A novel kinase function of a nucleoside-diphosphate-kinase homologue in Porphyromonas gingivalis is critical in subversion of host cell apoptosis by targeting heat-shock protein 27
Abstract
We have previously shown that a homologue of a conserved nucleoside-diphosphate-kinase (Ndk) family of multifunctional enzymes and secreted molecule in Porphyromonas gingivalis can modulate select host molecular pathways including downregulation of reactive-oxygen-species generation to promote bacterial survival in human gingival epithelial cells (GECs). In this study, we describe a novel kinase function for bacterial effector, P. gingivalis-Ndk, in abrogating epithelial cell death by phosphorylating heat-shock protein 27 (HSP27) in GECs. Infection by P. gingivalis was recently suggested to increase phosphorylation of HSP27 in cancer-epithelial cells; however, the mechanism and biological significance of antiapoptotic phospho-HSP27 during infection has never been characterised. Interestingly, using glutathione S-transferase-rNdk pull-down analysed by mass spectrometry, we identified HSP27 in GECs as a strong binder of P. gingivalis-Ndk and further verified using confocal microscopy and ELISA. Therefore, we hypothesised P. gingivalis-Ndk can phosphorylate HSP27 for inhibition of apoptosis in GECs. We further employed P. gingivalis-Ndk protein constructs and an isogenic P. gingivalis-ndk-deficient-mutant strain for functional examination. P. gingivalis-infected GECs displayed significantly increased phospho-HSP27 compared with ndk-deficient-strain during 24 hr infection. Phospho-HSP27 was significantly increased by transfection of GFP-tagged-Ndk into uninfected-GECs, and in vitro phosphorylation assays revealed direct phosphorylation of HSP27 at serines 78 and 82 by P. gingivalis-Ndk. Depletion of HSP27 via siRNA significantly reversed resistance against staurosporine-mediated-apoptosis during infection. Transfection of recombinant P. gingivalis-Ndk protein into GECs substantially decreased staurosporine-induced-apoptosis. Finally, ndk-deficient-mutant strain was unable to inhibit staurosporine-induced Cytochrome C release/Caspase-9 activation. Thus, we show for the first time the phosphorylation of HSP27 by a bacterial effector—P. gingivalis-Ndk—and a novel function of Ndks that is directly involved in inhibition of host cell apoptosis and the subsequent bacterial survival.
1 INTRODUCTION
Nucleoside diphosphate kinases are highly conserved multifunctional enzymes expressed in both eukaryotes and prokaryotes that balance the pools of nucleoside triphosphates, such as ATP or GTP, by catalysing the transfer of orthophosphates (Chakrabarty, 1998; Ray & Mathews, 1992; Spooner & Yilmaz, 2012). Ndks form either homo-tetramers or -hexamers with phosphotransferase activity that function in a variety of cellular housekeeping functions such as DNA cleavage/repair, transcriptional regulation, cell proliferation, and apoptosis (Atanasova et al., 2016; Kumar et al., 2005; Yu, Rao, & Zhang, 2017). In addition to homeostatic functions, bacterial and human Ndk homologues have been implicated in the progression of a variety of different chronic diseases (e.g., cancer) and opportunistic infections (Atanasova & Yilmaz, 2014; Chakrabarty, 1998; Yu et al., 2017). Bacterial Ndks are cytoplasmic proteins that are also secreted, as is the case with pathogens such as Mycobacterium tuberculosis, Pseudomonas aeruginosa, and Porphyromonas gingivalis involved in chronic opportunistic infections (Spooner & Yilmaz, 2012; Sun et al., 2013). In the context of host–pathogen interaction specifically, these prokaryotic Ndk homologues play roles in immune evasion, inhibition of reactive-oxygen-species (ROS) through NADPH-oxidase complexes, and regulation of host apoptotic factors (Chakrabarty, 1998; Choi et al., 2013; Keith, Hynes, Sholdice, & Valvano, 2009; Sun et al., 2013; Yilmaz et al., 2008; Yu et al., 2017). Typically, bacterial Ndks regulate bacterial proteins through phosphorylation on histidine residues by transferring phosphates from active sites (ATP or GTP) on the enzyme to the histidine residue (Attwood & Wieland, 2015). In eukaryotes, however, serine, threonine, and tyrosine residues are considered the major targets for phosphorylation, whereas histidine phosphorylation is less defined (Adam & Hunter, 2017). Human Ndk (encoded by the nm23 gene) has previously been shown to extend outside of the standard histidine kinase function and phosphorylate on serine/threonine residues as well (Engel et al., 1995). Intriguingly, bacterial Ndks have not been shown to phosphorylate on any residues other than histidine; however, the significantly conserved homology of eukaryotic and prokaryotic Ndks could suggest shared kinase functions (Schneider et al., 2001). To date, this has never been investigated.
The cellular biology of Gram (−) opportunistic bacterium, P. gingivalis in the oral mucosal cells is complex. It has been shown that intracellular invasion of P. gingivalis does not induce apoptosis or necrosis and can render human primary gingival epithelial cells (GECs) resistant to cell death induced by proapoptotic molecules later in infection (Yao et al., 2010; Yilmaz et al., 2008; Yilmaz, Jungas, Verbeke, & Ojcius, 2004). P. gingivalis is a successful coloniser of the oral cavity and a major contributor to the aetiology of chronic severe periodontitis has recently been associated with other chronic conditions including the cancers of orodigestive tract (Atanasova & Yilmaz, 2014; Atanasova & Yilmaz, 2015; Olsen & Yilmaz, 2016).
Previous studies have investigated the biological importance of secreted Ndk as an effector for P. gingivalis, to intracellularly survive and replicate in human GECs (Spooner & Yilmaz, 2012; Yao et al., 2010; Yilmaz, 2008; Yilmaz et al., 2004; Yilmaz et al., 2008; Yilmaz, Watanabe, & Lamont, 2002). Recent studies have characterised P. gingivalis-Ndk as a putative virulence factor translocated extracellularly of the host epithelial cells in a time-dependent manner during infection (Choi et al., 2013; Spooner & Yilmaz, 2012; Yilmaz, 2008; Yilmaz et al., 2008). The extracellular release of P. gingivalis-Ndk from host epithelial cell does not occur as a result of compromised host membranes or cell death (Choi et al., 2013; Yilmaz et al., 2008) but rather occurs in a regulated mode using host trafficking machinery via Myosin-9 and leaving host cells through pannexin-1 hemi-channels (Atanasova et al., 2016). However, P. gingivalis-Ndk can also be found in the cytoplasm of the infected host cells (Atanasova et al., 2016; Choi et al., 2013).
Furthermore, P. gingivalis-Ndk is biologically active with similar GTP and ATP hydrolysis activity as other Ndk homologues in both higher organisms and bacterial species (Choi et al., 2013; Sun et al., 2013). The ATP hydrolytic activity of P. gingivalis-Ndk (~0.1 μM s−1; Choi et al., 2013; Yilmaz et al., 2008) is especially important in diminishing the danger signal extracellular ATP (eATP)/P2X7-receptor signalling in primary GECs that generates robust cellular ROS upstream of hypochlorous acid production, a potent eliminator of invading pathogens (Choi et al., 2013; Roberts et al., 2017; Roberts & Yilmaz, 2015; Yilmaz et al., 2008). Moreover, P. gingivalis-Ndk is also of importance in the modulation of P2X7-dependent inflammasome activation and the attenuation of secretion of pro-inflammatory cytokine IL-1β as well as pro-inflammatory danger signal HMGB1 in primary GECs (Johnson et al., 2015; Olsen & Yilmaz, 2016). However, despite the increasing key importance of Ndk in bacterial survival pathways in host cells, many studies, while observing phenotypic changes attributed to Ndk, do not study specific molecular Ndk-interactors potentially involved in the antiapoptotic pathways. In our previous studies, we demonstrated that P. gingivalis can inhibit intrinsic apoptosis and activates prosurvival phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT signalling in primary GECs. However, the bacterial factor(s) likely involved in this infection induced antiapoptotic phenotype of the host cells has never been characterised before (Lee et al., 2017; Yao et al., 2010; Yilmaz et al., 2004).
In this study, we investigate the putative interaction between P. gingivalis-Ndk and heat-shock protein 27 (HSP27) as identified by liquid chromatography-tandem mass spectrometry. HSP27 is a small mammalian cell phosphoprotein (Arrigo, 1998) that regulates apoptosis (Paul et al., 2002), lowers ROS levels through increased glutathione levels (Arrigo et al., 2005; Mehlen, Hickey, Weber, & Arrigo, 1997; Preville et al., 1999), and stabilises actin microfilaments (Guay et al., 1997) in response to oxidative stress or potent proapoptotic drugs such as staurosporine. Recently, there is increasing evidence that point to an important role for HSP27 in tumorigenesis and metastasis in a variety of cancers (Cornford et al., 2000; Deyhimi & Azmoudeh, 2012; Kapranos et al., 2002; Katsogiannou, Andrieu, & Rocchi, 2014; Vidyasagar, Wilson, & Djamali, 2012; Zimmermann et al., 2012); therefore making HSP27 a potential therapeutic target (Acunzo, Andrieu, Baylot, So, & Rocchi, 2014; Deyhimi & Azmoudeh, 2012; Leonardi et al., 2002). Moreover, a recent investigation has briefly demonstrated the increase of HSP27 in oral cancer epithelial cells infected with P. gingivalis and its importance in the activation of matrix metalloproteinase 9 (MMP9) in infected cells (Inaba et al., 2014). However, HSP27 has not yet been investigated in the context of P. gingivalis colonisation or survival in the oral cavity; neither have the molecular mechanisms of HSP27 phosphorylation during infection been defined.
Our results demonstrate a novel kinase function of a bacterial Ndk, specifically P. gingivalis-Ndk, to directly bind and phosphorylate a host protein, HSP27, on specific serine residues. In addition, we illuminate the significance of the Ndk–HSP27 interaction in promoting primary GEC survival during the infection by inhibiting proapoptotic Cytochrome C release and Caspase-9 activation. Thus, we demonstrate for the first time the phosphorylation of HSP27 by a bacterial effector—P. gingivalis-Ndk—which confers an antiapoptotic phenotype to primary GECs. This suggests HSP27 is a critical molecule for P. gingivalis-induced suppression of host cell apoptosis that appears essential for the microorganism's prolonged intracellular survival in host. Therefore, the findings of this study may translate to identifying targeted strategies to disrupt the establishment of P. gingivalis and other chronic opportunistic pathogens employing Ndk for successful colonisation and persistence strategy in human mucosa.
2 RESULTS
2.1 Identification of HSP27 as a putative Ndk interactor by mass spectrometry
Nucleoside diphosphate kinase can work both directly or indirectly with molecules in the host cell to facilitate membrane trafficking, manipulate metabolic systems, and affect host survival signalling pathways (Atanasova et al., 2016; Atanasova & Yilmaz, 2014; Atanasova & Yilmaz, 2015; Chakrabarty, 1998; Choi et al., 2013; Spooner & Yilmaz, 2012). As a secreted virulence factor from P. gingivalis, Ndk can be present in the host cytoplasm (Atanasova et al., 2016; Choi et al., 2013) and carries both ATPase (Choi et al., 2013; Yilmaz et al., 2008) and GTPase functions (Figure S1). The intracellular function of P. gingivalis-Ndk, however, has not been characterised. Therefore, we examined possible host cytoplasmic interactors from infected primary GEC lysates with recombinant P. gingivalis-Ndk using a glutathione S-transferase (GST)-pull down assay and performed a liquid-chromatography-tandem-mass-spectrometry analysis of the proteins coimmunoprecipitated with P. gingivalis-Ndk. This analysis revealed heat-shock protein beta-1/HSP27 (IPI00025512.2) as a strong binder to Ndk, with over 95% specificity and over 99.9% threshold in the detection, at a setting of five or more matching peptides (Keller, Nesvizhskii, Kolker, & Aebersold, 2002; Nesvizhskii, Keller, Kolker, & Aebersold, 2003; Figure S2). HSP27 is an ATP-independent oligomeric phosphoprotein that functions as a negative regulator of intrinsic apoptosis and oxidative stress (Paul et al., 2002; Rogalla et al., 1999) and thereby promoting cell survival. Therefore, the interaction between Ndk and HSP27 may be critical for P. gingivalis in promoting resistance against intrinsic apoptosis in primary GECs (which has been previously described), which is important for the intracellular life of P. gingivalis in the oral mucosa.
2.2 P. gingivalis-Ndk binds directly to HSP27 and increases phosphorylation on activating serine residues 78 and 82
We confirmed the mass spectrometry results by detecting HSP27 specifically in the eluted proteins from the GST-pull down assay by Western blotting (Figure 1a). Further confirmation of the Ndk/HSP27 interaction was assessed using confocal microscopy that demonstrated a high colocalisation rate (Manders coefficient of 0.92) of transfected GFP-Ndk with native HSP27 in primary GECs (Figure 1b). Moreover, an enzyme-linked immunosorbent assay (ELISA) binding assay using purified recombinant (rNdk) and HSP27 showed a concentration-dependent increase in HSP27 binding to Ndk (Figure 1c).
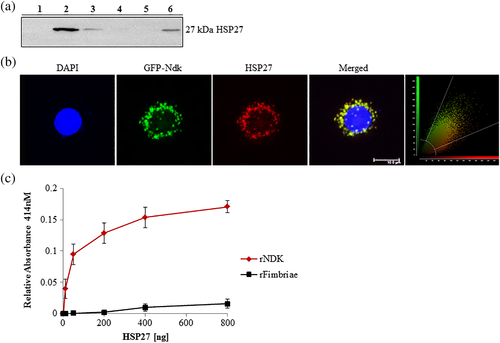
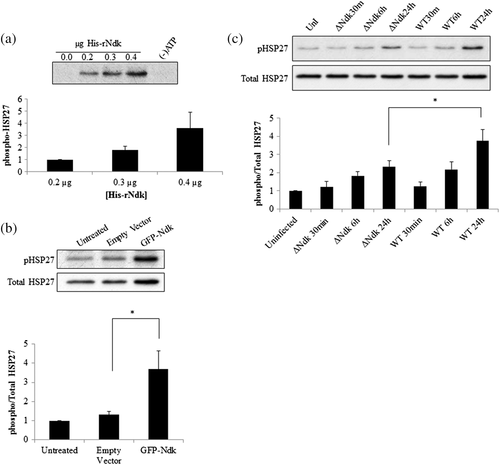
We then examined the biological significance of the Ndk/HSP27 interaction by assessing the phosphorylation state of HSP27 on activating residues serine 78 and 82 (Gusev, Bogatcheva, & Marston, 2002; Lambert, Charette, Bernier, Guimond, & Landry, 1999) using an in vitro phosphorylation assay. This in vitro reaction demonstrates P. gingivalis-Ndk can directly phosphorylate HSP27 in the presence of ATP and affects HSP27 phosphorylation in a concentration-dependent manner (Figure 2a). Furthermore, overexpression of GFP-Ndk in primary GECs significantly increased phospho-HSP27 as compared with a control empty vector (Figure 2b). In the presence of wild-type P. gingivalis infection or its isogenic ndk-deficient mutant strain infection, Western blot analysis revealed a gradual significant increase in phospho-HSP27 in primary GECs infected with wild-type P. gingivalis as compared with the ndk-deficient P. gingivalis in 24 hr (Figure 2c). Slightly increased levels of phosphoHSP27 are still present in ndk-deficient-infected GECs, which is most likely due to the host responding to microbial presence, because HSP27 is a well-characterised cellular stress response protein (Rogalla et al., 1999; Takayama, Reed, & Homma, 2003; REFs). Taken together, these data show the specific ability of P. gingivalis-Ndk to increase HSP27 phosphorylation on activating residues.
2.3 P. gingivalis requires HSP27 to block staurosporine-induced cell death via Ndk in primary GECs
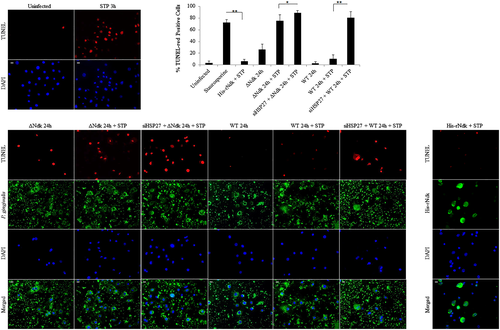
We have previously shown the ability of wild-type P. gingivalis to inhibit intrinsic apoptosis mediated by a potent proapoptotic agent, staurosporine in primary GECs through the time-dependent modulation of Bcl-2 family proteins and the activation of PI3K/AKT prosurvival pathway (Yao et al., 2010; Yilmaz et al., 2004). However, the importance of HSP27 as an antiapoptotic molecule during the infection of GECs has not been defined. Therefore, we first assess the potential role of HSP27 in protecting primary GECs against the staurosporine-induced cell death in the presence of infection. We visualised and quantified apoptotic cells using fluorescent microscopy analysis of a TUNEL-TMR red assay that stains nicked DNA. As observed before, wild-type P. gingivalis infection protected against staurosporine-induced cell death, whereas the ndk-deficient infection failed to show any significant resistance against apoptosis as compared with staurosporine treatment only (Figure 3). On the other hand, down-regulation of HSP27 by siRNA (Figure S3) in the presence of staurosporine, however, significantly increased cell death (abolished the protection against apoptosis) during wild-type infection and further exacerbated cell death in the presence of ndk-deficient P. gingivalis infection (Figure 3a,b). Transfection of the His-tagged recombinant P. gingivalis-Ndk protein into staurosporine-treated primary GECs, however, reversed the effects of staurosporine and thus reduced cell death. Taken together, these results show the key importance of HSP27 as a protective molecule against cell death in primary GECs during infection and P. gingivalis-Ndk plays a critical role for this function of HSP27.
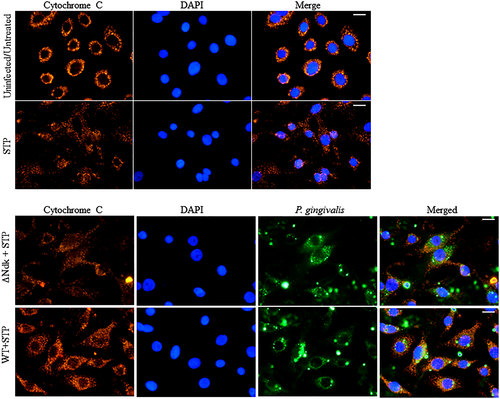
2.4 P. gingivalis-Ndk is vital for reduced Cytochrome C release and Caspase-9 activation during staurosporine-induced cell death
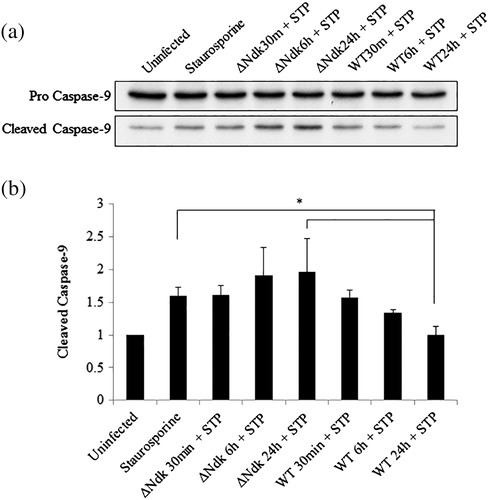
It is well established that HSP27 protects the cell from programmed cell death via the intrinsic apoptosis pathway specifically acting as a negative regulator of Cytochrome C release (Paul et al., 2002). Furthermore, we have previously reported the ability of wild-type P. gingivalis to inhibit staurosporine-induced cell death by abrogating Cytochrome C release and inhibition of mitochondrial membrane depolarisation in primary GECs (Yilmaz et al., 2004). However, the role of P. gingivalis effector Ndk in the modulation of intrinsic apoptosis has never been investigated previously. Therefore, we analysed the ability of ndk-deficient or wild-type P. gingivalis infection to abrogate staurosporine-induced Cytochrome C release using immunofluorescence microscopy. Our results show that ndk-deficient P. gingivalis is unable to maintain Cytochrome C sequestration inside the mitochondria whereas the wild-type P. gingivalis infected cells displayed intact mitochondria and thus inhibited staurosporine-induced Cytochrome C release (Figure 4). Cytochrome C release activates procaspase-9 that leads to the formation of an apoptosome (Chinnaiyan, 1999; Elmore, 2007). Therefore, we examined the active cleaved form of Caspase-9 in primary GECs infected with wild-type or ndk-deficient P. gingivalis. Western blot analyses reveal that ndk-deficient P. gingivalis infection significantly increases proapoptotic Caspase-9 activation over 24 hr as compared with wild-type (which is comparable to untreated; Figure 5). These suggest that inhibition of intrinsic apoptosis during P. gingivalis infection in GECs can occur in a stepwise fashion through its effector, Ndk. Ndk's induction of HSP27 leads to enhanced blockings of Cytochrome C release from mitochondria and Caspase-9 activation. HSP27, with an ability to interact with a large number of proteins in a sequential manner, has been established to tightly control/inhibit the intrinsic apoptotic pathway via direct binding to Cytochrome C (Paul et al., 2002; Takayama et al., 2003).
3 DISCUSSION
The regulation of phosphorylation has critical importance in determining the function of a protein and has significant importance in enabling the host cell to quickly respond to stimuli. The function and specific protein interaction of HSP27 is regulated by its phosphorylation state that promotes the formation of small oligomers that can interact with target molecules such as proapoptotic Cytochrome C (Katsogiannou et al., 2014; Paul et al., 2002). This leads to inhibition of apoptosis. The unphosphorylated state of HSP27 forms larger oligomers but can also modulate key cellular functions (Hayes, Napoli, Mazurkie, Stafford, & Graceffa, 2009; Lentze & Narberhaus, 2004). For example, in the unphosphorylated state, HSP27 can cap actin filaments, thus inhibiting polymerisation, (Benndorf et al., 1994) whereas phosphorylated HSP27 promotes polymerisation (Guay et al., 1997; Huot, Houle, Spitz, & Landry, 1996). Another example of dual roles regulated by phosphorylation can be seen in the modulation of glutathione levels by phosphorylated HSP27 (Arrigo et al., 2005; Preville et al., 1999; Rogalla et al., 1999).
P. gingivalis, a facultatively intracellular bacterium, can replicate and survive in GECs without being overtly detrimental to the host and modulate the epithelial cell response as an effective strategy for host immune evasion and persistence (Yilmaz et al., 2008). Our findings summarised in Figure 6, show the novel ability of P. gingivalis-Ndk to bind and directly phosphorylate HSP27 on serine residues 78 and 82. The in vitro phosphorylation assay and use of recombinant Ndk constructs confirm that the observed increase in HSP27 phosphorylation is significantly due to P. gingivalis-Ndk. In this way, P. gingivalis-Ndk serves as a phospho-regulator of HSP27 in primary GECs during the infection. Increased phospho-HSP27 contributes to protection of the infected cell from Cytochrome C/Caspase-9 dependent cell death, which are hallmark characteristics of the intrinsic apoptosis pathway (Elmore, 2007). This mechanism of action of HSP27 has been well defined in literature (Elmore, 2007; Paul et al., 2002) albeit it has not been characterised in human GECs. Therefore, we show for the first time that HSP27 is largely critical in conferring apoptotic resistance to GECs during P. gingivalis infection.

Nucleoside diphosphate kinase are present in a variety of different organismal species and operate as a multifunctional enzyme in the both prokaryotic and eukaryotic cells (Chopra et al., 2004; Dar, Prasad, Varshney, & Chakraborti, 2011; Spooner & Yilmaz, 2012). Typically, Ndk enzymes mediate phosphorylation of histidine residues (Adam & Hunter, 2017; Attwood & Wieland, 2015) and, to our knowledge, only one study, using human form of Ndk (designated as Nme), has reported phosphotransferase activity leading to phosphorylation on serine and threonine residues (Engel et al., 1995). As we have previously reported, P. gingivalis-Ndk, which can be secreted from the microorganism intracellularly during the host infection and translocated extracellularly in a time-dependent mode, can deplete eATP thereby hindering P2X7-receptor signalling and subsequent cellular ROS generation (Choi et al., 2013; Yilmaz et al., 2008). The substantial effects of Ndk and HSP27 interaction are markedly detected early as 6 hr postinfection and maximised at 24 hr postinfection. The largest increase in phosphorylation of HSP27, which we show is directly attributed to Ndk, is observed at 24 hr postinfection causing inhibition of cell death. Therefore, over the course of infection, P. gingivalis may use its effector Ndk in a multifaceted approach to sustain a safe environment for its intracellular life and survival in the primary GECs. It is tempting to suggest that other opportunistic pathogens that possess Ndk (Spooner & Yilmaz, 2012; Yu et al., 2017) may utilise this effector molecule for modulation of cellular signalling that critically involved in pathogen persistence in the host (Atanasova et al., 2016; Choi et al., 2013; Kim et al., 2014; Kim, Paek, Jin, Park, & Ha, 2014).
Therefore, this study provides a novel framework to build on the potential effects of the phospho-regulatory role of Ndk on HSP27 during the infection of GECs. Furthermore, P. gingivalis has recently been shown to increase HSP27 in oral cancer epithelial cells functioning upstream of MMP9 important for degrading the ECM and promoting invasion of cancer cells (Inaba et al., 2014). HSP27 is overexpressed in many cancer cell types leading to poor prognoses (Arrigo et al., 2005; Cornford et al., 2000; Guay et al., 1997; Katsogiannou et al., 2014; Mehlen et al., 1997; Vidyasagar et al., 2012). Hence, it has been recently investigated as a viable therapeutic target in cancer therapies using antisense oligonucleotides and/or pharmacological inhibitors to diminish HSP27 (Baylot et al., 2011; Hsu et al., 2011; Vidyasagar et al., 2012).
Therefore, given the role of HSP27 for the protection against chemically induced—intrinsic—apoptosis of primary GECs in the presence of P. gingivalis, HSP27 may be an essential target to promote clearance of P. gingivalis in the oral mucosa. Further, the rising association and proposed causative roles of microbes with cancer (Atanasova & Yilmaz, 2015), also suggests that the insight gained in this study of the Ndk-HSP27 interaction, could point to future targeted therapeutic strategies against other chronic diseases (e.g., cancer) recently associated with P. gingivalis infection. Interestingly, Human nm23/nucleoside diphosphate kinase (Nme) has also been investigated for its involvement in the metastatic potential of tumour cells, where it can be found in elevated levels secreted from tumour cells both in vitro and in vivo (Huwer et al., 1997; Niitsu et al., 2003; Niitsu et al., 2003; Okabe-Kado et al., 1992). The shared conserved homology of bacterial and human Ndks (Schneider et al., 2001; Yilmaz et al., 2008; Yu et al., 2017) compels the examination of different Ndk species and whether they may function similarly in the context of tumorigenesis and metastasis.
In summary, our study demonstrates a novel kinase function for P. gingivalis-Ndk in phosphorylating host cell HSP27 on specific serine residues, not previously described for any bacterial effector and/or Ndk family of proteins. Furthermore, our findings suggest the specific role of HSP27 for host cell survival in the context of P. gingivalis-mediated resistance against intrinsic apoptosis in primary GECs. Ultimately, the bacterial control of the host cell death pathways via its effector molecule, Ndk is essential for optimal intracellular life and establishment of P. gingivalis infection in the oral mucosa. The knowledge gained from this study may have significant implications for future therapeutic strategies for chronic microbial diseases and other conditions where Ndks are a virulence factor.
4 EXPERIMENTAL PROCEDURES
4.1 Bacterial and primary cell culture
Primary human GECs were obtained and cultured as described previously (Oda & Watson, 1990; Yilmaz et al., 2002; Yilmaz et al., 2008). Briefly, gingival tissue was collected from patients who were selected anonymously and randomly from those presenting for tooth crown lengthening or impacted third molar extraction under the approved guidance of the University of Florida Health Science Center Institutional Review Board (IRB, human subjects assurance number FWA 00005790). No patient information was collected and informed consent was obtained by all subjects. Cells were cultured in serum-free keratinocyte growth medium (KGM, Lonza) at 37 °C in 5% CO2.
P. gingivalis ATCC 33277 strain and its isogenic ndk-deficient mutant strain were cultured to midlog phase in Trypticase soy broth supplemented with yeast extract (1 μg/ml), menadione (1 μg/ml), and hemin (5 μg/ml) at 37 °C under anaerobic conditions and harvested as previously described (Yilmaz et al., 2008). Erythromycin (10 mg/ml) was added to the media as a selective agent for the growth of the mutant strain. The bacterial pellets were resuspended in Dulbecco's phosphate-buffered saline (PBS) containing calcium and magnesium (HyClone), and bacteria were quantified using a Klett-Summerson photometer. GECs were infected with the bacteria at a multiplicity of infection of 100 in all experiments.
4.2 GTPase assay
His-tagged recombinant P. gingivalis-Ndk (1.4 μg; GenScript) was incubated at 25 °C with 1 mM GTP for 30 min, and the liberated inorganic phosphate (Pi) was measured using the GTPase colorimetric assay kit (Innova Biosciences; Babraham, Cambridge, UK) according to manufacturer instructions. BSA (10 μg) was used as a negative control. To overcome high background due to the presence of Pi in the initial buffers that rNdk or BSA were stored in, we pretreated with PiBind resin (Innova Biosciences) overnight at 4 °C with gentle mixing followed by centrifugation at low speed (100× g for 1 min). The supernatant was checked for Pi contamination according to the kit manual (Innova Biosciences). The amount of Pi produced was determined from a standard curve expressing GTPase activity as μmol of Pi generated per mol of rNdk per minute.
4.3 GST-pull down assay and liquid chromatography-tandem mass-spectrometry (LC-MS/MS) analysis
To identify putative cellular binding partners for P. gingivalis-Ndk, we conducted GST-mediated pull-down assay. Primary GECs were infected with P. gingivalis and after 1 hr of infection, cells were washed with DPBS and then fractionated using a mitochondria/cytosol fractionation kit (BioVision). The N-terminal GST-tagged recombinant P. gingivalis-Ndk (100 μg; GenScript) was immobilised onto 25 μl of glutathione resin (ThermoFisher Scientific) at 4 °C for 1 hr. The immobilised Ndk was then incubated with 100 μg of the collected GEC proteins from the fractionated lysates (either mitochondrial of cytosolic) at 4 °C overnight. In order to eliminate non-specific binding (false positive) to the immobilised glutathione, we incubated 100 μg of GEC proteins with 25 μl of glutathione resin in the absence of recombinant Ndk and eluted the bound proteins to serve as a negative control. Eluted proteins from the GST-pull down assay were visualised using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent Coomassie staining. Stained protein band(s) found only in the recombinant Ndk pull-down as compared with the negative control were submitted to LC-MS/MS analysis, performed at the Interdisciplinary Center for Biotechnology Research of the University of Florida (Atanasova et al., 2016). Briefly, the MS/MS samples were analysed using Mascot software (Matrix Science, London, UK; version 2.4.0) and Scaffold (version Scaffold-4.0, Proteome Software Inc., Portland, OR) was used to validate the MS/MS peptide and protein identifications. In the Scaffold software, results representing 95% or higher specificity and over 99.9% threshold of detection, at a setting of no less than five matching peptides for each detected protein, were considered significant.
Mass spectrometry confirmation through Western blotting: The GST-pull down was performed as described above and the eluted immune-precipitated proteins were run on an SDS-PAGE polyacrylamide gel and probed for HSP27 as described in Western blotting methods.
4.4 Confocal microscopy
GECs were seeded directly on glass coverslips in four-well cell culture plates and were transfected with a plasmid, pEGFP-C1 (gift from Dr. Fredrick Southwick) transformed with a P. gingivalis ATCC 33277 NDK insert (gene PGN_1337) at 90% cell confluence using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. pEGFP-C1 vector, not containing an insert (empty vector), was used as control. After 48 hr of transfection, the cells were fixed with 10% neutral buffered formalin for 30 min and then permeabilised with 0.01% Triton X-100 for 30 min. The fixed cells were incubated with mouse monoclonal anti-HSP27 antibody (Santa Cruz) at a dilution of 1:100 at room temperature for 1 hr. Following the incubation with the primary antibody, the cells were washed three times with PBS and subsequently incubated with secondary antibody, Alexa Fluor568 goat anti-mouse at a dilution of 1:500 (Invitrogen), at room temperature for 1 hr. After antibody staining, the cells were washed with PBS and mounted on glass slides with mounting media containing 4′,6-diamidino-2-phenylindole (DAPI) and visualised using confocal microscopy (Zeiss). The colocalisation coefficient of P. gingivalis-Ndk and HSP27 was measured using NIH ImageJ with the JACoP plugin as described previously (Bolte & Cordelieres, 2006). The colocalisation rate was measured based on Manders coefficient ranging from 0 to 1, where zero indicates nonoverlapping and a value of 1 reflects a 100% overlap of the two analysed images (Choi, DeGuzman, Lamont, & Yilmaz, 2011).
4.5 Measurement of HSP27 and P. gingivalis-Ndk by ELISA binding assay
Ninety-six-well ELISA plates were coated with 300 ng His-tagged P. gingivalis-Ndk (GenScript) per well in carbonate/bicarbonate buffer (Sigma) at 4 °C overnight. The wells were blocked with PBS containing 0.05% Tween 20 and 0.5% BSA for 1 hr at room temperature. Varied amounts (0, 10, 50, 20, 400, and 800 ng) of purified recombinant HSP27 (Santa Cruz) were added to the immobilised Ndk and incubated for 2 hr at room temperature. After the ELISA plates were washed, bound HSP27 was detected with anti-HSP27 mouse antibody (Cell Signaling), followed by HRP-conjugated goat anti-mouse antibody (Cell Signaling). After washing, colorimetric substrate, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) in a citric acid-sodium phosphate buffer (Sigma) was added to the wells and incubated for 1 hr at room temperature. HRP activity was read at an absorbance of 414 nm using a Synergy MX plate reader (BioTek Instruments). Plates were coated with His-tagged recombinant P. gingivalis 33277 fimbriae (Yilmaz et al., 2002) as a control comparison for non-specific binding.
4.6 Western blot analyses
Western blot analysis was used to evaluate the level of phosphorylated HSP27 in GECs during P. gingivalis ATCC 33277 strain or ndk-deficient mutant strain infection: 30 min and 6 and 24 hr. Uninfected and infected GECs were collected at the same stage of growth to mitigate any effects of cellular ageing on the protein composition and HSP27 phosphorylation. Total proteins were extracted from the infected GECs using RIPA lysis buffer (Cell Signaling) plus protease and phosphatase inhibitors: 1 mM PMSF; 0.1 mM TLCK; 1 mM NaF; 2 mM N-ethylmaleimide; 1 mM sodium orthovanadate; and aprotinin (10 μg/ml). The protein concentration of each sample was measured using a standard Bradford assay (BioRad) and equal amounts of protein (20 μg) were loaded onto a 12% SDS polyacrylamide gel. After gel electrophoresis, the proteins were transferred onto a nitrocellulose membrane using wet-transfer system and the membrane was blocked in Tris-buffered saline with 0.1% Tween 20 (TBST) containing 5% non-fat dry milk. The membrane was immunoblotted overnight at 4 °C with antiphospho-HSP27 rabbit polyclonal antibody (R&D Systems) at a dilution of 1:1,000 in TBST. Horseradish peroxidase conjugated anti-rabbit antibody (Invitrogen) was used for secondary labelling at 1:1,000 in TBST for 1 hr at room temperature. The membrane was reprobed with a total HSP27 mouse antibody (Santa Cruz) and β-tubulin mouse antibody (Invitrogen) at 1:1,000 in TBST. A horseradish peroxidase conjugated anti-mouse (Invitrogen) was used to visualise both antitotal HSP27 and β-tubulin. Protein bands were visualised by enhanced chemiluminescence (ECL, GE Healthcare). Phospho- and total HSP27 was also detected by Western blot in primary GEC lysates after transfection with either control plasmid, pEGFP-C1, or a plasmid expressing Ndk, pEGFP-C1-Ndk, at 90% cell confluence using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol after 48 hr of transfection.
Western blot was also used to analyse the expression of pro and cleaved Caspase-9. GECs were infected with P. gingivalis ATCC 33277 or its isogenic ndk-deficient mutant for 30 min and 6 and 24 hr and were treated with 1.0 μM of staurosporine (Sigma) for 5 hr before collection. Total proteins were extracted from the GECs using RIPA buffer plus protease and phosphatase inhibitors as described before. Equal amounts of total protein from the samples were separated by SDS-PAGE and transferred on to a nitrocellulose membrane. Pro- and cleaved Caspase-9 were detected using overnight incubation at 4 °C with anti-Caspase-9 rabbit antibody (Cell Signaling) at a dilution of 1:1,000 in TBST. Secondary antibody, horseradish peroxidase conjugated anti-rabbit (Cell Signaling), was used at 1:1,000 in TBST, and protein bands were visualised using ECL. Protein band intensities were examined using NIH ImageJ.
4.7 In vitro phosphorylation
In order to confirm the role of P. gingivalis-Ndk in phosphorylation of HSP27, we performed in vitro phosphorylation of purified recombinant HSP27 (Santa Cruz) with recombinant His-tagged P. gingivalis-Ndk (GenScript) 50 mM Tris, pH 7.5; 2.5 mM MgCl2; and 0.3 mM ATP in a total volume of 20 μl at 30 °C for 30 min. Each reaction contained 0.2 μg of recombinant HSP27. Three reactions contained recombinant P. gingivalis-Ndk at different quantities: 0.2, 0.3, and 0.4 μg. The phosphorylation reaction was terminated by the addition of 4× Laemmli sample buffer (Bio-Rad). The proteins were separated by SDS-PAGE and HSP27 phosphorylation was detected as described in the methods section titled Western blotting. The intensities of protein bands were analysed using NIH ImageJ software.
4.8 Silencing of HSP27
Primary GECs were seeded in 6-well plates and transfected with SignalSilence HSP27 siRNA II (100 nM; Cell Signaling) or Control siRNA (100 nM; Life Technologies) using Lipofectamine RNAiMax Protocol (Invitrogen) for 48 hr. Cell lysates were collected and equal amounts of protein were collected for Western blot analysis as described. HSP27 was detected using an anti-mouse HSP27 antibody (Santa Cruz) followed by β-tubulin mouse antibody as a loading control (Invitrogen) at 1:1,000 in TBST. A horseradish peroxidase conjugated anti-mouse (Invitrogen) was used to visualise both anti-total HSP27 and β-tubulin. Protein band intensities were examined using NIH ImageJ.
4.9 TUNEL assay
Primary GECs were seeded in 4-well plates on glass inserts and transfected with SignalSilence HSP27 siRNA II (100 nM) or Control siRNA (100 nM). After 24 hr of transfection, GECs were infected with either wild-type P. gingivalis 33277 strain or isogenic ndk-deficient mutant strain for 24 hr. Staurosporine (1 μM; Sigma) was added for 3 hr before collection (21 hr postinfection). To examine P. gingivalis infection and nicked DNA, the infected and staurosporine treated GECs were fixed with 10% neutral buffered formalin for 30 min and permeabilised for 15 min in PBS containing 0.1% Triton X-100. The permeabilised cells were incubated with a polyclonal rabbit anti-P. gingivalis 33277 antibody at 1:1,000 dilution in PBS containing 0.1% Tween 20 for 1 hr. After washing with PBS-Tween 20, cells were immunostained with Alexa Fluor 488 goat anti-rabbit immunoglobulin G (Invitrogen) at 1:1,000 dilution at ambient temperature for 1 hr. The cells were then incubated with TUNEL-TMR Red (Sigma/Roche) reaction mix according to manufacturer's instructions for 1 hr at 37 °C. The immunostained cells were then washed with PBS and mounted on glass slides using Vectashield mounting media with DAPI and examined using wide-field fluorescence microscope (Zeiss Axio Imager A1). The images were captured using a cooled charge-coupled device camera controlled by QCAPTURE software (Qimaging). Quantification was then determined using cell counts for TUNEL-positive cells; n > 100.
His-rNdk Protein Transfection: Primary GECs were seeded in 4-well plates on glass inserts and transfected with 1 μg of His-rNdk (GenScript) using Pierce Protein Transfection Reagent (ThermoScientific) according to the manufacturer's protocol. Briefly, the His-rNdk was diluted in PBS and mixed with 2.5 μl/well of the dried transfection reagent for 5 min. The mixture was then added to the GECs for 21 hr and then Staurosporine (1 μM; Sigma) subsequently added for 3 hr. His-rNdk was detected using anti-rabbit His-tag antibody (GenScript) 1:50 and Alexa Fluor 488 goat anti-rabbit immunoglobulin G (Invitrogen) 1:1,000. The remaining steps for TUNEL and immunofluorescence were as described above. TUNEL-TMR red positive cells are represented as percent positive cells out of the total number of cells assessed; n > 100.
4.10 Immunofluorescence
To examine Cytochrome C distribution, GECs were infected with P. gingivalis ATCC 33277 strain or its ndk-deficient mutant strain and incubated for 21 hr. At 21 hr postinfection, the infected GECs were treated for 3 hr with staurosporine (1.0 μM; Sigma) and permeabilised with digitonin (50 μg/ml; Sigma) in PBS supplemented with KCl (100 mM) for 5 min on ice (Yilmaz et al., 2004). The cells were then immediately fixed in 10% neutral buffered formalin for 30 min and permeabilised for 30 min in PBS containing 0.01% Triton X-100. The permeabilised cells were incubated with mouse anti-Cytochrome C monoclonal antibody (BD Pharmingen) at 1:100 dilution and rabbit anti-P. gingivalis 33277 polyclonal antibody at 1:1,000 in PBS containing 0.1% Tween 20 for 1 hr. After washing with PBS-Tween 20, cells were immunostained with Alexa Fluor568 goat anti-mouse (Invitrogen) at 1:500 dilution and Alexa Fluor488 goat anti-rabbit (Invitrogen) at 1:1,000 dilution at room temperature for 1 hr. Digitonin treatment of GECs prior to fixation allowed selective permeabilisation of the plasma membrane and diffusion of cytosolic Cytochrome C out of the cells, so that the GECs with high cytoplasmic Cytochrome C levels have less fluorescence than the cells with intact mitochondria (Yilmaz et al., 2004). The immunostained cells were mounted on glass slides using Vectashield mounting media with DAPI and examined using wide-field fluorescence microscope (Zeiss Axio Imager A1). The images were captured using a cooled charge-coupled device camera controlled by QCAPTURE software (Qimaging).
ACKNOWLEDGMENTS
This work was supported by funding from the NIDCR grants R01 DE016593, T90 DE021990, T32 DE017551, and F31DE026065. This work does not represent the official views of the NIH and is solely the responsibility of the authors.
CONFLICTS OF INTEREST
The authors declare no conflict of interest and no competing financial interests.