In addition, Streptococcus pneumoniae ΔhtrA mutants revealed a significantly diminished virulence in models of bacteremia and pneumonia (de Stoppelaar et al., 2013; Ibrahim, Kerr, McCluskey, & Mitchell, 2004). Besides controlling stress responses, it appears that HtrA localises to the outer surface of the growing bacterial cell near the Sec apparatus, possibly controlling the quality of secreted proteins (Tsui, Keen, Sham, Wayne, & Winkler, 2011). Remarkably, bacterial communication and competence are also disturbed when HtrA is inactivated. These activities are adjusted when the cell density changes, and HtrA diminishes the quantity of the extracellular pheromones competence-stimulating peptide (CSP) and BlpC (Dawid, Sebert, & Weiser, 2009; Cassone, Gagne, Spruce, Seeholzer, & Sebert, 2012; Kochan & Dawid, 2013). Finally, HtrA of Streptococcus mutans affected the surface expression of several extracellular proteins such as glucan-binding protein B, fructosyltransferase, and glucosyltransferases (Biswas & Biswas, 2005). In addition, htrA mutation altered the surface expression of various glycolytic enzymes and proper biofilm formation by S. mutans, which is known as dental plaque (Biswas & Biswas, 2005).
4 THE HUNT FOR HTRA INHIBITORS
Due to their important functions in the pathogenesis of numerous bacteria, HtrA proteins are regarded as potential targets for chemical inhibition. In particular, the secreted fractions of HtrAs that are involved in targeting of host cell surface factors and subsequent dissemination of microbes are especially attractive. The pioneering work in the field of the anti-HtrA compounds was done using E. coli HtrA as a model. The design of the inhibitor was based on a typical active site serine inhibitor, chloromethyl ketone, attached to a substrate resembling peptide. The resulting compound did inhibit E. coli HtrA; however, its specificity was rather low (Hauske et al., 2009). A similar approach was used to generate the antichlamydial HtrA compounds. The tripeptide sequences V-P-V or A-P-V were modified at the C-terminal part with a phosphonate electrophile that irreversibly binds to the active site serine. As a result, two effective chemicals were obtained, called JO146 and JCP83. The more potent JO146 was tested for its ability to prevent the development chlamydial infections in various cell cultures (Gloeckl et al., 2013). The experiments proved the effectiveness of the compound when added to the midreplicative phase of chlamydial developmental cycle without obvious toxicity in the human, mouse, or koala cells (Gloeckl et al., 2013; Lawrence et al., 2016). Moreover, JO146 was lethal against numerous Chlamydia spp., including Chlamydia trachomatis (Ong et al., 2013), Chlamydia pneumonia, and Chlamydophila pecorum (Lawrence et al., 2016). The latter two species cause severe infections in koalas (Jackson, White, Giffard, & Timms, 1999), so further optimization of JO146 for better specificity may result in a novel drug to treat chlamydiosis in koalas. HtrA from H. pylori is another example of a successfully targeted protease in vitro. In this case, a compound termed HHI characterised by a nonpeptide rhodamine derivative scaffold, was selected through virtual screening of databases and proved to be an effective inhibitor (Hoy et al., 2010; Lower et al., 2011). HHI became a template for further improvements leading to selection of a new lead compound with better chemical properties (Perna et al., 2015). Both chemicals are expected to act in the active center of the protease. These inhibitors were found to block cleavage of E-cadherin in vitro and also to prevent disruption of intercellular adhesion in a gastric epithelial cell culture model. Interestingly, one compound was generated to target a putative allosteric site of H. pylori HtrA, and it efficiently inhibited cleavage of E-cadherin (Perna et al., 2014). A peptide-based inhibitor was also designed from an E-cadherin cleavage site; however, its mode of action has not yet been determined (Schmidt, Perna, et al., 2016). Summing up, targeting the HtrA proteases offers a new possibility to prevent development of certain bacterial infection. However, several precautions should be taken to prevent potential side effects of anti-HtrA treatment. In particular, the HtrA inhibitors should be precisely optimised for high specificity; otherwise, the host's HtrA homologues or other serine proteases will be deactivated as well.
5 CONCLUSIONS
Life threatening infectious diseases represent a challenge in every society. In recent decades, the ongoing spread of antibiotic resistances has become an increasing concern both for Gram-positive and Gram-negative pathogens. The development of new antibiotic compounds has dramatically decreased, and drug resistances are now a priority in the medical sector worldwide. Facing this new hazard, solutions are being discussed to recommend alternative treatments. Here, we reviewed a novel class of bacterial virulence factors, the secretion and function of HtrA proteases, yet reported only for a small number of bacterial pathogens (Table 1). Although the precise mechanisms by which they contribute to the infection process is not always known, some general trends appeared. Numerous host cell targets of HtrAs and resulting clinically relevant phenotypes have been highlighted (Figure 2). Thus, we propose that the export of HtrA is a more widespread strategy among many additional pathogens. Consequently, exported HtrAs represent a highly attractive new group of antibacterial targets. One aim should be the specific interference with pathogens and inhibition of extracellular HtrA by compounds that do not penetrate the bacteria and thus do not affect the colonisation and survival of commensals. While many of the current antibiotics also affect commensal bacteria, the search for pathogen-selective HtrA inhibitors represents a very encouraging drug discovery strategy. In addition, the search of novel candidates for vaccine development is necessary and extracellular HtrAs also seem to be highly promising targets (Bartolini et al., 2013; Chitlaru et al., 2017). In the light of this tendency, there is an urgent need for further studies to unravel the molecular mechanisms of HtrA protease functions in more detail both under physiological and pathological conditions. Taken together, targeting of HtrA proteases is an inspiring way to elaborate new therapies for bacterial diseases but has just begun and needs more research before any clinical trials could be attempted.
ACKNOWLEDGMENTS
The work of S.B. is supported by Grant A04 in CRC-1181 of the German Science Foundation (DFG), J. S. G by Grant UMO-2016/21/B/NZ2/01775 of National Science Center (Poland) and S. W. by Grant P-24074 from Austrian Science Fund (FWF). We thank Donata Figaj for her fine support in drawing the HtrA structures in Figure 1b-e.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.




