A randomized, multicenter, double-blinded parallel study to evaluate the safety and performance of zoledronate-coated versus uncoated dental implants in partially edentulous patients
Abstract
Objective
To evaluate patient safety, implants survival and implant stability of the bisphosphonate (zoledronate) as a coating on dental implants in patients requiring oral rehabilitation in the posterior maxilla.
Materials and Methods
In this multicenter, double-blind, randomized controlled study, 62 patients were randomized to receive either zoledronate-coated or uncoated control implants in the premolar or molar area of the maxilla, using a one stage-protocol. Due to dropouts and exclusion 49 patients completed the study. The implants were examined by resonance frequency analysis (RFA) using an implant stability quotient (ISQ) scale at the time of insertion, and at 8 weeks, and after 12 weeks prior to prosthetic restoration. Radiographs were taken prior to surgery, directly after insertion, and during the follow-up at 12 weeks, 6 months, and 1 year to analyze changes in marginal bone levels (MBL). Finally, all complications and adverse effects (AE) were observed and recorded.
Results
Out of 62 included patients, 49 patients completed the study. No AE were reported by patients receiving zoledronate-coated implants. There was no statistically significant difference between the zoledronate-coated or uncoated implant groups when comparing ISQ levels at insertion and after 12 weeks of healing, the mean of the ISQ values demonstrated a change of 4.64 (95% confidence interval: 15.46; 5.79, p = 0.43) between the two groups. At 8- and 12-weeks, ISQ values remained stable (range 62–70). Radiographic analysis showed no statistically significant difference in MBL between the two implant groups after 1 year of loading neither at the mesial side (p = 0.99) or the distal side (p = 0.97). MBL for coated implants were 0.57 mm at the mesial side and 0.46 mm at the distal side. For the uncoated implants, MBL was 0.48 mm at the mesial side and 0.47 mm at the distal side.
Conclusion
The zoledronate-coated dental implants are safe to use in a one-stage surgery protocol in patients requiring oral rehabilitation in the posterior maxilla, after 1 year of loading. There were no statically significant changes in implant stability and marginal bone levels measured by intraoral radiographs in comparison to uncoated control implants.
Summary Box
What is known
According to Abtahi and colleagues1 dental implants coated with the bisphosphonates (BPs) ibandronate and pamidronate gave the implants better Osseo integrative properties and were safe to use without any adverse effects. The aim of our multicenter, double-blind, randomized controlled study was to investigate if a more potent BP zoledronate was safe to use and if the same conclusions could be drawn by us as the Abtahi and colleagues,1 with respect to implants stability, and implant survival.
What this study adds
The use of potent BPs for local use as a coating on dental implants have been a controversial subject since their systemic use in patients treated with severe bone metabolic disorders or patients suffering from hypercalcemia due to bone metastatic disease is known to cause medication related osteonecrosis of the jaw. The other benefit of investigating the local delivery of the antiresorptive drug zoledronates enhanced osseointegrative properties dental implants might enable patients with compromised bone to benefit dental rehabilitation of edentulism. Two systematic review of the literature surrounding BPs locally delivered from Kellesarian and colleagues2 and by Stavropolus and colleagues,3 regarding the effect of antiresorptive drugs on implant therapy, confirmed the need of further investigation within this field.
1 INTRODUCTION
The use of bone-anchored implants for the functional rehabilitation of partially edentulous patients is nowadays considered a routine procedure with a high level of success.4, 5 Historically, challenges with respect to implant stability and survival have been addressed by surface modification as well as changes to the macro design. Implants with modified surfaces have demonstrated higher implant survival than those with machined surfaces.6 However, there is an unmet need in the clinical setting to improve the success rate of dental implant treatment in patients who have impaired healing capacity in compromised bone, such as osteoporosis or poorly controlled diabetes, and irridiated patients.7 These patients are difficult to reconstruct and rehabilitate in a predictable way.
The incorporation of bisphosphonates (BPs) into implant coatings is an innovative approach to modify healing after implant placement and to improve implant stability. BPs are antiresorptive drugs that have been used in modern medicine for treating osteoporosis, hypercalcemia, Paget's disease, and to control the spread of bone metastasis in malignant disorders.8 BPs increase bone density and thereby bone strength by directly targeting the osteoclasts.9, 10 Mechanistically, BPs block farnesyl pyrophosphate synthase (FPPS), an essential enzyme of the mevalonate pathway that is needed for the proper functioning of osteoclasts. By blocking FPPS, BPs induce apoptosis of osteoclasts, thereby inhibiting bone resorption.11, 12 However, BPs also stimulate osteoblasts and control osteoblast metabolism by enhancing their overall anabolic effect.13-15 The dual effects of BPs, both in catabolic and anabolic processes of bone turnover, are dose-dependent.11, 16, 17
Several BPs are used clinically in the medical field, and among these, zoledronate is the most potent and has the highest affinity for FPPS and shows the highest accumulation in bone.8 Zoledronate is typically administered by intravenous (iv) injection and is used to manage patients with advanced diseases who are at risk of pathological fractures, cancer-induced bone loss, or hypercalcemia.18 Nevertheless, the systemic administration, iv or oral, of zoledronate and other BPs poses challenges and has safety risks, such as a potential risk for developing medication-related osteonecrosis of the jaw (MRONJ).19, 20 BPs are poorly absorbed from the gastrointestinal tract and have a high affinity for hydroxyapatite crystals in bone. About half of the absorbed drug accumulates in the bone matrix and the rest is excreted in the urine. As BPs are resistant to hydrolysis under physiological conditions, they have an extremely long half-life.19
It was previously shown that BPs incorporated into the coating of a dental implant could modify osteoblast and osteoclast activity at the target bone, and at the right dose, enhance osseointegration.14, 21, 22 Several preclinical and clinical studies have examined the biological and clinical performance of BP-coated implants. Karlsson and colleagues,7 used an in vivo model of rat tibia, and showed that titanium implants coated with alendronate could release this BP in the vicinity of the implant, within 500 μm, for 8 weeks. Andersson and colleagues23 compared the relative stability of two BP-coated implants placed in rat tibia and found that the coating with zoledronate resulted in stronger implant fixation in comparison to pamidronate; in each case, the chosen BP was fixated with cross-linked fibrinogen. Experimental and clinical data demonstrated that the local release of BPs from titanium dental implant coatings could increase the overall amount of bone around the implant by reducing bone resorption and leading to the formation of newly woven bone.7, 21 BP-coated implants has also presented higher removal torque values.24
As concerns have been expressed by the dental community about the potential of BPs to induce MRONJ,25 the primary outcome of the study has been to evaluate the safety of zoledronate-coated implants, given that zoledronate is the most potent BP commercially available. The secondary outcome was to compare early changes in implant stability with the zoledronate-coated test implants and uncoated control implants.
The hypothesis was that early stability would be improved in the coated implant group versus the uncoated implant group as determined by change in implant stability quotient (ISQ) values. Another hypothesis was that there would be less marginal bone loss (MBL) in the coated implant group versus the uncoated implant group after 1 year of function.
2 MATERIALS AND METHODS
The study was designed as multicenter, double-blind, parallel, randomized controlled study, and registered at the Swedish Medicines Agency and conducted in compliance with ISO 14155:2011 (EN), the Medical Device Directive (MDD), and independent ethics committee of the University of Gothenburg (DNR117-17). The study was conducted in respect of the Declaration of Helsinki.
2.1 Inclusion and exclusion criteria
A total of 62 patients were initially included in the study conducted at three different clinics in Sweden (the Department of Oral and Maxillofacial Surgery at Näl Regional Hospital in Trollhättan, the Holmgatans Tandläkarmottagning private practice in Falun Sweden, and the Dingletandläkarna, private practice, Dingle, Sweden). Patients requiring dental implant therapy were recruited from incoming referrals from dental practitioners in the neighboring regions.
Inclusion and exclusion criteria were following:
- Male or female subjects aged >18 years.
- Subjects should be willing to take part, able to understand the information given to them, and give written consent.
- Subject diagnosed with partial edentulism, in need of at least one dental implant in the posterior upper jaw, premolars to the first molar region.
- Suspicion of immune compromission or known medication with immunosuppressant.
- Current participation or participation within the last 6 months in another clinical investigation.
- Known sensitivity/allergies to any of the test materials or any of their ingredients, such as bisphosphonate, titanium, or human fibrinogen.
- Significant current or past medical history of hepatic, renal, cardiac, pulmonary, digestive, hematological, neurological, or psychiatric disease, hypercalcemia, previous or ongoing malignancy in the head and neck region, or uncontrolled diabetes type I.
- Pregnant and lactating females or those actively seeking to become pregnant in the next 3 months.
- Previous (last 5 years) or ongoing bisphosphonate treatment.
- Significant resorption of the alveolar process prior to implant insertion requiring bone grafting.
- Subject with extraction(s) performed in the position of implant placement within the last 2 months.
- Subject with need of >6 implants or a full bridge.
- The final prosthetic construction in need of support from neighboring teeth.
- Known drug or alcohol abuse.
Patients with diabetes type I were excluded since it in the opinion of the investigator could compromise the safety of the subject or affect the outcome of the investigation.
Smoking and moderate alcohol consumption were allowed to capture a broader spectrum of the population receiving dental implant therapy.
2.2 Randomization and implants
The patients were randomized to either the test implant (zoledronate-coated) or control implant (uncoated) group using a double-blinded computer-based randomization procedure.
Prior to study start, a treatment allocation according to the randomization list provided by Statistiska Konsultgruppen (Göteborg, Sweden) was performed.
Statistiska Konsultgruppen also provided treatment code envelopes. The code envelopes were sent to each participating site before study start.
Subjects were randomly assigned to receive either coated or uncoated implants according to a 1:1 ratio. A balanced block design was utilized, where the sequences and sizes of blocks could also be randomized. This was done to maintain a balance in subject numbers across all centers in the investigation at any one time, provided recruitment rates were similar. Once generated, the central randomization schedule was kept blinded for the study monitor, investigators and site personnel, and data management staff. After unblinding the study to analyze the data, the monitor, investigator, and site personnel, were still blinded regarding the treatment allocation.
2.3 Coating of implants
AddBIO One ExHex dental implants, a replica of Brånemark MkIV implants, 4.0 mm in diameter and 10 mm in length, were manufactured, blasted, and thoroughly cleaned prior to coating on a moderately rough surface according to the manufacturer. All processes were performed at ELOS Medtech Timmersdala (Timmersdala, Sweden). In a dedicated class 7 cleanroom (EN ISO 14644-1:2015), 130 implants were coated with a fibrinogen multilayer incorporating zoledronic acid in a process modified from Tengvall and colleagues.26 The coating procedure was performed by a validated process using a custom-built coating robot (AddBIO AB, Linköping, Sweden). The implants were incubated first in 0.5 mg/mL buffered medical-grade fibrinogen (RiaSTAP®; CSL Behring, Germany) for 10 min at 50°C and then in 0.2 mg/mL buffered zoledronic acid (active pharmaceutical substance, zoledronic acid monohydrate, CAS 165800-06-6, POLPHARMA S.A. Pharmaceutical Works, Starogard Gdański, Poland) for 10 min. After drying, the implants were single packed in titanium tubes with screw on lids, a sterile barrier Tyvek pouch, and a moisture barrier pouch before placement in cardboard boxes. The implants were sterilized with γ-irradiation at 17–25 kGy (Synergy Health, Ede, the Netherlands). Batch control analysis showed: bioburden of <4 CFU (Coloni forming unit) per implant (n = 3), fibrinogen amount of 4.2 μg/implant (n = 3), and zoledronic acid amount of 0.7 μg/implant (n = 8) (BioClin Laboratories, Althone, Ireland). The zoledronate-coated implants and uncoated implants were visually indistinguishable.
2.4 Surgical procedures for dental implant placement
2.4.1 Local anesthesia and premedication
The operation was performed using a one-stage protocol, under local anesthesia, xylocaine/adrenaline 2%. One hour prior to surgery, the patients received 2 g amoxicillin (Amimox®; or Clindamycin® 600 mg in case of allergy) and 1 g paracetamol. A crestal incision was made and the mucoperiosteal flap was raised in the posterior maxilla. The implant site was prepared with sequential drilling for an implant of 4 mm diameter and 10 mm length according to the clinical protocol for the Nobel-Brånemark Mark IV system (Nobel BioCare AG, Zurich, Switzerland). Thereafter, a healing abutment was applied and the mucoperiosteal flap was sutured. Intraoral baseline radiographs, using a parallel technique, were taken. All patients were provided with post-operative instructions, including post-operative analgesic, and rinsing with chlorhexidine solution 1 mg/mL for 7 days. All patients were recalled after 14 days for suture removal and a clinical follow-up and evaluation of healing.
2.5 Resonance frequency analysis measurements and implant stability quotient
Resonance frequency analysis (RFA) (Ostell Mentor; Integration Diagnostics, Gothenburg, Sweden) was used to measure ISQ at implant insertion, 14 days, 8 weeks, and 12 weeks prior to prosthetic restoration. The measurement was conducted in the mesio-distal and buccolingual directions for each implant. RFA measures the rigidity of the bone-implant contact. This method has been used extensively to evaluate implant stability,27, 28 and a clinically relevant scale has been developed. The ISQ scale range is between 1 and 100, whereby values between 55 and 85 correspond to an acceptable level of stability from a clinical standpoint.29, 30
2.6 Radiographic evaluation
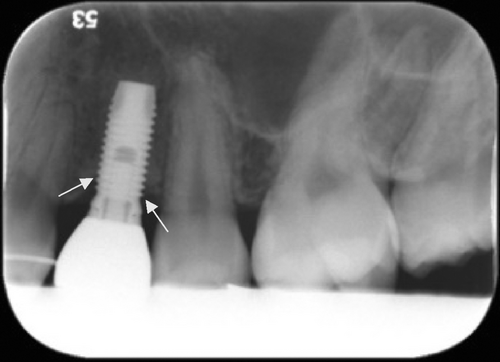
Radiographic evaluation was performed by digital intraoral periapical radiographs at baseline prior to insertion, directly after insertion, and after 8 weeks, 6 months, and 1 year of follow-up. A single investigator (RAM), who was blinded to the patient data, measured the level of marginal bone in relation to a defined radiographical edge on the implants at three different times and a mean was calculated. The distance calculated was from the reference point to the first implant-bone contact (see Figures 1 and 2). The level of marginal bone was defined as the distance from the fixture/abutment junction, where the bone level was marked at the mesial and distal aspect of each implant.31 Changes in marginal bone height were calculated as the difference between each thread and expressed in millimeter. The software used for the measurements was Planmeca Romexis® Dental Software version 5 and the screen used was the standard 24-inch Dell® screen.
2.7 Statistical analysis
For the comparison between the test and control groups, Fisher's nonparametric permutation test was used for continues variables, Mantel-Haenszel chi-square test for ordered categorical variables, chi-square test for non-ordered categorical variables, and Fisher's exact test for dichotomous variables. All significance tests were two-sided and performed at the 5% significance level.
2.8 Sample size calculation
The sample size calculation was based on the results of the study by Abtahi and co-workers,21 whereby the standard deviation (SD) for the change in ISQ values from insertion to 6 months was 5.3 and 5.4 in the test group and control group, respectively. Fisher's nonparametric permutation test with 90% power was used to calculate the required sample size needed to observe a clinical difference in the change of the five highest ISQ values from insertion to Week 12 between the test and control implant groups. Based on this, a minimum of 28 evaluable participants were needed per group assuming an SD for a change in ISQ of 5.6 and a significance level of 0.05. At least 31 participants were needed in the test and control group to compensate for a 10% dropout rate (ie, 62 participants in total).
2.9 Safety analysis
All adverse effects (AE) are coded according to the MedDRA dictionary (version 20.1) and tabulated by preferred term and system organ class codes.
- Leads to a death or permanent impairment to a body structure or body function.
- Leads to a serious deterioration in the health of the subject that either resulted in a life-threatening illness or injury.
- A permanent impairment of a body structure or a body function.
- In-patient hospitalization or prolonged hospitalization.
- Medical or surgical intervention to prevent life-threatening illness or permanent impairment to a body structure or a body function.
- Leads to fetal distress, fetal death or a congenital abnormality or birth defect.
Device deficiencies (DD): inadequacy of an investigational medical device related to its identity, quality, quantity, reliability, safety, or performance. DD include malfunctions, misuse, or user errors, labeling errors that did or could have led to an adverse event or adverse device effect irrespective of whether the device was used or not.
3 RESULTS
Patient demographics and characteristics at baseline are presented in Table 1. Out of 62 patients included in the study, 49 patients completed the study. Twelve patients were excluded due to failure to show up for recalls and one patient was excluded because the study protocol was not followed when loading the implant. A total of eight patients were reported as smokers and these were evenly distributed between the two groups. The alcohol consumption was minor to moderate in both groups.
| Total (n = 49) | Zollid One ExHex (coated) (n = 26) | One ExHex (uncoated) (n = 23) | p-Value | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 59.0 (13.9) | 57.2 (14.5) | 60.9 (13.3) | 0.37 |
| Median (min; max) | 59.0 (18.0; 83.0) | 58.5 (18.0; 83.0) | 60.0 (27.0; 79.0) | – |
| Sex | ||||
| Male | 24 (49.0%) | 13 (50.0%) | 11 (47.8%) | |
| Female | 25 (51.0%) | 13 (50.0%) | 12 (52.2%) | 1.00 |
| Implant site distribution | ||||
| Maxilla—16 | 3 (6.1%) | 0 (0.0%) | 3 (13.0%) | |
| Maxilla—15 | 9 (18.4%) | 4 (15.4%) | 5 (21.7%) | |
| Maxilla—14 | 10 (20.4%) | 5 (19.2%) | 5 (21.7%) | |
| Maxilla—24 | 9 (18.4%) | 6 (23.1%) | 3 (13.0%) | |
| Maxilla—25 | 8 (16.3%) | 5 (19.2%) | 3 (13.0%) | |
| Maxilla—26 | 10 (20.4%) | 6 (23.1%) | 4 (17.4%) | 0.44 |
| Alcohol status | ||||
| Never | 12 (24.5%) | 5 (19.2%) | 7 (30.4%) | |
| Monthly | 17 (34.7%) | 10 (38.5%) | 7 (30.4%) | |
| Weekly | 20 (40.8%) | 11 (42.3%) | 9 (39.1%) | 0.53 |
| Smoking status | ||||
| Never | 37 (75.5%) | 18 (69.2%) | 19 (82.6%) | |
| Former | 4 (8.2%) | 2 (7.7%) | 2 (8.7%) | |
| Current | 8 (16.3%) | 6 (23.1%) | 2 (8.7%) | 0.20 |
- Abbreviation: SD, standard deviation.
A clinical visual inspection and evaluation solely, by the operating surgeons showed that there were no signs of red and swollen soft tissue around the implants at 8 weeks, 12 weeks, or 12 months in any of the two groups. No serious AE were reported during the total duration of the study. Two implants were lost during the 12-month follow-up period, one in the test group due to device failure, during the prosthodontic procedure. The healing abutment was applied with excessive force and the implant was detourqued prior to the prosthetic restoration. One implant in the control group was removed due to lack of osseointegration after 12 weeks. Both events can be considered as early failures.
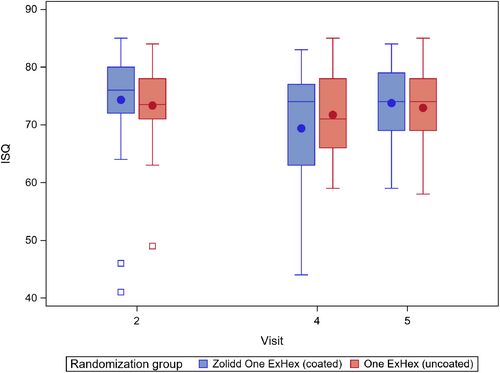
The ISQ analysis showed that there was no significant difference in stability from baseline to 12 weeks between coated and uncoated implant groups (Table 2). At 12 weeks, the mean of the ISQ values demonstrated a mean change of 4.64 (95% confidence interval: 15.46; 5.79, p = 0.43) between the two groups. At 8- and 12-weeks, ISQ values generally remained stable (ranging from approximately 62–70 units). These values indicate that both the control group and test group achieved optimal primary stability after implant placement (see Figure 3).
| Zollid One ExHex (coated) | One ExHex (uncoated) | |||
|---|---|---|---|---|
| Measure | All implants | All implants | Difference | p-Value |
| Mean (SD) | Mean (SD) | Mean | ||
| Median (min; max) | Median (min; max) | |||
| # Implants= | # Implants= | 95% (CI) | ||
| ISQ (all implants) at baseline | 75.2 (8.1) 78.0 (41.0; 85.0) # implants = 57 |
71.3 (12.6) 74.0 (0.0; 84.0) # implants = 49 |
3.6 (2.1) (−0.5; 7.8) |
0.086 |
| ISQ (all implants) at 2 weeks follow-up | 69.9 (14.3) 75.0 (0.0; 83.0) # implants = 54 |
67.6 (18.8) 72.0 (0.0; 85.0) # implants = 48 |
1.2 (3.6) (−6.1; 8.4) |
0.74 |
| Change in ISQ from Week 2 to Week 8 (all implants) | −5.0 (13.8) −1.0 (−71.0; 8.0) # implants = 54 |
−3.5 (17.1) −2.0 (−75.0; 26.0) # implants = 48 |
−1.8 (3.3) (−8.4; 4.9) |
0.59 |
| ISQ (all implants) at 8 weeks after surgery | 71.9 (12.8) 75.0 (0.0; 84.0) # implants = 54 |
69.1 (19.4) 74.5 (0.0; 85.0) # implants = 46 |
2.6 (3.4) (−4.4; 9.5) |
0.46 |
| Change in ISQ from Week 2 to Week 12 (all implants) | −3.0 (14.2) −2.0 (−71.0; 33.0) # implants = 54 |
−2.7 (17.0) −0.5 (−75.0; 23.0) # implants = 46 |
−0.4 (3.1) (−6.6; 5.9) |
0.91 |
- Abbreviations: CI, confidence interval; ISQ, implant stability quotient; SD, standard deviation.
There were no statistically significant differences in MBL between the coated and uncoated implant groups after 1 year of loading. Radiographic analysis showed no statistically significant difference in MBL between the coated and uncoated implant groups after 1 year of loading, neither at the mesial side (p = 0.99) nor the distal side (p = 0.97). MBL for coated implants were 0.57 mm at the mesial side and 0.46 mm at the distal side. For the uncoated implants MBL was 0.48 mm at the mesial side and 0.47 at the distal side (Table 3.).
| Zolidd One ExHex (coated) (n = 32) | One ExHex (uncoated) (n = 30) | p-Value | Difference between groups, mean (95% CI) | |
|---|---|---|---|---|
| From baseline to after 1 year of loading (mesial) | −0.467 (0.952) −0.6 (−1.8; 2) n = 24 |
−0.477 (1.054) 0 (−2.4; 3) n = 26 |
0.99 | 0.010 (−0.547; 0.560) |
| From baseline to after 1 year of loading (distal) | −0.458 (0.870) −0.45 (−1.8; 2) n = 24 |
−0.473 (0.973) −0.6 (−2.4; 3) n = 26 |
0.97 | 0.015 (−0.503; 0.523) |
- Abbreviation: CI, confidence interval.
4 DISCUSSION
The local administration of BPs at the bone-implant interface has the potential to optimize implant treatment by enhancing osseointegration in patients with compromised bone quality, that is, osteoporosis, uncontrolled diabetes, and bone metabolic disorders. In order to establish the safety and functionality of this novel treatment protocol, it was initially applied in a healthy patient group. In the present randomized, multicenter, double-blind parallel study, we evaluated the safety and performance of zoledronate-coated versus uncoated dental implants in partially edentulous patients. There were no observations of any serious AE and no evidence of MRONJ or BP-related risks with the zoledronate-coated implants. There were no reports of allergy-related AE or other hypersensitivities to the coating components. Moreover, the zoledronate-coated implants led to similar ISQ values as uncoated control implants, at 12 weeks suggesting favorable primary stability is achieved.
Bone quantity, bone quality, and surgical technique are major factors influencing the primary stability of dental implants.30 In the present study, we investigated implant placement in the posterior maxilla, where bone quality is poor. The higher quantity of cancellous bone in the posterior maxilla, compared with the mandible, makes it challenging to achieve implant stability and elevates the risk of implant failure.32 The data showed that ISQ values generally remained constant, and we found that primary stability was initially high and remained consistently high up to 12-weeks (primary efficacy outcome, ranging from approximately 62–70 units) in both groups. There were no statistically significant differences between ISQ values at Week 8 or 12 compared to Day 1. ISQ values >65 indicate favorable implant stability,33 and an average value of ~70 ISQ units has been reported for dental implants over time. When initial stability is low, ISQ values may increase over time as mechanical stability is reinforced by bone remodeling However, when initial stability is high (ISQ ≥70), as observed in the present study, the values tend to plateau and do not increase further.34 In this study, there was no statistically significant difference in MBL, obtained via radiographic measurements, between the zoledronate-coated and uncoated implants after 1 year of loading. This is consistent with the findings of Abtahi and colleagues, who showed no statistically significant differences in MBL between prototype zoledronate-coated implants versus uncoated control implants.34
Due to dropouts, the number of patients available for analysis was reduced and this affected the statistical analysis. Although the present clinical study demonstrated high implant survival and ISQ values in combination with stable MBL, there was no statistical significance between the zoledronate-coated and uncoated control implants. The observed variability in baseline ISQ values also made it difficult to detect changes between the groups and could be attributed in part to differences in the skill level of participating surgeons. An interesting observation was that in some patients who received zoledronate-coated implants at one of the investigating clinics, we observed an initial loss of marginal bone height that was regained after 1 year (see Figure 4). Unfortunately, there were few patients, and the results were not statistically significant. Further, a relatively short healing period, 12 weeks, was evaluated in the current work. This contrasts with the study by Abtahi and colleagues, which used implants coated with pamidronate and alendronate, BPs that are less potent than zoledronate, and incorporated a 6-month healing period before evaluation.21 Another potential factor could be related to the zoledronate coating, for example, the amount of zoledronate released from the implants and the resulting biological effects.7 It is possible to speculate whether the present study set-up fully capitalized on the zoledronate effect and consider how future studies could be designed to optimize the findings.
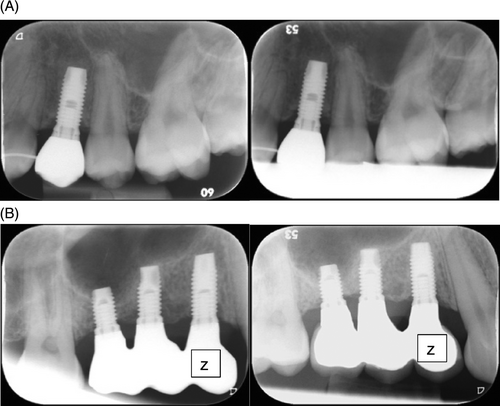
Clinically, zoledronate has the highest affinity for hydroxyapatite among BPs, with applications in the fields of orthopedics and oncology.10 However, it also has a documented risk profile, including MRONJ. The potential risk of MRONJ is a concern among the dental community regarding the use of BPs, including zoledronate, in procedures such as extractions, conventional dental treatment, and reconstructive bone-anchored implant treatment. In our investigation, both the zoledronate-coated and uncoated control implants were associated with few AE and demonstrated biocompatibility and safety for their intended use. Furthermore, there were no signs of MRONJ at 1-year post-surgery and no AE associated with inflammation or necrosis. The only AE that were judged as related to treatment were implant loss (ie, device failure) in one case, which was due to a loose healing abutment. Taken together, our findings suggest that coated Zollid One ExHex dental implants containing zoledronate for local release profile and did not show a risk of developing MRONJ after 1 year of function.
There is a further need to characterize zoledronate-coated dental implants also in an in vivo setting in order to define the exact biological response taking place. This is important because an imbalance of the immune system could have a pivotal role in the pathophysiology of perimplant disease or the foreign body reaction.35, 36 The immune system can activate osteoclasts, which originate from fusing macrophages, and cause perimplant disease in either a bacteria-driven or aseptic fashion. The major question of interest is if zoledronate can inhibit osteoclasts, delay the progression of perimplant disease, and thereby improve healing and enhance osseointegration after dental implant treatment. Further research is also needed to better understand the benefits and risks of BPs within the dental field, including repeated and long-term safety studies, and dose-optimization studies for the local application of zoledronate and other BPs.
Even though the data did not demonstrate a distinct clinical advantage of zoledronate-coated implants in terms of improved ISQ or MBL values, it is plausible to hypothesize that a zoledronate coating might improve the long-term prognosis of the implants and increase their resistance to perimplant disease and subsequently implant-loss.
5 CONCLUSION
The present clinical study demonstrated no AE, high implant survival and stable marginal bone levels with zoledronate-coated implants, and no significant differences versus uncoated controls. There is a need to characterize zoledronate-coated dental implants in vivo through to further understand the dynamics of the tissue healing. Additionally, further controlled trials are necessary to assess longer-term safety and optimize the dosage with these implants.
AUTHOR CONTRIBUTIONS
All authors have made a substantial contribution to the concept or design of the article and Reza A. Mokhtari, Morgan Olsson, Per-Olov Östman, and Christer Dahlin have provided the acquisition and collection of the data. Reza A. Mokhtari and Christer Dahlin have been analyzing and interpreted the data for the article. Christer Dahlin revised the content critically. All authors approved the final version to be published.
FUNDING INFORMATION
Funding for this study was provided by Nu-Sjukvården, Trollhättan, the Västra Götaland regional council for research and development, Trollhättan, Sweden and the implants were provided by AddBIO AB, Linköping, Sweden.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




