Cryptosporidium parvum disrupts intestinal epithelial barrier function via altering expression of key tight junction and adherens junction proteins
Abstract
Infection with the protozoan parasite Cryptosporidium parvum (CP) causes cryptosporidiosis, a widespread diarrhoeal disease. Impaired intestinal epithelial barrier function and increased permeability are most commonly associated with diarrhoeal diseases caused by enteric infections. However, studies on barrier disruption and underlying mechanisms in cryptosporidiosis are extremely limited. Epithelial tight junctions (TJs) and adherens junctions (AJs) are important in maintaining barrier integrity. Therefore, we examined the effects of CP infection on paracellular permeability and on the expression of the major TJ and AJ proteins utilising in vitro, ex vivo, and in vivo models. CP infection (0.5 × 106 oocysts/well in Transwell inserts, 24 hr) increased paracellular permeability (FITC-dextran flux) in Caco-2 cell monolayers and substantially decreased the protein levels of occludin, claudin 4, and E-cadherin. Claudin 3, zonula occludens-1 (ZO1) and α-catenin were also significantly decreased, whereas claudins 1 and 2 and β-catenin were not altered. Substantial downregulation of occludin, claudin 4, and E-cadherin was also observed in response to CP infection ex vivo in mouse enteroid-derived monolayers and in vivo in the ileal and jejunal mocosa of C57BL/6 mice. The mRNA levels of these proteins were also significantly decreased in CP-infected mouse ileum and jejunum but were unaltered in Caco-2 cells. Further, bafilomycin-A, an inhibitor of lysosomal proton pump, partially abrogated CP effects on occludin expression in Caco-2 cells, suggesting a potential role of posttranslational mechanisms, such as induction of protein degradation pathways, in mediating the effects of the parasite. Our studies suggest that disruption of barrier function via downregulation of specific key components of TJ and AJ could be a major mechanism underlying CP infection-induced diarrhoea.
1 INTRODUCTION
The protozoan parasite Cryptosporidium species infects the epithelial cells of the small intestine to cause cryptosporidiosis, a widespread diarrhoeal disease, increasingly being recognised as an alarming global health problem (Checkley et al., 2015; Shirley, Moonah, & Kotloff, 2012; Shoultz, de Hostos, & Choy, 2016; Sparks, Nair, Castellanos-Gonzalez, & White Jr, 2015). The Global Enteric Multicenter Study (GEMS; Kotloff et al., 2013), and most recently, a global network study of malnutrition and enteric diseases (MAL-ED; Platts-Mills et al., 2015) reported this neglected parasite as one of the four major enteric pathogens causing life-threatening diarrhoea in infants associated with growth stunting, high mortality, and morbidity. Oocysts of the parasite entering human hosts via faecal–oral route invade the epithelial cells of the small intestine and reproduce on the apical surface of the epithelium. Cryptosporidiosis is now viewed as an important cause of diarrhoeal diseases in children and adults worldwide. In immunocompromised patients, such as those with AIDS, cryptosporidiosis can lead to persistent diarrhoea and even death (Ryan & Hijjawi, 2015; Shirley et al., 2012; Ward, 2017). In developing countries, persistent infections with Cryptosporidium intensified by malnutrition have been associated with impaired physical and cognitive development in children (Checkley et al., 2015). Despite being recognised as a global health problem, however, treatment options for cryptosporidiosis are severely limited primarily due to lack of understanding of the mechanisms of Cryptosporidium-induced diarrhoea.
Diarrhoeal diseases caused by enteric infections could involve impaired epithelial barrier function (Guttman & Finlay, 2009; Viswanathan, Hodges, & Hecht, 2009) as well as dysregulation of fluid and ion transport processes (Field, 2003; Hoque, Chakraborty, Sheikh, & Woodward, 2012). Intestinal epithelial barrier defects occur mostly via disruption of epithelial junctional complexes (AJCs) such as tight junctions (TJs) and AJs (Guttman & Finlay, 2009; Mehta, Nijhuis, Kumagai, Lindsay, & Silver, 2015; Shen, Su, & Turner, 2009; Viswanathan et al., 2009). TJs provide the barrier required for the maintenance of the electrochemical gradients necessary for efficient transcellular ion transport (Viswanathan et al., 2009) whereas AJs serve as mechanical linkage between adjacent epithelial cells (France & Turner, 2017). These junctions comprise a variety of transmembrane proteins, coupled with cytoplasmic adaptor proteins and the actin cytoskeleton, to attach adjacent cells together thereby forming intercellular seals (France & Turner, 2017). Breaching of this barrier has profound effects on human health and disease, as barrier defects have been linked with the onset of inflammation, diarrhoea, and other extra-intestinal effects. Various enteric pathogens have developed specific strategies to alter or disrupt these structures as part of their pathogenesis, resulting in either pathogen invasion, or other consequences such as diarrhoea (Guttman & Finlay, 2009; Vogelmann, Amieva, Falkow, & Nelson, 2004). Understanding the strategies that microorganisms use to commandeer the functions of AJC defines an important area of research in microbial pathogenesis (Guttman & Finlay, 2009).
Few earlier studies showed decreased transepithelial resistance (TER) and increased paracellular permeability following Cryptosporidium parvum (CP) infection of intestinal epithelial cells in vitro or in vivo (Di Genova & Tonelli, 2016; Roche, Martins, Cosme, Fayer, & Guerrant, 2000). However, very little is known about the mechanisms of impaired barrier function or the effects on the expression of TJ and AJ assembly proteins in response to Cryptosporidium infection.
Our current studies demonstrated decreased expression of specific TJ and AJ assembly proteins in response to CP infection that could contribute, at least partially, to disruption of barrier function and associated diarrhoea.
2 RESULTS
2.1 Time-course of CP invasion of Caco-2 monolayers
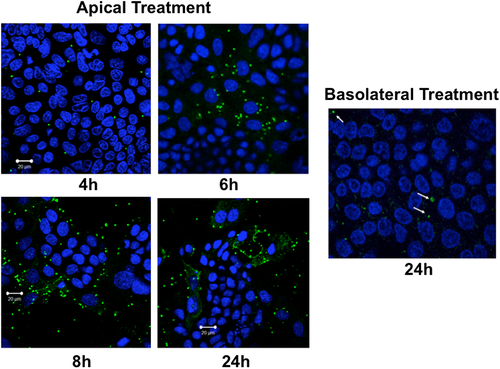
We measured time-course of cellular entry of excysted oocysts (sporozoits) of CP by direct immunofluorescence labelling of the parasite with Sporo-Glo reagent (Waterborne Inc., New Orleans, LA) following manufacturer's instructions. As shown in Figure 1, the parasite was stained intracellularly as early as 4 hr following treatment from apical surface of transwell-grown Caco-2 monolayers, with cellular entry gradually increasing till 24 hr. However, virtually there was no cellular entry of the parasite even after 24 hr when oocysts were added from basolateral side. For all the subsequent experiments in Caco-2 cells and mouse enteroid monolayers, parasite treatment was given from the apical surface for 24 hr.
2.2 CP infection increases paracellular permeability in Caco-2 monolayers
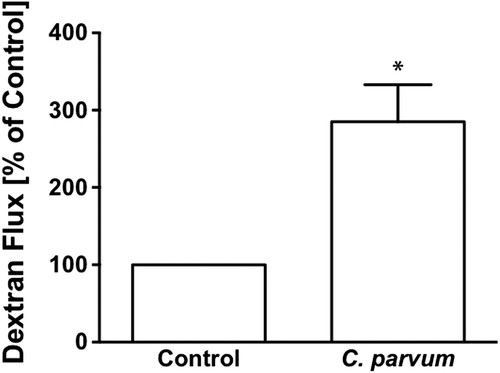
Measurement of apical to basolateral flux of FITC dextran was used to assess the CP infection effects on paracellular permeability in transwell-grown confluent Caco-2 monolayers. As shown in Figure 2, CP infection of Caco-2 monolayers from apical surface for 24 hr caused a threefold increase in FITC-dextran flux compared to untreated monolayers.
2.3 Differential effects of CP infection on the expression of TJ and AJ proteins
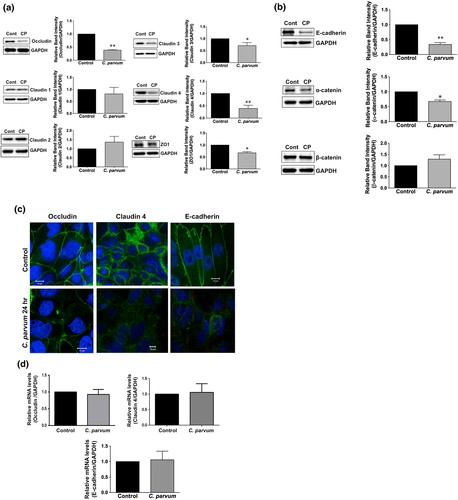
The intercellular AJC (France & Turner, 2017; Ivanov, Parkos, & Nusrat, 2010) regulates epithelial barrier function, permitting the passive entry of nutrients, ions, and water, while restricting pathogen access to underlying tissue compartments. The most apical TJ and its subjacent AJ constitute the AJC. Because TJ and AJ are common targets of most enteric pathogens, we sought to examine the effects of CP infection on the expression of key proteins that comprise TJ and AJ assembly. As shown in Figure 3a, CP infection of Caco-2 cells substantially decreased the protein levels of occludin and claudin 4, the TJ proteins. Although to a relatively lesser extent, the protein levels of claudin 3 and of the adaptor protein ZO1 were also significantly decreased. However, CP infection did not alter claudins 1 and 2. CP infection effects on AJ components are shown in Figure 3b. As evident, there was extensive downregulation of E-cadherin, a key component of AJ. Level of α-catenin, an AJ-associated protein, was significantly decreased, whereas there was no effect on β-catenin. We also measured the levels of occludin, claudin 4, and E-cadherin by immunofluorescence staining of the proteins in transwell-grown mouse enteroid-derived monolayers following CP infection for 24 hr. Similar to the results obtained in Caco-2 cells, CP infection substantially decreased immunostaining intensity of these proteins in mouse enteroids (Figure 3c). We next examined the mRNA levels of occludin, claudin 4, and E-cadherin, the TJ/AJ proteins that were substantially downregulated by CP infection in Caco-2 cells. As shown in Figure 3d, CP infection did not alter the mRNA levels of these proteins.
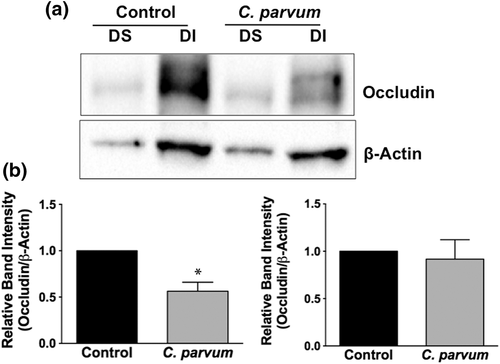
2.4 CP infection decreases mRNA and protein levels of occludin, claudin 4, and E-cadherin in mouse ileum and jejunum
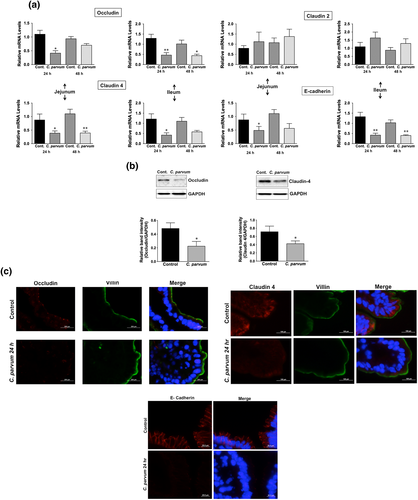
We next utilised in vivo model of CP infection in C57BL/6 mice to examine the effects on the expression of the key TJ and AJ proteins that were maximally downregulated in Caco-2 cells in response to infection by the parasite. Mice were infected with 1 × 107 oocysts/mouse by oral gavage for 24 and 48 hr. Unlike in Caco-2 cells, mRNA levels of occludin, claudin-4, and E-cadherin were significantly decreased in the mouse ileum and jejunum (Figure 4a) whereas there was no significant change in claudin 2 mRNA. We also measured protein levels of occludin, claudin 4, and E-cadherin by immunoblotting and/or immunofluorescence staining. CP infection decreased occludin and claudin 4 protein levels (Figure 4b) and immunostaining of occludin, claudin 4, and E-cadherin (Figure 4c) in immunofluorescence studies.
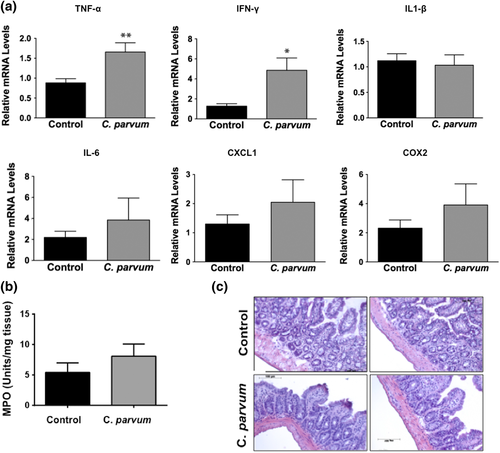
2.5 Effects of CP infection on cytokine levels and myeloperoxidase (MPO) activity in mouse ileum
In order to assess whether CP-induced alterations in the expression of TJ/AJ proteins at 24-hr post-infection were secondary to mucosal inflammation, we measured mRNA levels of key cytokines and MPO activity in ileal mucosa. As shown in Figure 5a, mRNA levels of TNF-α and IFN-γ were significantly increased whereas there were no significant effects on mRNA levels of IL-1β, IL-6, CXCL1, and COX2 in response to CP infection for 24 hr. Further, there was no effect of CP infection on MPO activity (Figure 5b), a measure of mucosal infiltration of neutrophils and marker of inflammation, in the mouse ileal mucosa. Haematoxylin and eosin (H&E) staining (Figure 5c) of infected versus control ileal mucosa also did not show marked changes in cellular morphology in response to CP infection. Further, although previous studies have shown decreased barrier function in response to TNF-α and IFN-γ (Al-Sadi, Guo, Ye, & Ma, 2013; Capaldo & Nusrat, 2009), these changes did not involve downregulation of expression of TJ/AJ proteins. Overall, our results could suggest that decreased expression of specific AJC proteins in mouse ileum in response to CP infection for 24 hr was not primarily due to inflammation.
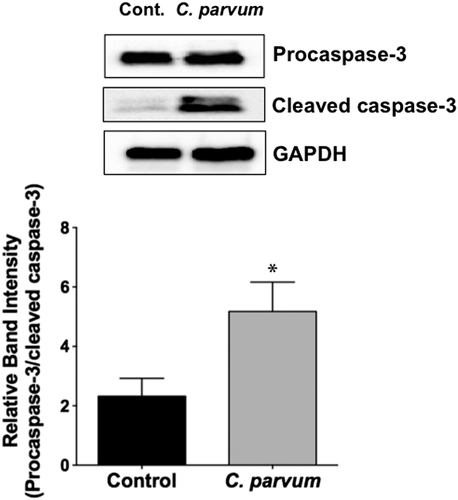
2.6 CP infection induces apoptosis in mouse ileum
Previous studies have shown CP induction of apoptosis of intestinal epithelial cells that was attributed to altered barrier function (Foster, Stauffer, Stone, & Gookin, 2012; Laurent & Lacroix-Lamande, 2017; McCole, Eckmann, Laurent, & Kagnoff, 2000). We examined the occurrence of apoptosis in the ileal mucosa in response to CP infection of mice via measurement of the levels of cleaved caspase-3. As shown in Figure 6, CP infection for 24 hr significantly increased the level of cleaved caspase-3 in the ileal mucosa, suggesting occurrence of apoptosis.
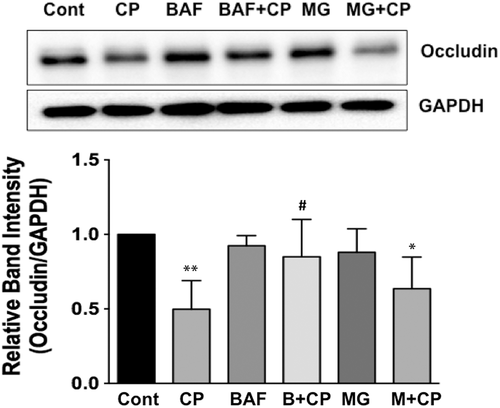
2.7 Bafilomycin A alleviates CP-induced downregulation of occludin in Caco-2 cells
Because CP infection substantially decreased occludin protein levels in Caco-2 cells but had no effects on occludin mRNA, we examined the role of posttranslational mechanisms, such as protein degradation pathways, in mediating CP-induced downregulation of occludin protein in Caco-2 cells. Ubiquitin-proteosomal system and lysosomal degradation are two cornerstones of cellular catabolism involved in intracellular protein degradation under normal physiology or in a broad array of pathological states (Korolchuk, Menzies, & Rubinsztein, 2010). Therefore, we utilised pharmacological inhibitors of these pathways to examine if CP infection triggered any of these pathways to degrade cellular occludin. Our results showed that bafilomycin-A, an inhibitor of lysosomal proton pump, partially abrogated CP-induced downregulation of occludin expression (Figure 7), whereas MG132, a proteosomal inhibitor, had no effect. These results suggest that CP induction of specific protein degradation pathways, at least partially, could account for its effects in downregulating occludin expression.
2.8 CP infection decreases low molecular weight (MW) form of Triton X 100-insoluble occludin
Occludin has been shown to partition into both Triton X-100-soluble and Triton X-100-insoluble fractions (Andreeva, Krause, Muller, Blasig, & Utepbergenov, 2001; Nusrat et al., 2001). Because of different levels of phosphorylation, occludin exhibits an MW in the range of 65–80 kDa on Western blot (Andreeva et al., 2001; Farshori & Kachar, 1999). A high-MW form of occludin, reflecting enhanced phosphorylation, appears only in the Triton X-100-insoluble fraction. The decreased expression of occludin in CP-infected cells reported above (Figure 3a) prompted us to examine whether infection also alters the distribution of the protein in the Triton X-100-soluble and Triton X-100-insoluble fractions. As shown in Figure 8, occludin showed a strong band of about 65 kDa [low-MW band] and a relatively weaker band of ~78 kDa (high-MW band representing hyperphosphorylated form) in the detergent-insoluble fraction of uninfected Caco-2 cells. Densitometric analysis showed 50–60% decrease in the levels of the low-MW form of occludin in the detergent-insoluble fraction in the CP infected cells compared to those in uninfected controls, whereas the level of the high-MW phosphorylated form was not significantly altered (Figure 8). Occludin level in the detergent-soluble fraction that showed a single band at 65 kDa was much lower, and CP infection did not seem to have any significant effect on occludin level in this fraction.
3 DISCUSSION
The objective of this study was to examine the mechanisms underlying disruption of intestinal epithelial barrier function in response to infection by CP. Infection by this parasite in immunocompetent hosts may cause acute, self-limiting, or recurrent diarrhoea, whereas in immunocompromised hosts, it may lead to fulminant diarrhoea, extra-intestinal manifestations, and death (Checkley et al., 2015; Ward, 2017). However, the molecular and cellular basis of Cryptosporidium infection-induced diarrhoea is poorly understood. Persistent infectious diarrhoeal diseases commonly affect mucosal barrier that separate the luminal contents from the body's internal milieu. This barrier, the first line of defence against external pathogens, is formed by a monolayer of intestinal epithelial cells and the substances that they secrete. This selectively permeable barrier prohibits passage of luminal microorganisms and their toxins while permitting flux of water, ions, and solutes, including nutrients. The epithelium maintains its selective barrier function through the formation of complex protein–protein networks, collectively known as AJC (Matter & Balda, 2003) that mechanically link adjacent cells and seal the intercellular space. The TJs and AJs are key components of AJC comprising transmembrane proteins that interact extracellularly with adjacent cells and intracellularly with adaptor proteins that link to the cytoskeleton (Groschwitz & Hogan, 2009). TJs seal paracellular gaps between adjacent epithelial cells (Tsukita, Furuse, & Itoh, 2001), whereas AJs are responsible for cell–cell contacts (Yap, Brieher, & Gumbiner, 1997). The TJ barrier exhibits both size and charge selectivity with two distinct routes across an intact epithelial monolayer, termed the pore and leak pathways (France & Turner, 2017). The pore pathway refers to a high-capacity, size-selective, and charge-selective route, whereas the leak pathway is a low-capacity pathway that has more limited selectivity. Both TJ and AJ assemblies are under intricate homeostatic regulation under normal physiology and are known to be dysregulated in various disease states (France & Turner, 2017).
Several diarrhoeal pathogens have been shown to disrupt AJCs in in vitro and in vivo models of infection (Groschwitz & Hogan, 2009; Nusrat et al., 2001; Vogelmann et al., 2004). Although limited studies demonstrated disrupted barrier function in cryptosporidiosis (Di Genova & Tonelli, 2016; Roche et al., 2000), very little is known about the alterations in the expression and/or assembly of TJ and AJ proteins and the underlying mechanisms. Because CP is known to infect and invade the cells of small intestinal epithelium, we used post-confluent Caco2 cell monolayers, morphologically and functionally mimicking the small intestinal mucosal barrier (Hidalgo, Raub, & Borchardt, 1989; Pignata, Maggini, Zarrilli, Rea, & Acquaviva, 1994), to examine the effects of infection by the parasite.
In order to gain molecular insights into CP-induced increase in FITC-dextran flux, indicating increased paracellular permeability or disrupted barrier function, we analysed the effects of infection on AJC components. We were able to show, for the first time, extensive downregulation of expression of the TJ proteins occludin and claudin 4, and of E-cadherin, a key component of AJ following CP infection. Claudin 3 was also significantly decreased, whereas claudins 1 and 2 were not altered. ZO1, the adaptor protein that links TJ assembly to the actin cytoskeleton and is also known to regulate leak pathway permeability (France & Turner, 2017) was also significantly decreased in response to CP infection. Similar to our observations in Caco-2 cells, occludin, claudin 4, and E-cadherin were extensively downregulated in CP infected mouse enteroid-derived monolayers and in the ileal and jejunal mucosa of mice administered CP for 24 and 48 hr. It is important to note that in Caco-2 cells parasite infection had no effects on the mRNA levels of occludin, claudin 4, and E-cadherin, whereas both their mRNA and protein levels were decreased in vivo in mouse jejunum and ileum. This could signify differential regulatory mechanisms in different mammalian species or could be due to the fact that multiple factors come into play in the compositionally complex in vivo environment of the native intestine. In either case, however, it appears that posttranslational mechanisms, at least partially, are important in CP infection-induced modulation of specific AJC protein expression. Indeed, our studies in Caco-2 cells further showed that bafilomycin A, an inhibitor of lysosomal proton pump, partially blocked the effects of CP infection on occludin suggesting the role of parasite-induced protein degradation pathways in reducing occludin levels. Additional studies are needed to determine the relative contribution of lysosomal pathways of protein degradation and/or other posttranslational mechanisms employed by CP in downregulating the expression of occludin and/or other TJ/AJ proteins.
The role of occludin in regulating TJ integrity has been suggested from studies showing increased TER (improved barrier function) following overexpression of occludin (Balda et al., 1996). Occludin has also been shown to regulate paracellular pore and leak pathways via its effects on claudins (France & Turner, 2017). The role of occludin in barrier regulation is, nevertheless, controversial because two studies have reported normal intestinal barrier function in unstressed occludin-deficient mice (Saitou et al., 2000; Schulzke et al., 2005). It appears from various studies that occludin could be a regulator of TJs, rather than an essential component, an interpretation that could explain the absence of intestinal barrier defects in unstressed occludin-deficient mice (France & Turner, 2017). Previous studies have also reported that levels of high- and low-MW occludin differing in phosphorylation levels could change in response to infection by enteric pathogens (Beau, Cotte-Laffitte, Amsellem, & Servin, 2007; Fiorentino, Levine, Sztein, & Fasano, 2014). However, the levels of high-MW form of the occludin in the detergent-insoluble fraction representing hyperphosphorylated occludin appeared to be unaltered in response to CP infection.
Claudins, critical components of TJ assembly, comprise a family of at least 24 proteins, whose differential expression and properties are believed to determine the tissue-specific variations in electrical resistance and paracellular permeability of epithelia (Gunzel & Yu, 2013). Claudin proteins are fundamental components of TJ strands. The consensus view is that the structure of the TJ, which forms the barrier, comprises barrier-enhancing claudins (such as claudin 4) that occlude the intercellular space and pore-forming claudins (such as claudin 2) that form channels supporting solute flow (France & Turner, 2017). Further, experimental evidence has shown that overexpression of claudin 4 increases whereas that of claudin 2 decreases TER in cultured epithelial cell monolayers. Conversely, increased claudin 2 and/or decreased claudin 4 have been reported to be associated with barrier disruption in response to inflammation and infection (Guttman & Finlay, 2009; Landy et al., 2016; Zhang, Wu, Xia, & Sun, 2013). In our studies, both in vitro and in vivo, CP infection substantially decreased claudin 4 but showed no effects on claudin 2. Earlier studies have shown that claudin 4 expression reduces paracellular permeability only when claudin 2 is coexpressed but not in monolayers lacking claudin 2 expression, suggesting that barrier-enhancing claudins regulate paracellular permeability via antagonising the function of pore-forming claudins (France & Turner, 2017). Therefore, although there was no effect on claudin 2 expression, CP infection increased paracellular permeability presumably because the substantially reduced level of claudin 4 could not counteract the pore-forming function of basal levels of claudin 2.
Components of the AJ provide a foundation for cell–cell interactions and are essential for TJ assembly. The major component of the epithelial AJ is E-cadherin, a single-spanning transmembrane protein capable of homotypic cell–cell interactions. E-cadherin is used as a receptor for adhesion and/or internalisation by several microorganisms, allowing microbial persistence in the host, avoidance of mechanical clearance, and increased pathogenesis (Costa, Leite, Seruca, & Figueiredo, 2013). Downregulation of E-cadherin has been shown in response to infections with enteropathogenic bacteria and in the inflamed mucosa in Crohn's disease (Kucharzik, Walsh, Chen, Parkos, & Nusrat, 2001; Schneider et al., 2010). Decreased expression of E-cadherin observed in our studies in response to CP infection could contribute to barrier dysfunction and increased permeability. However, it remains to be determined whether impaired cell–cell interactions secondary to decreased E-cadherin facilitates parasite entry or is an after effect of cellular invasion.
Earlier studies have shown production of chemoattractants in response to CP infection leading to immune cell infiltration to the site of infection (Laurent & Lacroix-Lamande, 2017). These studies, however, utilised longer time of infection (7–14 days) to examine the chronic effects, and some of the studies showing mucosal damage and neutrophil infiltration were in immunocompromised mice. Our current studies aimed at examining the early effects (24–48 hr) of cryptosporidiosis on TJ and AJ proteins in immunocompetent mice did not show significant mucosal damage or immune cell recruitment to the mucosa during this early infection period. Thus, CP infection seemed to reduce the expression of TJ/AJ proteins before initiation of any significant inflammatory response.
Various earlier studies have also shown induction of host cell apoptosis in response to CP infection and implicated its relevance to epithelial barrier integrity (Foster et al., 2012; Laurent & Lacroix-Lamande, 2017; McCole et al., 2000). Whether the occurrence of apoptosis in cryptosporidiosis benefits the parasite or the host, however, is not clear (Sasahara et al., 2003). CP has been shown to induce moderate apoptosis in vitro early after infection that was followed by induction of anti-apoptotic mechanisms via activation of NF-κB. These studies implicated that moderate levels of epithelial cell apoptosis could limit the host inflammatory response detrimental to the survival of the parasite. On the other hand, shedding of infected epithelial cells by apoptosis may benefit the host, because it allows maintenance of epithelial barrier integrity (McCole et al., 2000). Further studies utilising caspase inhibitors will be needed to establish whether CP-induced downregulation of key TJ/AJ proteins observed in our studies is a mechanism of disruption of barrier function independent of induction of apoptosis.
In summary, our studies for the first time evaluated the molecular basis of disruption of epithelial barrier function in response to CP infection via detailed analysis of the expression of TJ and AJ proteins that primarily regulate paracellular permeability. It has been suggested that subset of claudins including claudin 4 regulate pore pathway whereas leak pathway permeability is regulated by occludin and ZO1 (France & Turner, 2017). Therefore, increased paracellular permeability in response to CP appears to signify alterations of both pore and leak pathways of permeability. Additional studies will be needed for elucidating the mechanisms underlying CP-induced downregulation of specific TJ and AJ assembly proteins and the responses of host epithelial cells and their impact on barrier function. Further, effects of CP infection on the proteins of desmosomes, another AJC component, remain to be investigated.
4 EXPERIMENTAL PROCEDURES
4.1 Chemicals and antibodies
RNeasy kits for RNA extraction were obtained from Qiagen (Valencia, CA), and real-time quantitative RT-PCR kits were obtained from Stratagene (La Jolla, CA). Antibodies for occludin, claudins 1, 2, 3, 4, ZO1, α and β catenin were from Invitrogen (Carlsbad, CA), and E-cadherin and caspase-3 were from Cell Signaling (Danvers, MA). Bafilomycin A and MG-132 were from Calbiochem (Burlington, MA) and FITC-dextran was obtained from Sigma-Aldrich (St. Louis, MO).
4.2 Preparation of Cryptosporidium oocysts
Two major species of the parasite, namely, CP and Cryptosporidium hominis, cause disease in humans (Ward, 2017). We have utilised CP in our studies as this species, but not Cryptosporidium hominis, infects both human and mice. Suspensions of live oocysts of CP in PBS were obtained from Waterborne Inc. (New Orleans, LA). For in vitro and ex vivo studies, oocysts were excysted by incubating four volumes of the suspension with one volume of 20% bleach solution for 10 min at room temperature. The pellet obtained by centrifugation was washed several times with HBSS to remove traces of bleach and finally resuspended in MEM cell culture media (Invitrogen) containing 0.2% taurocholate to release infectious sporozoites for treatments.
4.3 Cell culture
Caco-2 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and grown at 37 °C in a 5% CO2-95% air environment. Cells were grown in MEM supplemented with 50 U/ml penicillin and 50 μg/ml gentamicin, and with 20% fetal bovine serum. For in vitro studies, cells were seeded on 6-, 12-, or 24-well plastic supports or 12-well transwell inserts and grown to confluency (12 days post-plating) before infecting with Cryptosporidium oocysts.
4.4 Mice
C57BL/6 mice (4–6 weeks old) obtained from Jackson Laboratories were maintained in the animal care facility of the Jesse Brown Veterans Affairs Medical Center (JBVAMC), Chicago. In vivo studies performed in these mice were approved by the Animal Care Committee of the University of Illinois at Chicago and JBVAMC. Mice were divided into four groups, two groups for infecting with CP oocysts for 24 and 48 hr each and the other two groups being the respective controls. On Day 1, each mouse in the 48-hr treatment group was gavaged with 1 × 107 intact (unexcysted) oocysts in 200-μl sterile PBS, whereas mice in the respective 48-hr control group received 200 μl of the vehicle. On Day 2, mice in the two treatment groups (24 and 48 hr) were gavaged with 1 × 107 intact oocysts and the control groups with the vehicle. Mice were euthanised on the third day post-CP infection. Intestines were resected, and mucosa was scraped for RNA extraction and lysate preparation. Sections (5-8 μm) of the different regions of intestine (ileum and jejunum) were immediately snap-frozen in optimal cutting temperature (OCT) embedding medium (Tissue-Tek OCT compound, Sakura) for immunofluorescence studies.
4.5 Preparation of crypt-derived mouse enteroids and enteroid-derived monolayers
Small intestinal crypt-derived enteroids from C57BL/6 mice were generated and cultured according to previously described protocol (Sato et al., 2011) with minor modifications. Briefly, small intestine was dissected and opened longitudinally and cut into small (~1 cm) pieces after cleaning with ice-cold PBS (penicillin, 100 I.U./ml/streptomycin, 100 μg/ml). The tissues were rocked in PBS with 2 mM EDTA for 30 min at 4 °C and then switched to PBS with 54.9 mM d-sorbitol and 43.4 mM sucrose. The tissues were then vortexed for 1–2 min and filtered through a 70-μm sterile cell strainer. The crypts were collected by centrifugation at 150 g for 10 min at 4 °C. Approximately 500 crypts were suspended in 50-μl growth factor reduced phenol-free Matrigel (BD Biosciences, San Jose, CA). Next, a 50-μl droplet of Matrigel/crypt mix was placed in the centre of the well of a 12-well plate. After 30 min of polymerisation, 650 μl of Mini gut medium (advanced DMEM/F12 supplemented with HEPES, L-glutamine, N2 and B27) was added to the culture, along with R-Spondin, Noggin, and EGF. The medium was changed every 2–3 days. For passaging, enteroids were removed from Matrigel and broken up mechanically by passing through a syringe and needle (27G, BD Biosciences), then transferred to fresh Matrigel. The passage was performed every 7–10 days with a 1:4 split ratio. For preparing enteroid-derived monolayers, enteroids were mechanically disrupted with a p200 pipette and cultured as monolayers on Matrigels for 96 hr as described previously (Sato et al., 2011) with minor modifications.
4.6 Determination of paracellular permeability
Paracellular permeability of the Caco-2 monolayers grown on 12-well transwell inserts (untreated or apically treated with 0.5 × 106 oocysts/well for 24 hr) was measured as apical to basolateral flux of FITC- Dextran (FD-4, M.W. 4 kDa, Sigma) as described previously (O'Hara, Feener, Fischer, & Buret, 2012).
4.7 Real-time PCR
RNA was extracted from Caco-2 cells or homogenised mouse intestinal mucosal samples using Qiagen RNeasy kits. RNA was reverse transcribed and amplified using a Brilliant SYBR Green qRT-PCR Master Mix kit (Stratagene). Human and mouse TJ and AJ protein genes were amplified with gene-specific primers utilising human and mouse GAPDH, respectively, as internal controls, as previously described by us (Kumar et al., 2016). Primer sequences of the genes used in this study are presented in Table 1.
| Gene | Primer sequence (5′-3′) |
|---|---|
| Human occludin |
F: CCCCATCTGACTATGTGGAAAGA R: AAAACCGCTTGTCATTCACTTTG |
| Human claudin 4 |
F: GGCGTGGTGTTCCTGTTG R: AGCGGATTGTAGAAGTCTTGG |
| Human E-cadherin |
F: ATTTTTCCCTCGACACCCGAT R: TCCCAGGCGTAGACCAAGA |
| Mouse occludin |
F: CCTCCAATGGCAAAGTGAAT R: CTCCCCACCTGTCGTGTAGT |
| Mouse claudin 2 |
F: TTAGCCCTGACCGAGAAAGA R: AAAGGACCTCTCTGGTGCTG |
| Mouse claudin 4 |
F: AGCAAACGTCCACTGTCCTT R: AATCCACCTCCACCCTTCTT |
| Mouse E-cadherin |
F: CAGCCTTCTTTTCGGAAGACT R: GGTAGACAGCTCCCTATGACTG |
| Mouse IL-1β |
F: GCAACTGTTCCTGAACTCAACT R: ATCTTTTGGGGTCCGTCAACT |
| Mouse IFN-γ |
F: ATGAACGCTACACACTGCATC R: CCATCCTTTTGCCAGTTCCTC |
| Mouse CXCL1 |
F: AAAGATGCTAAAAGGTGTCCCCA R: AATTGTATAGTGTTGTCAGAAGCCA |
| Mouse GAPDH |
F: TGTGTCCGTCGTGGATCTGA R: CCTGCTTCACCACCTCTTGAT |
- Note. F = forward primer; R = reverse primer.
4.8 Immunoblotting
Whole cell lysates (Caco-2) and mouse intestinal mucosal lysates were prepared as described previously by us (Kumar et al., 2016; Singh et al., 2014). Protein concentrations in the lysates were measured by the Bradford reagent (Bio-Rad, Hercules, CA). Protein extracts (50 μg of total proteins) were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Levels of TJ/AJ proteins were immunodetected using specific antibodies and visualised by enhanced chemiluminescence reagents (ECL Plus; GE Healthcare, London, UK).
4.9 Immunofluorescence staining in mouse intestinal tissues
Sections of small intestinal tissues from different mice groups were snap frozen in OCT embedding medium. For immunostaining, 5-μm frozen sections were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature (RT). Fixed sections were washed in PBS and permeabilised with 0.3% Nonidet P-40 for 5 min; sections were then encircled with water repellant pap pen (fisher) and blocked with 5% normal goat serum (NGS) for 1 hr at RT. Tissues were then incubated with the appropriate primary antibodies at a 1:100 ratio including anti-rabbit occludin (Invitrogen), E-cadherin (Cell Signaling), claudin 4 (Invitrogen), and anti-mouse villin (Invitrogen) in 1% NGS PBS for 2 hr at RT. After washing several times in PBS, sections were incubated with Alexa fluor-594-conjugated goat anti-rabbit IgG and Alexa Fluor 488-conjugated goat anti-mouse IgG (Invitrogen) at a ratio of 1:100 in 1% NGS for 1 hr at RT. Sections were then washed in PBS and mounted under cover slips using Slowfade Gold antifade with DAPI reagent (Invitrogen). Sections were stored at −80 °C until imaged. Images were acquired using Olympus BX51/1X70 fluorescence microscope equipped with X100 oil immersion objective.
4.10 H&E staining and determination of MPO activity
Cryopreserved mouse ileal tissues from each group were sectioned (5 μm) using a cryostat microtome. The sections were washed in PBS to remove excess OCT and fixed in 4% paraformaldehyde for 20 min. These tissues were washed again in PBS and stained with H&E stain kit from ScyTek Laboratories Inc. (Logan, UT) per the manufactures protocol. The slides were then mounted with permount (Fisher scientific, Waltham, MA) and stored at room temperature until use. Images from each group were acquired with Zeiss Axiocam acc1 microscope (Oberkochen, Germany) at 20× magnifications. MPO activity in the ileal mucosa was determined by the method of Krawisz, Sharon, and Stenson (1984) with minor modifications. MPO activity was normalised to the amount of protein in the supernatant as measured by Bradford method and calculated as units per gram protein and expressed relative to control (considered as 100).
4.11 Immunoblotting of occludin in Triton X-100 soluble and insoluble fractions
Triton X-100 soluble and insoluble fractions were prepared as described previously (Fiorentino et al., 2014). Briefly, monolayers were harvested on ice in lysis buffer (1% Triton X-100, 100 mM NaCl, 10 mM HEPES, 2 mM EDTA, 4 mM Na3VO4, 40 mM NaF and a protease inhibitor cocktail [Complete Mini, Roche Molecular Biochemicals, Mannheim, Germany] and phosphatase inhibitors [Sigma]). Lysates were rotated at 4 °C, 30 min, centrifuged (14,000 g for 30 min at 4 °C) and the supernatant representing the Triton X-soluble fraction was collected. The remaining pellet was resuspended in lysis buffer supplemented with 1% SDS and sonicated and centrifuged (14,000 g for 5 min at 4 °C). The supernatant, representing the Triton X-insoluble fraction, was collected. Samples were used immediately or stored at −80 °C. Protein concentration was quantified by the Bradford method. Proteins in the samples were separated on SDS polyacrylamide gel and probed with anti-occludin antibody in immunoblotting.
4.12 Statistical analyses
Data are presented as means ± SEM of three to four independent experiments. Differences between controls versus treated groups were analysed using one-way analysis of variance with Tukey's test or Student's t test. Differences were considered significant at p ≤ .05.
ACKNOWLEDGMENTS
These studies were supported by NIH (NIDDK; DK-54016, DK-81858, DK-92441 [PKD], DK-109709 [WAA], DK-105118 [JS]); Senior Career Research Scientist Award (PKD) and Career Research Scientist Award (WAA) from the Department of Veteran Affairs; VA merit review grants (BX 002011 [PKD] and BX 000152 [WAA]; and Bill and Melinda Gates Foundation (OPP1058288 [AB]).
CONFLICT OF INTEREST
All authors declare that there is no conflict of interests.




