A MORN1-associated HAD phosphatase in the basal complex is essential for Toxoplasma gondii daughter budding
Summary
Apicomplexan parasites replicate by several budding mechanisms with two well-characterized examples being Toxoplasma endodyogeny and Plasmodium schizogony. Completion of budding requires the tapering of the nascent daughter buds toward the basal end, driven by contraction of the basal complex. This contraction is not executed by any of the known cell division associated contractile mechanisms and in order to reveal new components of the unusual basal complex we performed a yeast two-hybrid screen with its major scaffolding protein, TgMORN1. Here we report on a conserved protein with a haloacid dehalogenase (HAD) phosphatase domain, hereafter named HAD2a, identified by yeast two-hybrid. HAD2a has demonstrated enzyme-activity in vitro, localizes to the nascent daughter buds, and co-localizes with MORN1 to the basal complex during its contraction. Conditional knockout of HAD2a in Toxoplasma interferes with basal complex assembly, which leads to incomplete cytokinesis and conjoined daughters that ultimately results in disrupted proliferation. In Plasmodium, we further confirmed localization of the HAD2a ortholog to the basal complex toward the end of schizogony. In conclusion, our work highlights an essential role for this HAD phosphatase across apicomplexan budding and suggests a regulatory mechanism of differential phosphorylation on the structure and/or contractile function of the basal complex.
Introduction
The Apicomplexa are obligate intracellular parasites infecting a wide range of hosts, including several species that infect humans and cause significant diseases. Plasmodium falciparum, the causative agent of the most severe form of human malaria, impacts almost 40% of world's population while Toxoplasma gondii infection is present in almost one third of humans today (Montoya and Liesenfeld, 2004; White et al., 2013). Unlike the severe and often fatal consequences of malaria, Toxoplasma infection is usually asymptomatic. Clinical toxoplasmosis is associated with an immature immune system such as in the developing fetus, causing a range of birth defects, or associated with immunocompromised or immunosuppressed conditions causing life-threatening encephalitis or myocarditis (Montoya and Liesenfeld, 2004). In general, cell and tissue destructions incurred by the fast lytic replication cycles and the ensuing host immune response are associated with disease caused by any apicomplexan parasite.
Studies of the lytic replication cycle have relied heavily on Toxoplasma as a model for other Apicomplexa (Kim and Weiss, 2004; Francia and Striepen, 2014). Toxoplasma cell division, termed endodyogeny, employs an internal budding process wherein two daughter cytoskeleton buds are assembled within the confinements of the mother cell providing the scaffold for organelle assembly and partitioning (Nishi et al., 2008; Anderson-White et al., 2012). The cortical cytoskeleton is composed of flattened alveolar vesicles (the inner membrane complex, or IMC), an intermediate filament protein network comprising a set of IMC proteins (Anderson-White et al., 2011) and 22 sub-pellicular microtubules emanating from the apical end (Gubbels and Morrissette, 2013). Daughter buds are assembled in an apical to basal direction, nucleated by the centrosome (Hu et al., 2002; Chen and Gubbels, 2013; Suvorova et al., 2015). The centrosome is also nucleating the assembly of Plasmodium merozoites in a process termed schizogony (Mahajan et al., 2008; Kono et al., 2012). This budding mode organizes the formation of several daughter buds from the periphery of a single mother cell, after passing multiple rounds of nuclear division. The apicomplexan division modes are unified by the timely connection of final nuclear division and cytokinesis during daughter cell budding (Francia and Striepen, 2014). After the daughter cytoskeleton reaches the widest point, it starts to taper down toward the basal end. This constriction is driven by the basal complex, which is also conserved across the Apicomplexa (Ferguson et al., 2008). In Toxoplasma, contraction of the basal complex was first described by the discovery of MORN1 (Gubbels et al., 2006; Hu et al., 2006), a scaffolding protein of the basal complex that also localizes to the apical end and the spindle pole (Heaslip et al., 2010; Lorestani et al., 2010). Unlike the contractile ring in mammalian cells, constriction of the basal complex is independent of actin (Shaw et al., 2000; Gubbels et al., 2006) and members of the ESCRT-III machinery, completing the final separation of mammalian cells, are absent from the Toxoplasma genome (Tomavo et al., 2013). Furthermore, microtubules do not localize to the basal complex, suggesting that constriction is driven by an unknown mechanism. One suggested but not yet validated candidate is the contractile filament forming protein Centrin2, which is recruited to the basal complex at the onset of constriction (Hu, 2008). However, it is largely unknown which proteins constitute the basal complex, how it is assembled and how its assembly and constriction are regulated.
To enhance our understanding of the biology of the basal complex we have focused on identifying the proteome of the basal complex and its architecture. In recent years we identified a sub-set of intermediate filament-like IMC proteins in the basal complex (Anderson-White et al., 2011). In another effort toward this goal we performed a parasite fractionation combined with an immunoprecipitation step to enrich the basal complex and determine its composition by mass spectrometry (Lorestani et al., 2012). We identified several new components of the basal complex in this effort, including a 14-3-3 protein and MSC1a, which associates only with the mature basal complex in mother parasites. However, we did not observe much overlap between different approaches that identified basal complex proteins (Hu et al., 2006; Anderson-White et al., 2011; Lorestani et al., 2012), suggesting that our current understanding of basal complex composition is still incomplete.
Here we set out with an independent approach to identify additional basal complex components based on the role of MORN1 as a scaffolding protein (Lorestani et al., 2010). Using MORN1 as bait in a yeast two-hybrid (Y2H) screen we identified an uncharacterized protein co-localizing with MORN1 in the basal complex during constriction. A putative HAD phosphatase domain validated for activity in vitro was found in this protein, and therefore, we named it HAD2a. The loss of HAD2a causes aberrant assembly of the basal complex resulting in a failure to complete cytokinesis and a severe proliferation defect. Orthologs of HAD2a are conserved across the Apicomplexa and related organisms sharing an inner membrane complex, which together make up the Alveolata and suggest this protein specifically acts in the biology of this cytoskeletal structure. Indeed, we confirmed this hypothesis by subcellular localization of the P. falciparum HAD2 ortholog to a ring-like structure at the basal end of forming merozoites, indicative of an involvement in the final steps of daughter cell budding.
Results
Identification of HAD2a
To identify proteins directly interacting with MORN1 we mined a tachyzoite cDNA library by Y2H screening. Because bait comprising full-length MORN1 resulted in auto-activation, we used two partial constructs, a N-terminal construct (MORN1 aa 1–144) and a C-terminal construct (MORN1 aa 196–363) (Fig. S1), not causing auto-activation. A total of ~120 000 yeast colonies were screened for Y2H interactions across both constructs, which resulted in 44 yeast clones corresponding with 33 unique Toxoplasma genes (Fig. S1, Table S1). Among these was MORN1 itself (Y2H clone ‘MORN1 N-terminal interacting candidate’ (MNIC-13) as well as Hsp20 (clones MNIC-6 and ‘MORN1 C-terminal interacting candidate’ (MCIC-29), the latter is known to localize to the IMC and the basal end of the parasite (de Miguel et al., 2008). We focused our attention on genes that encode hypothetical proteins and shortlisted nine because of their gene expression profile (peaking in S/M phase of parasite replication; Behnke et al., 2010) and our ability to obtain sub-cellular localization information (Table S1). Yeast clone MNIC-18 became our lead candidate as its product showed strong interaction with MORN1 across three different Y2H interaction reporter assays (Fig. 1A). Sequence analysis identified a haloacid dehalogenase (HAD) phosphotransferase domain (Fig. 1B and C). Based on these data, our phenotypic observations described in the succeeding texts and the recent characterization of a HAD phosphatase in P. falciparum, PfHAD1, (Guggisberg et al., 2014) with a putative ortholog in Toxoplasma, this gene was named HAD2a. A BLAST search identified three additional paralogs, which we subsequently named HAD2b-d (HAD2b: TGME49_246110; HAD2c: TGME49_271960; HAD2d: TGME49_223870). HAD2a-d share a conserved motif of ~200 amino acids (Fig. 1B) flanked on both the N- and C-terminus with unique regions. The conserved region is shared among the Alveolates and is characterized by a DxDx(T/V) motif (Fig. 1C, red; Fig. S2) representative of the HAD superfamily of phosphotransferases (Burroughs et al., 2006; Allen and Dunaway-Mariano, 2009; Seifried et al., 2013). The majority of these evolutionarily wide-spread enzymes are phosphatases that use Asp as a nucleophile (the first residue in the DxDx(T/V) motif) in a Mg2+-dependent fashion for catalytic activity (Allen and Dunaway-Mariano, 2009). Consistent with this, the three Asp residues known to associate with the Mg2+ ion are conserved in this group of Toxoplasma proteins (Fig. 1C, red). Moreover, a modelling search of crystal structures deposited on SwissProt provided the best model for HAD2a and HAD2b using the crystal structure of a HAD family hydrolase from Pseudomonas aeruginosa (Fig. S3). Furthermore, the key residues for the four conserved motifs defining the HAD hydrolases according to Burroughs et al. (Burroughs et al., 2006) are conserved in this family of Toxoplasma HADs (Fig. 1C, yellow/red). The unmodeled loop between motifs I and II is known as the cap domain (Fig. 1C), which endows phosphatase specificity and impacts the ability to recognize either micromolecule or macromolecule as substrates (Burroughs et al., 2006; Seifried et al., 2013). Between the four members of this HAD family we noticed a considerable divergence in the cap domain suggesting different substrate specificities. Collectively, these data imply that this group of proteins is a family of phosphatases.
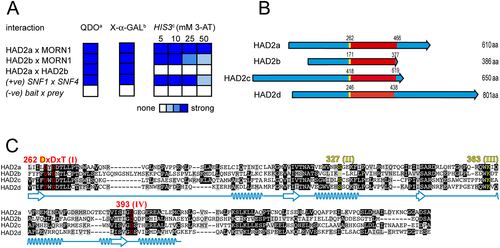
HAD2a and HAD2b interact with MORN1 by yeast two-hybrid and possess a conserved HAD phosphatase domain.
A. Summary of performed yeast two-hybrid screens. Full-length clones are used (bait × prey). Yeast transformants were tested for: (a) Growth on quadruple drop-out, which indicates a two-hybrid interaction. (b) Conversion of X-α-GAL substrate (measures strength of the LacZ α-galactosidase reporter), which is a colorimetric assay for the MEL1 reporter gene. (c) Grown on plates with increasing 3-amino-triazole (3-AT) concentration, which poisons the histidine biosynthetic complex (the strength of the HIS3 reporter) and provides a qualitative indication of interaction strength. The positive control (+ve) is a well validated control (Fields and Song, 1989). The negative control (−ve) represents empty bait and prey plasmids. The shade of blue reflects signal strength as indicated in the legend.
B. Schematic representation of HAD2a and paralogs HAD2b-d. Identified proteins share a conserved ~200 aa HAD phosphatase domain (red) but are diverse at the N- and C-terminus. The begin of the DxDxT motif in the HAD domain is marked in yellow.
C. Alignment of the HAD domains identified in Fig. 1B. The conserved Asp residues of the HAD domain are highlighted in red, and further, conserved HAD signature motifs are highlighted in yellow. Prediction of secondary structure shown in light blue (beta-strand indicated by arrows, alpha helices indicated by helices; prediction based on modelling to Pseudomonas HAD phosphatase; Fig. S3). Identical amino acids are highlighted in black and amino acids with functional-related side groups are highlighted in grey.
HAD2a and HAD2b display phosphatase activity
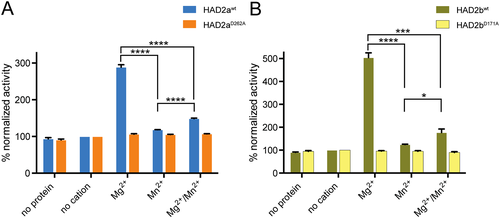
As shown in Fig. 1C, sequence analysis and modelling identified a HAD domain conserved across this family of proteins. To provide direct evidence for the predicted phosphate hydrolase activity we expressed and purified recombinant 6xHis-tagged HAD2a and HAD2b proteins and performed phosphatase activity assays. We also generated constructs wherein the first nucleophilic Asp residue in the conserved DxDx(T/V) motif is mutated into an Ala residue (HAD2aD262A and HAD2bD171A), a mutation known to abrogate phosphatase activity (Collet et al., 1999; Li et al., 2003). We assessed the purity of the proteins by SDS-PAGE and Coomassie Brilliant Blue staining and demonstrated that the purified HAD2a and HAD2b proteins, besides some contaminants in the case of HAD2a, display strong protein bands around the predicted 64 and 42 kDa protein sizes respectively (Fig. S4). Expression and purification of HAD2aWT and HAD2bWT as well as their respective active site mutants resulted in comparable purity within the pairs (Fig. S4). We then used para-Nitrophenyl Phosphate (p-NPP) to test for enzyme activity of the recombinant proteins (Takai and Mieskes, 1991). p-NPP is a non-specific substrate for alkaline and acid phosphatases with a demonstrated ability in detecting Ser/Thr as well as Tyr protein phosphatase activity (Zhang et al., 1992; Zhuo et al., 1993). Using this assay, we readily detected phosphatase activity for HAD2a and HAD2b, which was abolished in the active site mutants (Fig. 2). To test which co-factors are prerequisites for phosphatase activity we utilized the known cations Mg2+ and Mn2+ (Zhuo et al., 1993) and detected a vital dependence on Mg2+ for catalytic activity of both HAD2a and HAD2b (Fig. 2). Taken together, HAD2a and HAD2b have demonstrable Mg2+-dependent phosphatase activity consistent with their HAD domain.
HAD2a dynamically associates with the mostly basal cytoskeleton
The strong Y2H interaction between HAD2a and MORN1 together with their closely matched mRNA expression profiles suggested that these proteins might localize to the same subcellular compartments in vivo. To test this we first determined the dynamics of HAD2a and HAD2b localization by fluorescence microscopy. We generated specific polyclonal HAD2a and HAD2b antisera raised against the full-length 6xHis-tagged proteins to study their localization. The antisera are specific as in Toxoplasma lysates the HAD2a and HadD2b antisera recognize bands of 64 and ~40 kDa, corresponding with predicted molecular weights of 64.5 and 41.7 kDa respectively (Fig. S5A and B). We assessed the dynamics of HAD2a throughout daughter cell budding using IMC3 as a development marker for both mother and daughter cortical cytoskeletons. In nearly all parasites we observe HAD2a at the basal complex of the mother (Fig. 3A, asterisks). In early daughter buds we observed HAD2a predominantly in the forming basal and apical complexes (Fig. 3A, early budding, arrows). During mid-budding, the signal disperses slightly over the whole nascent daughter cytoskeleton though remains associated with the apical and basal complex (Fig. 3A, mid budding, arrow). In late buds when the basal complex is constricting, HAD2a transitions into a nearly exclusive association with the basal complex of the daughter buds (Fig. 3A, late budding, arrows). To determine whether HAD2a co-localizes with MORN1 we co-stained parasites expressing Myc2-MORN1. Indeed, we observe co-localization of both proteins at the basal complex of mother (asterisk) and daughter (arrow) cells (Fig. 3B). To assess whether the localization of HAD2a to the basal complex is dependent on MORN1 we stained for HAD2a in an inducible MORN1 knockdown cell line (Lorestani et al., 2010). Upon the loss of MORN1, HAD2a still localizes to the cortex of the daughter buds; however, we can no longer detect HAD2a signal at the basal complex (Fig. 3C, +ATc, arrows). This indicates that HAD2a localization to the daughter buds is independent of MORN1 but that HAD2a association with the basal complex requires MORN1. Taken together, HAD2a dynamically associates with the daughter cytoskeleton in early buds but transitions to the basal complex when it starts to contract and is retained in the basal complex in the majority of mature parasites.
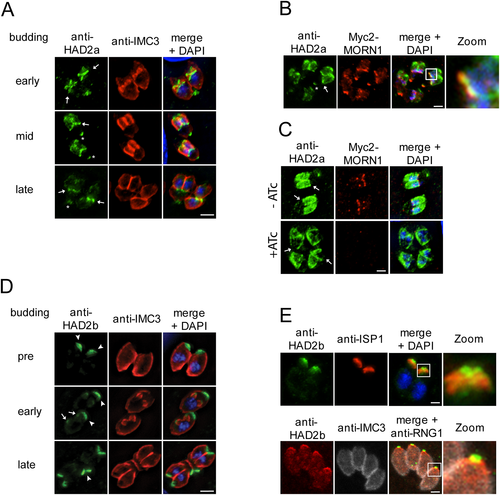
Intracellular localization of HAD2a and HAD2b during Toxoplasma endodyogeny.
A. HAD2a localizes to forming daughter buds and the basal complex during endodyogeny. HAD2a antiserum (green) was used to co-localize HAD2a with IMC3 (red) during different stages of budding (early to late), DNA is stained with DAPI (blue). HAD2a is present in the daughter buds and the basal complex (daughter cell basal complex: arrows; mother cell basal complex: asterisks) in early and mid-buds but relocates nearly exclusively to the basal end upon conclusion of budding.
B. Using a Myc2-MORN1 expressing cell line we co-localized HAD2a (green) and MORN1 (red) in the daughter (arrow) and mother basal complexes (asterisk). Zoom = 4x of boxed area.
C. Localization of HAD2a to the basal complex is dependent on MORN1. HAD2a (anti-HAD2a, green) localization to the basal complex is lost upon induction of MORN1 knockdown with ATc (+ATc) in inducible MORN1 knockdown parasites (Lorestani et al., 2010). Upon loss of MORN1 HAD2a still localizes to the daughter cytoskeleton but is absent from the expected basal complex location (arrows).
D. HAD2b localizes to the apical IMC in Toxoplasma. HAD2b antiserum (green) was co-localized with the parasite's cytoskeleton (IMC3, red), DNA was detected with DAPI (blue). HAD2b is present in the apical cap of the mother (arrowheads) as well as of the daughter cell (early budding, arrows). HAD2b present at the mother's apical end also migrates with the conoid to the residual body (late budding, arrowhead), which implies a physical connection to the conoid.
E. HAD2b localizes to the apical extreme of the IMC but is not part of the conoid. Top panel: Co-localization of anti-HAD2b (green) and anti-ISP1 (red) indicates HAD2b is positioned apical to the apical cap marker ISP1. Lower panel: HAD2b (red) localizes posterior of the apical polar ring marker RNG1 (anti-RNG1, green) and is therefore in the strict definition not part of the conoid. The cortical cytoskeleton is stained with anti-IMC3 (white). Zoom = 4× of boxed area. Scale bars = 5 µm.
HAD2b associates with the apical cytoskeleton
We determined that HAD2b also strongly interacts with MORN1 in Y2H experiments (Fig. 1A). We also assessed whether HAD2a and HAD2b interacted with each other, but we did not observe any interaction between these two proteins (Fig. 1A). By IFA HAD2b localizes to the apical end of the cortical cytoskeleton in both mother (arrowhead) and daughter (arrow) parasites (Fig. 3D). During endodyogeny HAD2b is present in the early daughter buds (Fig. 3D, early budding, arrows). In emerging parasites we also recognized a strong HAD2b signal at the base of daughter buds where the mother's IMC is disassembling (Fig. 3D, late budding, arrowhead). Such a signal is suggestive of association with the conoid as the conoid is not disassembled till very late in division and is observed as a signal migrating in an apical to basal direction with the retracting mother cytoskeleton (Morrissette and Sibley, 2002). To further refine to which apical cytoskeleton structure HAD2b localizes we co-stained with markers for IMC sub-compartment protein 1 (ISP1), which localizes to the apical cap alveolus of the IMC (Beck et al., 2010), and RNG1, which localizes to the apical polar ring serving as the sub-pellicular microtubule organizing centre at the base of the conoid (Tran et al., 2010) (Fig. 3E). We observed that HAD2b sits apical of the ISP1 signal in mature parasites. On the other hand, the HAD2b signal partially overlaps with the RNG1 signal, but RNG1 extends further apically. Together, these data indicate that HAD2b is not part of the conoid but is associated with the very end of the IMC where it connects to the apical polar ring. Notably, MORN1 also has a minor presence at this position (Gubbels et al., 2006; Hu et al., 2006), suggesting that this localization could be dependent on MORN1. In conclusion, HAD2b associates with MORN1 by Y2H and localizes to the apical end of the IMC at the interface with the conoid.
HAD2a is an essential gene in Toxoplasma
Because our primary interest was to identify new proteins and mechanisms of the contractile-related machinery in T. gondii, we focused our efforts on HAD2a because of its co-localization with MORN1 during endodyogeny. We engineered a conditional knockout cell line for HAD2a using conditional diCre-LoxP technology (Andenmatten et al., 2012). In this LoxP-HAD2a line the had2a ORF is replaced with a LoxP flanked, Ty-tagged had2a allele that can be excised upon activation of diCre-mediated recombination by rapamycin treatment. We exchanged the single YFP ORF against a double YFP in the transfection plasmid to enhance detection of YFP when expressed under the endogenous had2a promoter following diCre recombination (Fig. 4A). A clonal LoxP-HAD2a line was established, and successful gene-swap and genomic integration of the transfected plasmid were verified by diagnostic PCR (Fig. 4B). We verified expression of the Ty-tagged HAD2a allele by western blot using Ty and the specific HAD2a antisera, which confirmed a protein of ~64 kDa in the LoxP-HAD2a as well as in a diCre control cell line (Fig. S5B). To induce recombination we incubated LoxP-HAD2a parasites overnight with 50 nM rapamycin and detected an average of ~10% recombination efficiency. Loss of Ty-HAD2a signal and expression of YFP-YFP in had2aKO parasites were confirmed by IFA experiments using anti-Ty and anti-GFP antisera (Fig. 4C, S5C). Loss of HAD2a did not result in a change of HAD2b expression and localization (Fig. S5D), indicating that there is no phenotypic compensation between these two proteins. To obtain sufficient YFP-positive had2aKO mutants for subsequent phenotypic characterization we applied fluorescence-activated cell-sorting (FACS) resulting in highly enriched YFP-positive parasite samples with only a minor (<10%) YFP-negative parasite population. Excision of the had2a ORF was verified by diagnostic PCR (Fig. 4D), and sorted parasites were used for standard plaque-assays taking the YFP-negative samples as controls for parasite fitness. Plaque assays revealed a severe proliferation defect for YFP-positive parasites, shown by 85% less plaques formed in comparison to control parasites (Fig. 4E). Residual plaques in YFP-positive samples could be assigned to residual YFP-negative parasites as evidenced by fluorescence microscopy performed four days after FACS sorting (Fig. 4F). Taken together, our results demonstrate an essential function for HAD2a in the lytic replication cycle of T. gondii.
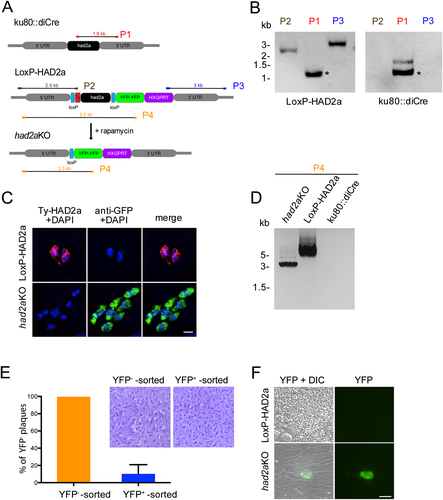
HAD2a is essential for completing the Toxoplasma lytic cycle.
A. Schematic representation of the conditional had2aKO strategy using the diCre system. The endogenous had2a ORF in ku80::diCre parasites is replaced with a LoxP-flanked ty-had2a allele via homologous recombination in the 5′- and 3′-UTR of the had2a locus. Rapamycin induces Cre-mediated recombination thereby deleting the ty-had2a ORF and activating YFP-YFP expression under the endogenous had2a promoter. Expected PCR products of diagnostic PCR in B and D) are indicated as P1-P4.
B. Diagnostic PCR reactions P1-P3 (as in panel A) confirm replacement of the endogenous had2a locus with the transfected LoxP-HAD2a plasmid. Aspecific PCR bands are marked with an asterisk.
C. Ty-tagged HAD2a (red) expression is observed in LoxP-HAD2a parasites but not in had2aKO mutants incubated for 16 hr with 50 nM rapamycin. YFP (green) expression is only detected in parasites after rapamycin treatment. DNA is visualized by DAPI (blue). Scale bar = 10 µm.
D. Diagnostic PCR (P4 in panel A) confirms excision of the had2a ORF in YFP+ FACS-sorted parasites. Control parasites show presence of had2a locus. Ku80::diCre gDNA served as a control for the PCR.
E. FACS-sorted YFP-positive parasites form ~85% less plaques than YFP-negative parasites. Values show the mean of eight plaque assays from three independent FACS experiments + s.d., normalized against the YFP-negative sorted control. Plaque assays show a representative example of a crystal violet stained host cell layer for YFP-negative and YFP-positive sorted parasites following seven days of growth.
F. Although plaques were detected in YFP-positive sorted parasites, immunofluorescence detection after four days indicated that YFP-positive had2aKO parasites form no plaques in contrast to control YFP-negative LoxP-HAD2a parasites. Scale bar = 50 µm.
had2aKO parasites display a cytokinesis defect
Having established an essential role for HAD2a we dissected the phenotypic consequences of had2a loss. We compared intracellular division of had2aKO parasites to LoxP-HAD2a controls using IMC3 antiserum 24 h after host cell invasion and observed a severe division defect in the had2aKO population (Fig. 5A). Defects first appeared in intracellular vacuoles harbouring ~4 parasites. IMC3 staining revealed enlarged had2aKO parasites with two separated nuclei in the absence of daughter buds. Furthermore, we observed vacuoles in which had2aKO parasites passed several rounds of budding eventually leading to a distorted shape, lacking the rosette appearance of LoxP-HAD2a control parasites (Fig. 5A). When quantified 24 and 48 h after invasion, we found 57% and 82% respectively of the counted had2aKO vacuoles to display division defects, likely produced by incomplete separation of dividing tachyzoites (two nuclei per parasite in absence of large daughter buds and/or distorted shape in intracellular vacuoles) (Fig. 5B).
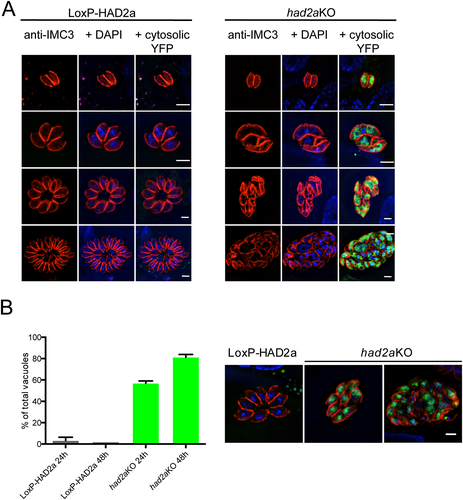
had2aKO parasites have a defect in daughter budding.
A. Comparison of intracellular growth of LoxP-HAD2a and had2aKO parasites. LoxP-HAD2a parasites show normal growth through multiple rounds of budding, had2aKO parasites show abnormal growth indicated by improper daughter cell segregation and distorted parasite shape in the vacuole. Parasite cytoskeletons were stained with IMC3 antiserum (red). Detection of cytosolic YFP (green) indicated successful had2aKO, DNA was stained with DAPI (blue). Scale bar = 5 µm.
B. Quantification of vacuoles with misshaped parasites in the LoxP-HAD2a and had2aKO populations. Parasites were incubated with 50 nM rapamycin for 16 h and stained with IMC3 antiserum 24 or 48 h after host cell invasion. More than 100 vacuoles were counted and morphology assessed by microscopy. Representative examples for vacuoles of LoxP-HAD2a and had2aKO parasites are shown on the right. had2aKO parasites are mostly conjoined or show a distorted appearance in the vacuole. An average of n = 3 + SD is plotted.
The sequence in Fig. 5A suggests an escalating phenotype rather than a clean arrest. To best understand the role of HAD2a the most information lies in the first step wherein the phenotype manifests, which differentiates subsequent secondary defects. Because the earliest changes in morphology and budding are reminiscent of the previously described phenotype for parasites with a loss of MORN1, where the basal complex fails to assemble and results in severely impaired daughter cell segregation (Heaslip et al., 2010; Lorestani et al., 2010), we focused our phenotype dissection on this step in had2aKO phenotype development. To validate the conjoined appearance of the had2aKO parasites we used SAG1 antiserum to visualize the parasite's plasma membrane and RNG1 antiserum to highlight the parasite's apical end (Fig. 6A). We found conjoined parasites carrying two apical ends as highlighted by RNG1 staining (Fig. 6A, arrows) but lacking a basal end, a characteristic previously reported for the double-headed and multi-headed parasites upon loss of MORN1 (Heaslip et al., 2010; Lorestani et al., 2010). We further confirmed the formation of double-headed parasites with the apical marker ISP1 (Fig. 6A, lower panel) and validated the continuous connection between the two ‘heads’ by differential interference contrast (Fig. 6A and Fig. S5C and D). To verify that the observed had2aKO conjoined parasites completed budding we stained with anti-IMC1 and anti-GAP45 antibodies. GAP45 is associated with the IMC only when division is concluded, allowing us to distinguish between mother and daughter cytoskeletons (Gaskins et al., 2004). In LoxP-HAD2a control parasites a closure of the basal end that separated one cell from the other was always observed (Fig. 6B, asterisks), contrary to had2aKO conjoined parasites that lacked a tapered closure and displayed two completely segregated nuclei in one continuous cell (Fig. 6B). The absence of large daughter buds indicated that conjoined parasites had completed budding and the presence of small daughter buds implied that the next round of budding had initiated even though parasites failed to conclude cytokinesis of the previous cycle (Fig. 6B, arrows). We also noticed a gap in had2aKO parasites that was neither stained by IMC1 nor GAP45 (Fig. 6B, arrow head), indicating that there are two separate cytoskeletons and that the parasite failed to close the cytoskeletons after the mother cell's cytoskeleton disassembled.
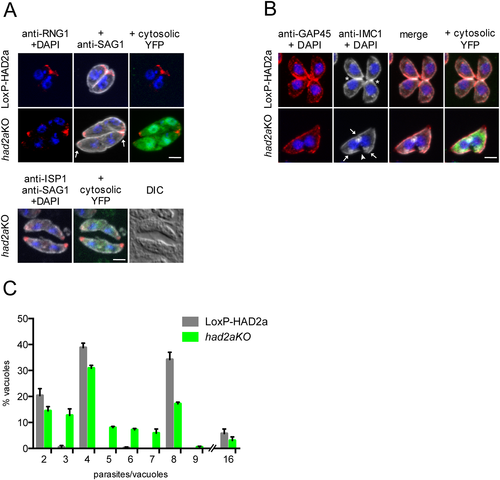
had2aKO parasites fail to conclude cytokinesis.
A. Top panel: LoxP-HAD2a and had2aKO parasites stained with anti-SAG1 (white) to highlight the plasma membrane and with anti-RNG1 (red) to visualize the apical end, DNA was stained with DAPI (blue). had2aKO parasites shared one plasma membrane and exhibited two apical ends (arrows) but lack a basal end. The nuclei were completely separated. Cytosolic YFP expression (green) identified had2aKO parasites. Lower panel: had2aKO parasites were stained with the apical cap marker ISP1 (anti-ISP1, red), anti-SAG1 (white). Differential interference contrast further illustrates the continuity between the double-headed parasite. Scale bar = 5 µm.
B. LoxP-HAD2a and had2aKO parasites stained with anti-GAP45 (red) and anti-IMC1 (white), DNA was detected with DAPI (blue). LoxP-HAD2a parasites had a closed basal end (asterisk) and one nucleus per cell, had2aKO parasites were found with two nuclei in a continuous cytosol in absence of complete basal closure (arrowhead). Newly initiated daughter cells were observed (arrows), indicating that these had2aKO parasites already initiated a new round of daughter cell formation before completing cytokinesis of the previous round. Scale bar = 5 µm.
C. Quantification of intracellular growth of had2aKO parasites. Parasites were incubated with 50 nM rapamycin for 4 h and stained with anti-IMC3 24 h after invasion. The number of parasites per vacuole was counted by microscopy. LoxP-HAD2a parasites were found to grow geometrically with the majority of vacuoles harbouring either four or eight parasites after 24 h. had2aKO parasites showed vacuoles with aberrant numbers of parasites because of formation of conjoined and not completely separated parasites. Average of n = 3 + SD is plotted.
By counting the number of parasites per vacuole 24 h after host cell invasion we found aberrant parasite numbers almost exclusively in had2aKO vacuoles compared with LoxP-HAD2a vacuoles that displayed regular endodyogeny and geometric increase in numbers (Fig. 6C). Together, these data strongly suggest that had2a knockout impairs the conclusion of cytokinesis in Toxoplasma endodyogeny.
had2aKO parasites fail to constrict the basal complex
The formation of conjoined parasites and imperfect basal end closure upon had2a loss suggests that HAD2a might act on the basal complex and/or MORN1 itself. To first verify that had2aKO parasites possess the scaffold of the basal complex we tested if MORN1 localization is impaired in had2aKO parasites. Hereto we stably expressed mCherry-tagged MORN1 in LoxP-HAD2a parasites. As expected, we localized mCherry-MORN1 to the spindle pole and the mature basal complex in LoxP-HAD2a parasites (Fig. 7A, basal complex marked with an asterisk). In had2aKO parasites we also localized mCherry-MORN1 to the spindle pole and detected the MORN1- labelled basal complex at either the parasites' basal end (Fig. 7A, arrow second panel) or at the mid point of cells where conjoined parasites were fused (Fig. 7A, arrow third panel). We rarely observed basal complexes in segregating parasites for the uninduced parent line (LoxP-HAD2a). This indicates that loss of HAD2a does not interfere with the association of the basal complex scaffolding protein MORN1 with the basal extremity of the IMC. Furthermore, we noticed an unusually large diameter of the basal complex for both the midpoint basal complexes and at the basal end of had2aKO parasites as compared with LoxP-HAD2a controls. Measurement of the basal complex diameter in LoxP-HAD2a controls showed an average of 0.94 µm, whereas had2aKO parasites had a much wider average basal complex diameter of 1.47 µm, with 83% of the analysed had2aKO parasites displaying a basal complex width greater than 1 µm (compared with only 29% of the LoxP-HAD2a controls) (Fig. 7B). Thus, these data indicate that some had2aKO parasites are still able to complete cytokinesis but with incomplete basal complex contraction. Mechanistically, MORN1 recruitment does not appear to be hampered in had2aKO parasites but basal complex contraction is impaired.
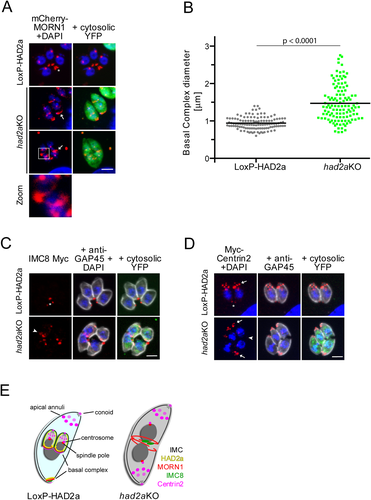
Basal complex contraction is impaired in had2aKO parasites.
A. In LoxP-HAD2a parasites stably expressing mCherry-MORN1 (red). mCherry-signal localized to the spindle pole and the basal complex (asterisk, upper panel). Upon induction of the had2aKO (green) mCherry-MORN1 still localizes to the basal complex of single parasites (upper panel; note that these parasites are dividing) or the midpoint of conjoined parasites (lower panel). Arrows: the basal complex of the mature parasite (second panel) and in conjoined double-headed parasites (third panel) exhibits a wider diameter (quantified in B). Zoom (4x of boxed area): double-basal complex rings at the interface between conjoined parasites as illustrated in E. DNA was stained with DAPI (blue). Scale bar = 5 µm.
B. Quantification of basal complex diameter. The basal complex diameter as highlighted by mCherry-MORN1 was measured in LoxP-HAD2a and had2aKO parasites. A significantly wider basal complex diameter was detected in had2aKO parasites, indicating that these parasites fail to contract the basal complex. n = 100, horizontal bars mark average. Significance tested by unpaired t-test.
C. Localization of basal complex protein IMC8. The IMC8 locus was endogenously tagged with a triple Myc tag in LoxP-HAD2a parasites and localized in had2aKO following diCre recombination. IMC8 localized to the basal complex of LoxP-HAD2a parasites (asterisk). In had2aKO IMC8 was detected at the midpoint of conjoined parasites (arrowhead). The nucleus is stained with DAPI. Scale bar = 5 µm.
D. Localization of Centrin2 was assessed in a LoxP-HAD2a cell line stably expressing Myc2-Centrin2 (red). Localization of Centrin2 to the apical end, the apical annuli, centrosome and basal complex was detected in LoxP-HAD2a parasites. Arrows highlight Centrin2 at the apical end whereas basal localization is marked by an asterisk. Expression of cytosolic YFP (green) indicates had2aKO parasites, the parasite's cortex was highlighted with anti-GAP45 antibody (white). In had2aKO parasites Centrin2 localizes normally to the to the apical end and apical annuli of both heads (arrows) but we failed to detect a basal complex signal for Centrin2 (arrowhead) at the conjoined parasites. DNA was stained with DAPI (blue).
E. Model for subcellular localization of select basal complex components in LoxP-HAD2a and had2aKO tachyzoites. Indicated are HAD2a (yellow), MORN1 (red), Centrin2 (magenta) IMC8 (green) and the cortical IMC (black). Mother cell cytoplasm is labelled in light blue; daughters are highlighted in grey. Upon loss of HAD2a the apical to basal orientation of the tachyzoite is lost because of formation of a double-headed parasite. The basal complex (indicated by MORN1) partially assembles and fails to constrict and to separate the nascent daughter cells.
The Toxoplasma basal complex has been resolved into three sub-compartments. Starting from the apical end, a ring harbouring MORN1, IMC9 and IMC13 is followed by a structure harbouring IMC5 and IMC8, which in turn is bordered at the very basal end by Centrin2 (Anderson-White et al., 2011). We demonstrated the presence of MORN1 and asked next whether the other structures are formed. As a representative of the middle compartment we endogenously tagged IMC8 with a triple Myc tag in LoxP-HAD2a parasites and determined its localization after induction of the had2aKO (Fig. 7C). We observed the previously reported basal localization in uninduced parasites (Fig. 7C, asterisks), whereas loss of HAD2a resulted in a clustered IMC8 Myc signal at the mid-point (Fig. 7C, arrowhead). These data indicate that this basal complex component is still recruited to the right place, but does not assemble into a proper ring.
To test for Centrin2 localization in had2aKO parasites, we stably transfected the LoxP-HAD2a cell line with a Myc2-Centrin2 expression plasmid and induced the had2aKO. We confirmed Centrin2 localization at the apical end, the apical annuli, the centrosome and the basal complex in the uninduced parasites (Fig. 7D) (Hu et al., 2006; Hu, 2008). In the conjoined phenotype we detected Centrin2 at the apical end and the apical annuli at both ends of double-headed had2aKO parasites (Fig. 7D, arrows). However, we were unable to detect Centrin2 at the midpoint of conjoined parasites where we expected to find the basal complex (Fig. 7D, arrowhead). However, the typically weak signal of Centrin2 at the basal complex (Hu et al., 2006; Hu, 2008) might be below the detection limit and therefore this result in not conclusive.
In summary, the absence of HAD2a does not affect recruitment of MORN1 to the basal complex but results in defective formation of the IMC8 sub-compartment. Moreover, the absence of HAD2a results in an overall wider basal complex diameter. Thus, HAD2a operates downstream of MORN1 recruitment to the basal complex in properly assembling basal complex sub-structures and HAD2a absence results in defective basal complex contraction and a cytokinetic defect (Fig. 7E).
The Plasmodium HAD2 ortholog resides in the cytokinetic ring of budding merozoites
Using a standard BLASTP approach we identified a P. falciparum protein that although divergent at the N- and C-terminus, showed considerable identity to the HAD-like domain of TgHAD2a (Fig. S6). The identified gene product (PF3D7_1205200) is annotated as a putative HAD domain ookinete protein (PlasmodDB.org) and gene expression peaks during late schizogony of the Plasmodium asexual replication cycle, consistent with a putative role in cell division (Bozdech et al., 2003). It is furthermore recognized as part of the established Plasmodium invasion-related network, PlasmoINT (Hu et al., 2010), which contains both invasion and division-related proteins. To approach the subcellular localization of Plasmodium HAD2 (PfHAD2) we replaced the endogenous ORF with a GFP-tagged version in 3D7 P. falciparum parasites (Fig. 8A). Successful replacement of the 3′ region was verified by diagnostic PCR (Fig. 8B) and subcellular localization assessed by microscopy (Fig. 8C). PfHAD2 was first visible in schizont stage parasites (~10 nuclei) localizing to several ring-like structures at the periphery of the mother cell (Fig. 8C, early budding). With proceeding schizogony, the PfHAD2 rings expanded and engulfed the separated nuclei and finally moved to the basal end of the nascent merozoites (Fig. 8C, mid budding). Upon conclusion of schizogony, PfHAD2 rested basal of the nascent merozoite's nuclei (Fig. 8C, late budding). The dynamic of PfHAD2 was further confirmed by co-localizing the protein with a marker of the parasite's IMC, GAPM2 (Bullen et al., 2009) (Fig. 8D). PfHAD2 first appeared as a wider ring compared with GAPM2 in early schizogony and eventually guides the IMC during merozoite formation to rest at the basal end of the cell (Fig. 8D, early – late budding). Thus, although considerably different in the amino acid sequence, Plasmodium HAD2 is shown to participate in the budding process, implying a conserved function for HAD2 in apicomplexan cell division.
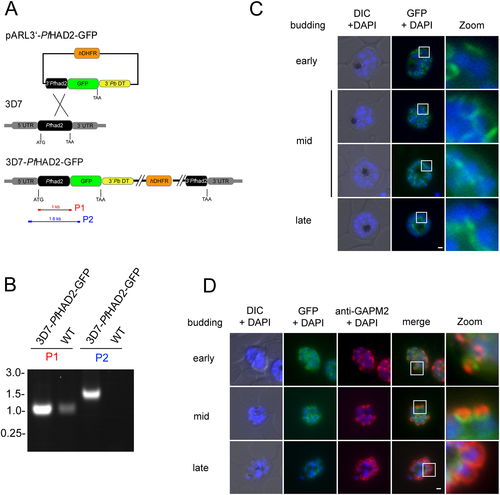
The Plasmodium falciparum HAD2 ortholog reveals identical subcellular localization to the basal complex during schizogony.
A. Schematic of the used integration strategy. The 3′ end of the endogenous Plasmodium HAD2 ORF was replaced with a GFP-tagged copy. Human DHFR (orange), GFP (chartreuse), P. berghei dhfr-ts 3′ UTR (yellow). Expected PCR products for diagnostic PCR (in B) are indicated (P1 and P2).
B. Successful 3′ replacement was verified by diagnostic PCR using gDNA from 3D7-PfHAD2-GFP parasites. Parental 3D7 gDNA was used as a control.
C. Live-cell microscopy of 3D7-PfHAD2-GFP parasites. Schizont stage parasites were incubated with DAPI (blue) to stain DNA. During early stages of Plasmodium budding PfHAD2 localizes to the periphery of the schizont to ring-like structures. With budding progression (early to mid) the PfHAD2 rings enlarge and engulf the divided nuclei. With conclusion of budding (late) the PfHAD2 signal localizes basal to the nuclei, posterior to its originating point in the periphery. Zoom = 5× of boxed areas.
D. Co-localization of PfHAD2 with the IMC marker protein GAPM2. PfHAD2 (green) localizes to the originating point of the nascent merozoites as indicated by anti-GAPM2 (red) co-staining. DNA was stained with DAPI (blue). PfHAD2 rings are associated with the GAPM2 signal during formation of daughter buds before resting at the basal end (late) of nascent merozoites. Zoom = 5× of boxed areas. Scale bar = 1 µm.
Discussion
Apicomplexan parasites have adapted several unique replication strategies allowing fast rounds of intracellular asexual proliferation (Francia and Striepen, 2014). Although different budding modes can give rise from 2 to 1000s of daughters, cytokinesis occurs concurrent with the final round of nuclear division. Regardless of the budding mode, the basal complex is pinching off the nascent daughter cells in the late steps of budding (Ferguson et al., 2008).
In order to reveal mechanistic detail we pursued the identification of new Toxoplasma basal complex proteins and utilized a Y2H system, taking MORN1 as bait for this constrictive compartment. Out of the 33 identified unique genes we only identified HAD2a and yeast clone MCIC-15 (TGME49_255880) to co-localize with MORN1. The latter gene encodes a hypothetical protein that is conserved across most Apicomplexa, localizes to both the basal and apical end, but we note that the splice version we tested is quite different from the annotated ORF, and therefore, this assignment is tentative (data not shown). Besides, these candidates we found multiple genes several times (e.g. succinate-Coenzyme A ligase with two hits and ribosomal proteins RPS20 and RPS27 each with four hits, neither of which appears to be biologically relevant hits for MORN1). Although these interaction partners are probably spurious as these proteins do not reside in the same compartment as MORN1, we interpret their multiple identifications as an indication to have accomplished a practical level of library saturation under the chosen restrictive conditions, at least for highly expressed proteins.
Our subsequent characterization of HAD2a identified the protein as a phosphatase, which phosphatase activity was strongly dependent on Mg2+. These features together with the DxDx(T/V) motif classifies HAD2a as a member of the diverse group of HAD phosphotransferases. Members of this group can be found in all three superkingdoms of life acting in various cellular processes and accepting either small or macromolecular substrates. Although the overall sequence identity is usually low, the enzymatic HAD signature motifs are the unifying feature (Seifried et al., 2013). Phylogenetic profiling revealed that HAD2a only shares a ~200 aa region, the HAD domain, with its paralogs in Toxoplasma and its orthologs in the superphylum Alveolata (Fig. S2). HAD2a shows a gene expression profile peaking during the M/C phase of the intracellular division cycle trailing the profile of MORN1 ((Behnke et al., 2010); www.toxodb.org), which is consistent with the hierarchy of these proteins in the division process. In contrast HAD2b mRNA expression is barely detectable throughout the cell cycle, yet the protein product is easily detectable by IFA and western blot, and its localization suggests a putative role in organizing the apical end of the cytoskeleton during cell division. We determined that HAD2c localized to the cytosol and the nucleus using an exogenously expressed fusion reporter (results not shown), whereas we have so far not pursued HAD2d. Taken together, this diverse sub-cellular localization together with the divergent cap regions and the unique N- and C-termini suggests a diverse substrate and function range for the HAD2 family members.
Our subcellular localization and functional studies of HAD2a in Toxoplasma as well as its orthologous localization in Plasmodium indicate that it is critical for assembly and subsequent contraction of the basal complex. The association of HAD2a with the alveolar skeleton and the presence of putative orthologs throughout the Alveolata could therefore suggest a conserved function of HAD2a in this superphylum. Although it remains to be proven whether HAD2a is protein phosphatase, it is tempting to speculate that MORN1, the basal complex scaffolding protein, is a potential critical substrate of the phosphatase because of their close association. To date, no phosphorylation of MORN1 is evident from the available phosphoproteome data (Nebl et al., 2011; Treeck et al., 2011) that highlight a potential targeting site for HAD2a. MORN1 contains 13 Ser and 15 Thr residues many of which are identified as putatively phosphorylated using phosphorylation site prediction software (Obenauer et al., 2003; Blom et al., 2004) (data not shown). Substrate identification of HAD2a is the focus of our current work.
Regardless of the nature of the substrate of HAD2a, its loss results in incomplete assembly of the basal complex. We postulated that either the contractile motor and/or its infrastructure is not recruited, or if it is, it is not functional. Our data demonstrate the defective assembly of IMC8 in the basal complex, but our data are inconclusive for the supposed contractile motor Centrin2 (Hu, 2008). If Centrin2 is indeed absent, it is possible that this is a secondary result of the absence of IMC8. Regardless of the exact hierarchy of basal complex assembly, the most logical conclusion is that the absence of Centrin2 from the basal complex explains the less contracted basal complex.
Contrary to the previously described phenotypic assessments of MORN1 knockout/knockdown parasites, we did not detect an impact on apicoplast segregation in had2aKO parasites (Fig. S7). Parasites lacking MORN1 are described to fail in apicoplast division, indicating that either MORN1 itself or the formed ring-like structure controls segregation of this organelle (Heaslip et al., 2010; Lorestani et al., 2010). In LoxP-HAD2a and had2aKO parasites, the apicoplast was in close proximity to the nucleus and each nucleus showed a single apicoplast signal, as judged by streptavidin labelling (Jelenska et al., 2001) (Fig. S7).
The presence of HAD2a at the basal complex of Toxoplasma and PfHAD2 at the basal complex of Plasmodium points to a conserved function for these phosphatases in apicomplexan cytokinesis. Although the mode of cell division executed by Toxoplasma (endodyogeny) and Plasmodium (schizogony) differs from each other, unifying processes of daughter cell formation are evident (Francia and Striepen, 2014). Our work shows that HAD2a and PfHAD2 are members of their respective basal complex from early on in the budding process (Figs 3A and 8C and D). Functional orthologs of the basal complex IMC proteins are absent in Plasmodium and support for a functional Centrin2 ortholog is missing, an orthologous MORN1 is present in the basal complex (Ferguson et al., 2008). In combination with the significant sequence divergence between TgHAD2a and PfHAD2, the most parsimonious conclusion is that the basis of basal complex assembly is conserved, but the composition and execution of contraction are divergent. Besides, the recruitment of a potential motor component/complex to the basal complex, TgHAD2a/PfHAD2 could also be directly involved in the regulation of basal complex constriction.
The presence of a HAD phosphatase in the basal complex suggests that basal complex assembly and activity may rely on a signalling network of reversible phosphorylation. While the critical role for kinases and phosphatases in cytokinesis of organisms like animals, plants or yeast is well established (Skop et al., 2004; Pollard and Wu, 2010; Balasubramanian et al., 2012) no regulatory proteins have so far been identified to directly control late cytokinetic events in Toxoplasma or other Apicomplexa. However, circumstantial evidence consistent with our previously proposed model of basal complex constriction control is compiling. Recently, it was shown that pharmacological inhibition of the Toxoplasma protein kinase MAPK-L1 disrupts proper daughter cell segregation (Sugi et al., 2015). This inhibition led to formation of multi-headed parasites similar as described for MORN1 knockout parasites (Heaslip et al., 2010; Lorestani et al., 2010). Future work needs to clarify if MAPK-L1 acts directly on the constriction of the basal complex or plays a role in its correct positioning. Hints toward the latter are provided by a MAPK-L1 temperature-sensitive mutant, in which the connection between the basal complex and the posterior end of the daughter cells is lost (Suvorova et al., 2015). Additional support for a critical role of phosphorylation in the basal complex is the presence of a 14-3-3 protein (Lorestani et al., 2012). Across eukarya, 14-3-3 proteins bind phosphorylated proteins (van Heusden, 2005; Obsilova et al., 2008) and typically they transduce phosphorylation states, or regulate phosphorylation states by blocking phosphatase access. Overall, these data indicate a critical role for phosphorylation and as such kinases and phosphatases in the biology of the basal complex.
Experimental procedures
Toxoplasma strains
All Toxoplasma parasites used are RH strain derivatives and were grown in confluent human foreskin fibroblast (HFF) cells (Roos et al., 1994). Parasite transfections and selections using 1 μM pyrimethamine, 20 μM chloramphenicol, or a combination of 25 mg ml−1 mycophenolic acid and 50 mg ml−1 xanthine were performed as described previously (Roos et al., 1994).
Toxoplasma plasmid constructs
All primer sequences are provided in Supplementary Table S2. All exogenous YFP or Myc2-tagging plasmids were based on replacing functional modules flanked by the restriction enzymes between brackets in the following plasmid: (PmeI)ptub-(BglII)YFP(AvrII)YFP(EcoRV)/sagCAT or dhfrDHFR-TS (Anderson-White et al., 2011). Insertional tagging of genomic loci was performed by gene-swap in RH ku80::diCre parasites (Andenmatten et al., 2012). For the gene-swap approach, the p5RT70 promoter sequence was replaced by a DNA fragment encoding 2500 bp upstream of the annotated had2a start ATG via the KpnI and EcoRI restriction sites. The respective gene coding sequence was placed between the AvrII and PacI sites, a fragment of 2500 bp after the annotated stop codon was cloned in the gene-swap plasmid using the SacI sites.
To generate recombinant His6 N-terminal fusion proteins, full-length HAD2a and HAD2b CDS sequence were PCR-amplified and cloned into the pAVA0421 plasmid by LIC (Alexandrov et al., 2004). The Asp to Ala point mutants were generated by site directed mutagenesis.
Endogenous Myc tagging of IMC8 was performed by cloning 1.8 kb of the genomic DNA encoding the 3′-end of the ORF into (PmeI)ptub-(AvrII)Myc3(−)3′-dhfr(NotI)/DHFR (unpublished) via PmeI/AvrII cloning sites. The resulting plasmid was linearized by NheI before transfection of LoxP-HAD2a parasites.
All YTH constructs were made using Gateway Technology (Invitrogen). In short, full-length MORN1 (primer pair AttB1-F-MORN1-ATG and AttB2-R-MORN1-fl), the N-terminal (AttB1-F-MORN1-ATG and AttB2-R-MORN1-M6, including the 6th MORN domain) or the C-terminal half (AttB1-F-MORN1-M13and AttB2-R-MORN1-fl, from the 13th MORN domain onward) of MORN1 were amplified in a two-step PCR with the first round primer pairs indicated between brackets and the second round with universal AttB adapter primers Att-B1-adopt and Att-B2-adopt to complete the AttB-sites. To clone full-length HAD2a and HAD2b YTH constructs we used first round primer pairs AttB1-F-HAD2a/AttB1-R-HAD2a and AttB1-F-HAD2b/AttB1-R-HAD2b respectively. The amplicons were cloned by BP recombination into the pDONR201 vector. Subsequently, the inserts were transferred into plasmids pGBK-Att or pGAD-Att vectors by LR recombination. Plasmids pGBK-Att and pGAD-Att were derived from pGBKT7 and pGADT7 plasmids (Clontech), respectively, and converted into Gateway compatible vectors by cloning the reading frame B cassette (Invitrogen) into the SmaI site of each plasmid.
Yeast two-hybrid screen
Saccharomyces cerevisiae strain AH109 (MATa, trp1-901, leu2-3, 112, ura3-52, lys2-181, his3-200, ade2-101, gal4-952, gal80-538, LYS2::GAL1uas-GAL1tata-HIS3, GAL2uas-GAL2tata-ADE2, URA3::MEL1uas-MEL1tata-lacZ) (Clontech) was used. Yeast was transformed using the LiC2H3O2 method with either 2.5 µg of bait plasmid mixed with 1.0 µg of a pGAD-‘GOI’-AD prey plasmid or the RH two-hybrid cDNA library (Fig. S1). The library was generated from random cycling tachyzoites. Oligo-dT primed polyA+ RNA was directionally cloned by EcoRI/XhoI digestion into plasmid pGBK-Att. The library complexity was 2.4 × 106 recombinants with an average insert size of 1.65 kb and an empty plasmid background of under 1%.
Transformed yeast were plated on SD/Trp−/Leu− plates and incubated at 30°C until appearance of colonies. Plates were then replica-plated to a quadruple-dropout selective medium (QDO) (SD/His−/Ade−/Trp−/Leu−). Growing colonies were patched onto new QDO plates, which were subsequently replica plated to QDO/X-α-Gal and QDO/3-aminotriazole (3-AT) at 5, 10, 25 and 50 mM. Colonies growing on QDO/X-α-Gal and at least QDO + 10 mM 3-AT plates were selected as positive clones. Prey plasmids were isolated from positive colonies grown overnight in 5 ml of QDO/Leu− medium at 30°C using published methods (Hoffman and Winston, 1987) and transformed into E. coli DH5α. Minipreps were screened by HindIII digestion and distinct clones sequenced from the 5′-end insertion site using the standard T7 primer. Sequence results were analysed by BLAST searching the Toxoplasma genome (www.toxodb.org). For clones of interest the library insert was sequenced using primer T7 (refer to Supplementary Data file for all collected sequences).
Because full-length MORN1 resulted in autoactivation, two MORN1 baits (N-terminal: start till MORN domain 6; C-terminal MORN domains 13–15; Gubbels et al., 2006) were used independently to screen the tachyzoite cDNA Y2H library (Fig. S1). Approximately 120 000 total yeast transformants were screened for YTH interactions. Forty four total cDNAs were isolated following these screens and subjected to DNA sequencing numbered with MNIC or MCIC prefixes representing MORN1 N- or C-terminal interacting clone respectively. Nine genes were chosen for secondary screening by YFP-tagging and fluorescent microscopy. Results are summarized in Table S1.
Sequence analysis
HAD2a-d sequences were aligned with ClustalW (standard settings), manually adjusted and coloured with BoxShade (50% identity and similarity settings). For phylogram construction, the extended HAD domain as shown in the alignment in Fig. 1C was BLASTP searched against the NCBI Nr database. Representative sequence fragments covering the spectrum of hits were picked and aligned by ClustalW (standard settings). The phylogram was assembled in ClustalW (standard settings). The 3D-Model was generated by model searches against structures deposited on Swiss-model (http://swissmodel.expasy.org) using automated mode (Arnold et al., 2006). The obtained coordinates were visualized using PyMOL (www.pymol.org).
Recombinant protein expression and polyclonal antisera generation
His6-HAD2a expression was induced for 4 h with 1 mM IPTG at 37°C in BL21-DE3-pLysS Escherichia coli grown in 2xYT medium. The protein was purified from inclusion bodies as follows: the E. coli pellet is resuspended in lysis buffer (20 mM Tris HCl pH8.0, 25% sucrose, 1 mM EDTA) and sonicated. An equal volume of wash buffer 1 (200 mM NaCl, 1% deoxycholic acid, 1% NP-40, 20 mM Tris pH 7.5, 2 mM EDTA) was added and then pelleted at 5000*g for 10 min at 4°C. The pellet was resuspended in wash buffer 2 (0.5% TX-100, 1 mM EDTA), followed by addition of an equal volume of wash buffer 1 and centrifugation (repeated a total of two times). The purified and pelleted inclusion bodies were then refolded and solubilized using 8 M urea in 50 mM Tris pH 7.6, 1 mM β-mercaptoethanol, 0.025% Tween 20 for 3 h by end-over-end rotation at 4°C. The supernatant was collected following centrifugation (1500*g for 20 min at 4°C) and dialysed for 8–16 h periods at 4°C subsequently against 5, 4, 3, 2, 1, 0 M urea in 50 mM Tris pH 7.6, 1 mM β-mercaptoethanol, 0.025% Tween 20, followed by a final 24 hr dialysis against 0 M urea. His6-HAD2b expression and bacterial lysis was identical to His6-HAD2a. An end-concentration of 10 mM imidazole was added to the cleared lysate and applied to pre-a equilibrated Talon column (Clontech). The column was washed subsequently with 10 and 20 mM imidazole in wash buffer and the protein eluted with the supplied elution buffer containing 150 mM imidazole. All purified proteins were snap frozen and stored at −80°C till further use.
Polyclonal antisera were generated by rabbit immunizations (Covance, Denver, PA). HAD2a antisera were affinity purified against His6-HAD2a recombinant protein using published methods (Gubbels et al., 2006).
Toxoplasma immunofluorescence microscopy
LoxP-HAD2a parasites were incubated with 50 nM rapamycin for ~16 h to induce Cre-recombination and fixed for immunofluorescence after ~24 h with methanol or 4% PFA (permeabilized with methanol) as described previously (Anderson-White et al., 2011). The following antibodies were used: rat α-IMC3 (1:1000; Anderson-White et al., 2011); mouse α-RNG1 (1:1000, Tran et al., 2010; kindly provided by Naomi Morrissette, UC Irvine); MAb α-cMyc (1:50, Santa Cruz Biotech); MAb α-Ty (1:10, Bastin et al., 1996), kindly provided by Markus Meissner, University of Glasgow, Chris de Graffenried, Brown University and Peter Bradley UCLA; rabbit α-CherryRFP (1:200 000, kindly provided by Iain Cheeseman, Whitehead Institute); rabbit α-GFP (1:1000, Torrey Pines Biolabs); mouse α-GFP (1:500, Roche Diagnostics); rabbit α-GAP45 (1:1000, Con Beckers University of North Carolina); mouse α-IMC1 (1:1000, Garry Ward, University of Vermont); mouse α-ISP1 (1:500, Peter Bradley, UCLA); Alexa594 labelled α-SAG1 (1:500, J.-F. Dubremetz, Université de Montpellier) and Streptavidin labelled Alexa 594 (1:1000, Invitrogen) in blocking solution. Secondary antibodies were conjugated to the Alexa Fluor dyes (Invitrogen) and DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI). Imaging was performed on a Zeiss Axiovert 200 M wide-field fluorescence microscope equipped with standard DAPI, FITC, YFP and TRTC filter sets, a α-Plan-Fluar 100x/1.45 NA oil objective or with a Zeiss Axio Imager Z2 with ApoTome.2. Images were analysed and processed using Volocity (Improvision/Perkin-Elmer), Zeiss ZEN and ImageJ software.
Western blot
Western blots were performed as previously reported (Gubbels et al., 2006). Briefly, LoxP-HAD2a and DiCre control parasites were harvested by needle-lysing host cells ~24 h after host cells were infected. For each sample, 15 × 106 parasites were loaded on a 12% NuPAGE Bis-Tris gel (Invitrogen) and proteins transferred to a PVDF membrane (BioRad). After blocking with a 6% milk solution, blots were probed with mouse anti-Ty, 1:500 (Peter Bradley, UCLA) or mouse anti-IMC1, 1:6000 ((Mann and Beckers, 2001); kindly shared by Gary Ward, University of Vermont). After incubation with goat anti-mouse HRP, 1:10000 (Molecular Probes) membranes were developed with chemiluminescent HRP substrate (Millipore) and exposed to X-ray film. The same membranes were stripped and re-probed with rabbit anti-HAD2a or rabbit anti-HAD2b, 1:5000, with subsequent decoration of goat anti-rabbit HRP, 1:3000 (Molecular Probes).
Parasite sorting (FACS)
Cre-recombination in LoxP-HAD2a parasites was induced for 16 h by addition of 50 nM rapamycin to parasites inoculated in HFF cells. After incubation, parasites were mechanically released by passing them through a 26G needle and filtered through a 3 µm polycarbonate filter. YFP− and YFP+ parasites were sorted using a Becton Dickinson FACSAria cytometer (BD Biosciences) and recovered in PBS containing 15% FBS. YFP was excited using a 488 nm Argon blue laser and detected with a 530/30 nm band pass filter. Parasites were gated on FSC/SSC. Parasites were washed with ED1 media before plaque assays were inoculated. For microscopy of YFP+ parasites four days after FACS, parasites were inoculated in Lab-Tek II Chambers (Thermo Fisher) confluent with HFF cells and kept under standard conditions until observed with a Zeiss Axiovert 200 M wide-field fluorescence microscope and α-Plan-Fluar 63x/1.40 NA oil objective.
Basal complex diameter measurement
To measure the basal complex diameter, we induced had2a knockout in LoxP-HAD2a parasites co-expressing mCherry-MORN1 with 50 nM rapamycin overnight. Parasites were fixed in host cells after ~24 h with 4% PFA and permeabilized with methanol. mCherry-MORN1 was stained with anti-mCherry antibody, and 3D-images of vacuoles harbouring 2–4 parasites were taken with a Zeiss Axio Imager Z2 with ApoTome.2. mCherry-MORN1 signal was measured in total projection of 3D-Images using ImageJ software.
Phosphatase activity assays
Recombinant HAD2aD262A and HAD2bD171A were purified in parallel with wild type proteins under identical conditions (refer to recombinant protein expression). Proteins were separated with 12% NuPage SDS-PAGE (Invitrogen) and stained with Coomassie Blue. Phosphatase activity was assessed by conversion of the phosphatase substrate p-NPP (Sigma), based on published methods (Takai and Mieskes, 1991; Zhuo et al., 1993). One microgram of recombinant protein (His6-HAD2a, His6-HAD2b and respective D- > A mutants) was incubated with 27 mM p-NPP in 50 mM HEPES (pH 7.5), 1 mM DTT for 4 h at RT and OD405 nm of triplicate reactions was measured in a M5 SpectraMax spectrophotometer (Molecular Devices). Reactions were performed in presence of either 5 mM MgCl2, 0.5 mM MnCl2 or a combination of both salts and background corrected values are depicted as percentage of control (in absence of cation).
Plasmodium nucleic acids and construct
To tag Plasmodium had2 with GFP via homologous recombination, we amplified 864 bp of the had2 ORF 3′end from Plasmodium 3D7 genomic DNA and cloned it into the NotI/AvrII restriction sites of the pARL-1a-GFP expressing plasmid releasing the crt promoter (Struck et al., 2005). Single cross-over integration was confirmed by PCR. All primers are listed in Table S2.
Plasmodium parasite strain and transfection
P. falciparum 3D7 parasites at asexual stages were cultured in human O+ erythrocytes as previously published (Trager and Jensen, 1976). Parasites were transfected at the ring stage with 100 µg of purified plasmid DNA as described previously (Fidock and Wellems, 1997). We selected for transfected parasites using 2.5–5 nM WR99210 (Jacobus Pharmaceuticals) and obtained parasites with single cross-over integration in the had2 locus by growing them in alternating cycles of WR99210 presence/absence.
Plasmodium microscopy
Images of unfixed Plasmodium schizonts expressing PfHAD2-GFP were captured using a Zeiss Axioskop 2 Plus microscope with a Hamamatsu camera (Model C4742-95, Zeiss axiovision). DNA was stained with 1 µg ml−1 DAPI (Roche). To co-localize PfHAD2-GFP with the IMC marker protein GAPM2, we fixed schizont stage parasites with 4% formaldehyde and 0,0075% glutaraldehyde as previously published (Tonkin et al., 2004). Parasites were permeabilized with 0.1% Triton X100 and blocked with 3% bovine serum albumin and then incubated with anti-GAPM2 antibody (1:500; Kono et al., 2012). DNA was stained with DAPI.
Acknowledgements
We thank Drs. Con Beckers, Peter Bradley, Iain Cheeseman, J.-F. Dubremetz, Chris de Graffenried, Markus Meissner, Naomi Morrissette and Gary Ward for generously sharing reagents and Patrick Auttisier and Bret Judson for technical assistance with this work. Jacobus Pharmaceuticals for WR99210. K.E. is supported by the Deutsche Forschungsgemeinschaft. This work was supported by National Institutes of Health grants R01AI081924 and U54AI057159 through a New England Regional Center of Excellence in Biodefense and Emerging Infectious Disease developmental grant.
Conflict of interest
The authors declare that they have no conflict of interest.