Heterozygous missense variants of SPTBN2 are a frequent cause of congenital cerebellar ataxia
Funding information Italian Ministry of Health (Ricerca Finalizzata), Grant/Award Number: NET-2013-02356160 to E.B
Abstract
Heterozygous missense variants in the SPTBN2 gene, encoding the non-erythrocytic beta spectrin 2 subunit (beta-III spectrin), have been identified in autosomal dominant spinocerebellar ataxia type 5 (SCA5), a rare adult-onset neurodegenerative disorder characterized by progressive cerebellar ataxia, whereas homozygous loss of function variants in SPTBN2 have been associated with early onset cerebellar ataxia and global developmental delay (SCAR14). Recently, heterozygous SPTBN2 missense variants have been identified in a few patients with an early-onset ataxic phenotype. We report five patients with non-progressive congenital ataxia and psychomotor delay, 4/5 harboring novel heterozygous missense variants in SPTBN2 and one patient with compound heterozygous SPTBN2 variants. With an overall prevalence of 5% in our cohort of unrelated patients screened by targeted next-generation sequencing (NGS) for congenital or early-onset cerebellar ataxia, this study indicates that both dominant and recessive mutations of SPTBN2 together with CACNA1A and ITPR1, are a frequent cause of early-onset/congenital non-progressive ataxia and that their screening should be implemented in this subgroup of disorders.
1 INTRODUCTION
Congenital ataxias account for about 10% of non-progressive infantile encephalopathies and are characterized by severe neonatal hypotonia and developmental delay, followed by cerebellar ataxia in the first years of life. They are generally classified into pure cerebellar and syndromic forms and their inheritance can be autosomal recessive autosomal dominant or X-linked recessive (reviewed by1, 2). More than 20 genes are known to date (see Table S1). Homozygous loss of function mutations of SPTBN2 have been identified in four families with early-onset cerebellar ataxia associated with cognitive impairment, eye movements abnormalities and variable additional neurological signs, defined as autosomal recessive spinocerebellar ataxia 14 (SCAR14; MIM#615386).3-6 Heterozygous missense variants of SPTBN2 have been initially associated to autosomal dominant spinocerebellar ataxia 5 (SCA5; MIM#600224) characterized by a slowly progressive cerebellar syndrome with downbeat nystagmus and tremor, beginning in the third decade with a tendency toward anticipation in later generations.7-11 The identification of three unrelated cases of early-onset cerebellar ataxia and developmental delay carrying the same de novo pathogenic variant (p.R480W) in SPTBN2 has challenged the previous SCA5/SCAR14 genotype-phenotype correlation.12-15 We used targeted next-generation sequencing (NGS) in 100 unrelated patients with non-progressive congenital or early onset ataxia associated with cerebellar atrophy.
2 MATERIALS AND METHODS
2.1 Clinical reports
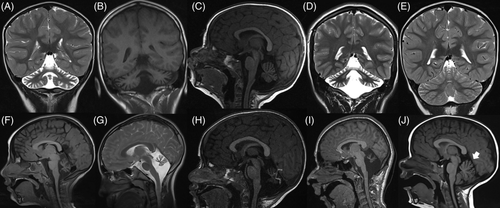
The clinical and neuroradiological features of the five probands (Figure 1, Table 1 and Data S1): congenital onset (within the first month of life), nonprogressive cerebellar symptoms (nystagmus, ataxia, hypotonia) and developmental delay, are almost constant findings. For detailed neuropsychological profiling of patients 1 and 5 (see Data S1 and Table S2).
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sex, age | F, 8 years | M, 18 years | M, 5 years | F, 18 years | F, 9 years |
| SPTBN2 variant | c.1309C > T | c.1310G > A | c.479 T > G | c.185C > T | c.888 T > C; c.6267G > A; |
| Protein | p.R437W | p.R437Q | p.F160C | p.T62I | p.Y272H; p.W2065* |
| Domain | 2nd SPEC | 2nd SPEC | ABD | ABD | 17th SPEC, PH |
| Age of onset | 10 months | 5 months | 5 months | 8 months | 12 months |
| First sign/symptom at onset | Psychomotor delay | Hypotonia | Psychomotor delay and strabismus | Psychomotor delay Microcephaly | Motor delay |
| Sitting unsupported | 1 year | 10 months | 13 months | 1 year | 8 months |
| Walk (ataxic) unassisted | 5 years | 2 years | Not acquired | 10 years | 2 years 4 months |
| First words | 2 years 6 months | 1 year | Not acquired | 3 years | 1 year 6 months |
| Ocular anomalies | − | Nystagmus (horizontal) | Strabismus | Nystagmus (horizontal and vertical) | − |
| Congenital hypotonia | + | + | + | + | + (mild) |
| Pyramidal signs | − | + (increased OTR) | − | − | − |
| Tremor | − | + (intention tremor) | − | − | − |
| Dystonia | − | − | − | − | − |
| Facial myokymia | − | − | − | − | − |
| Bulbar dysfunction | − | − | − | − | − |
| Cognitive delay | + | + | + | + | − |
| Behavioral problems | − | − | − | − | − |
| Additional findings | − | − | − | Mild bradykinesia | − |
| Cerebellar atrophy | + | + | + | + | + (mild) |
| Progression of atrophy | NA | + | + | NA | NA |
| Additional MRI findings | − | − | − | − | − |
| Course | Non-progressive | Non-progressive | Non-progressive | Non-progressive | Non-progressive |
- Abbreviations: ABD, actin-binding domain; n.d, not determined in the father, absent in the mother; OTR, osteotendineous reflexes; PH, Pleckstrin homology domain; SPEC, spectrin repeats.
2.2 NGS and bioinformatic analysis
All samples (from 100 index patients: 87 sporadic cases, eight probands with autosomal dominant, four with autosomal recessive and one with X-linked inheritance) were included in a NGS panel of genes whose mutations are causative of various forms of cerebellar ataxias (Table S3), including all known SCA genes. Genomic DNA was extracted from peripheral blood of the patients and their parents. Informed consent was obtained from all participating subjects according to the Declaration of Helsinki. The panel was designed using Nextera technology on a MiSeq platform (Illumina, San Diego, California), following the manufacturer's protocol, with expected coverage of 99% of the targeted genomic regions. Mapping of sequences against the hg19 reference genome was performed by Bowtie2. Bioinformatic tools HaplotypeCaller (GATK ver. 4.3) and ANNOVAR were used to call and annotate the variants, respectively. Subsequent filtering allowed to exclude intronic variants, synonymous variants not affecting splicing, and variants with frequency > 1% in human variation databases. Segregation was verified by Sanger sequencing in the families. Accession numbers are as follows: human SPTBN2 mRNA: NM_006946; human SPTBN2 protein: NP_008877. The pathogenicity of the identified missense variant was investigated using PolyPhen-2, SIFT, Mutation Assessor, and CADD, while conservation of the affected residue was assessed by ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). We filtered rare SPTBN2 variants in 15/100 ataxic patients (a list of all SPTBN2 variants found in our screening is available as Table S4); only one-third (5/15) of the variants were confirmed to be pathogenic (highly conserved, predicted to be damaging by in silico tools and molecular modeling), after segregation analysis and careful clinical evaluation of carrier parents, by two experienced pediatric neurologists.
2.3 Modeling
The p.T62I and p.F160C mutations were mapped on the PDB structure 6ANU (cryo-electron microscopy structure of F-actin complexed with spectrin beta chain). The p.Y272H mutation was mapped on the conformer 4 of the PDB structure 1WYQ (NMR structure of the second CH domain of human spectrin beta chain). To model the p.R437W and p.R437Q mutations, the homology model of SPTBN2 spectrin repeats 1 to 3 was built employing the following procedure. The amino acid residues 305 to 638 of SPTBN2 and the residues of the ROD domain of alpha-actinin from the PDB entry 1HCI (used as the template structure), were aligned (the two sequences share 33% amino acid identity; Data S1). All side chain atoms in the template structure were deleted, the amino acids were renamed and renumbered to the corresponding human SPTBN2 residues according to the above pairwise sequence alignment, and the modified PDB structure file was parsed to SIDEpro16 for side chain reconstruction.
3 RESULTS
3.1 Genetic data
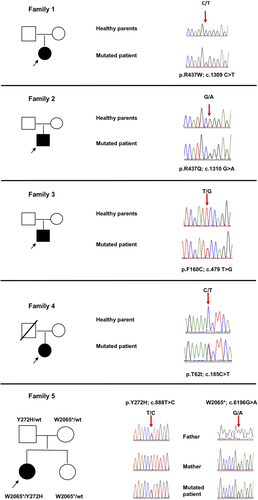
Six novel mutations in SPTBN2 were detected. In particular, four patients harbored heterozygous missense variants (NM_006946): c.185C > T;p.T62I, c.479 T > G;p.F160C, c.1309C > T;p.R437W, and c.1310G > A;p.R437Q and one patient harbored compound heterozygous variants (a nonsense c.6267G > A; p.W2065* and a missense c.888 T > C; p.Y272H) (Figure 2, Table 1). Segregation analysis demonstrated a de novo origin of the mutations in three cases (patients 1, 2 and 3) whereas in one case (patient 4) segregation analysis could not be completed because the father was not alive. All mutations are predicted deleterious or probably damaging by in silico prediction tools. None of the six mutations have been reported in available databases (ie, dbSNP146, 1000 Genomes, ExAC and GnomAD).
3.2 Molecular modeling
The p.T62I and p.F160C pathogenic variants fall in the first Calponin-homology (CH) domain of SPTBN2 (NP_008877) affecting a highly conserved residue. The replacement of Thr62 with an isoleucine residue introduces a novel hydrophobic interaction with Trp154, which modify the interface of the CH-1 domain and actin (Figure 3B). The replacement of Phe160 (highly conserved) with a cysteine causes the loss of the hydrophobic interactions with Trp66 and Leu98 (Figure 3C), modifying also in this case the interface of the CH-1 domain with actin. The p.Y272H mutation affects a highly conserved residue and disrupts the hydrophobic interactions with nearby residues in the second CH domain of SPTBN2 (Figure 3D). Superposition of the SPTBN2 CH-2 domain with the CH2 domain of human alpha-actinin, which, like SPTBN2 also presents two CH domains arranged in tandem, suggests that the Y272H amino acid substitution likely perturbs the interface between the two CH domains and possibly their mutual three-dimensional arrangements. The p.R437W or p.R437Q mutation implies the replacement of the cationic and highly conserved arginine 437 with the aromatic and large tryptophan (W) or the polar glutamine (Q) residue in a region that has structural importance for the second spectrin repeat (Figure 3E). Both the tryptophan and glutamine residues should cause important reorganizations in the structure of the spectrin repeat 2 also affecting its arrangement with the flanking repeats (spectrin repeats 1 and 3). The p.W2065* mutation causes the loss of a functional domain of SPTBN2 (Figure 3). Based on conservation and structural analysis, all the mutations presented in this work are expected to have important impacts on functional regions of the SPTBN2 protein, providing a basis for rationalizing their pathogenic effects.

4 DISCUSSION
Spectrins are heterotetrameric cytoskeletal membrane proteins composed of two main alpha subunits (SPTA1, SPTAN1) and five beta subunits (SPTB, SPTBN1, SPTBN2, SPTBN4, SPTBN5) (reviewed by Reference 17). The non-erythrocytic beta spectrin 2 subunit (beta-III spectrin) is a 2391-amino acid protein encoded by SPTBN2 (11q13.2) and expressed throughout the soma and dendritic tree of cerebellar Purkinje cells. SPTBN2 is required for the maintenance of dendritic architecture and for the trafficking and stabilization of several membrane proteins: Ankyrin-R, cell adhesion proteins, voltage-gated sodium channels, glutamate receptors (mGluR1) and transporters (EAAT4).18 It contains the actin/ARP1 binding site at the N-terminal (calponin-homology domains), 17 spectrin repeats involved in the formation of the heterotetrameric α-β-spectrin complex and a pleckstrin homology (PH) domain which binds phosphatidylinositol lipids, at the C-terminal.19 Loss of SPTBN2 in the mouse mutant results in altered dendritic morphology and density, changes in Purkinje cells intrinsic excitability: reduced sodium currents and deficits in glutamatergic neurotransmission.20-22
To date, three cases of dominant SPTBN2 (p.R480W) mutations and four cases of homozygous recessive mutations have been reported in early-onset ataxia (Figure S1). In a recent targeted exome analysis of patients with ataxic phenotypes, de novo or dominant SPTBN2 mutations were identified in 4/88 (4.5%) two of them presenting with infantile onset ataxia and developmental delay.23 Here, we show novel pathogenic SPTBN2 variants in 5% of our cohort of patients with congenital or early-onset non-progressive ataxia; of note that only one-third (5/15) of the rare SPTBN2 variants identified in our study were confirmed to be pathogenic. The five novel pathogenic missense variants localized in the actin-binding domain or in the spectrin-repeat domains of SPTBN2 and one stop variant localized in the PH domain of the protein. Thermodynamic changes caused by different mutations may affect the overall dimerization capability of the protein. The hotspot R480W mutation, located in the second spectrin-repeat domain (as the R437Q and R437W variants identified in the present study) has been predicted to increase protein stability, whereas SCA5-related T472M found in the same domain or L253P found in the actin-binding domain were either neutral or destabilizing the protein.14 Yet, the molecular mechanisms for differences in timing of onset, progression, mode of inheritance and presence of cognitive deficits in ataxic patients, remain unknown. Interestingly, the two brothers carrying a homozygous missense mutation reported by6 and patient 5, carrying a nonsense and a missense variant, appear to have a milder phenotype with normal cognitive development (Supplementary file and Table S2) if compared with previously reported SCAR14 patients with homozygous loss of function mutations.3-5 Similar frequencies have been observed for mutations in other SCA-associated loci such as ITPR124-26 or CACNA1A,27 whereas both autosomal recessive and dominant modes of inheritance associated with neurodevelopmental and/or neurodegenerative features have been reported in KCNC328 or GRM129, 30 mutated families. The challenge will be to decipher subtle changes underpinning Purkinje cells dysfunction both in early and later stages of these various forms of ataxia. This study further delineates the clinical, mutational and epidemiological spectrum of congenital and early onset SPTBN2-related ataxias and suggest that screening of SPTBN2 should be implemented in this subgroup of disorders.
ACKNOWLEDGEMENTS
We thank the patients and their families for participating to this study. This work was supported by grants from the Italian Ministry of Health (Ricerca Finalizzata NET-2013-02356160 to E.B).
CONFLICT OF INTEREST
The authors declare no conflict of interest.




