Detecting somatic mosaicism: considerations and clinical implications
Abstract
Human disease is rarely a matter of all or nothing; variable expressivity is generally observed. Part of this variability is explained by somatic mosaicism, which can arise by a myriad of genetic alterations. These can take place at any stage of development, possibly leading to unusual features visible at birth, but can also occur later in life, conceivably leading to cancer. Previously, detection of somatic mosaicism was extremely challenging, as many gold standard tests lacked the necessary resolution. However, with the advances in high-throughput sequencing, mosaicism is being detected more frequently and at lower levels. This raises the issue of normal variation within each individual vs mosaicism leading to disease, and how to distinguish between the two. In this article, we will define somatic mosaicism with a brief overview of its main mechanisms in concrete clinical examples, discuss the impact of next-generation sequencing technologies in its detection, and expand on the clinical implications associated with a discovery of somatic mosaicism in the clinic.
Mosaicism is defined by the presence of more than one cell population arising from a single zygote, by genic or genomic alterations, in a single individual. When distinct cell populations derive from different zygotes, it is defined as chimerism 1. True chimeras are rare, though microchimerism can arise in utero by an exchange of cells between mother and fetus or between dizygotic twins, although still remaining incredibly rare and hard to detect 2, 3. Mosaicism is more common 4, partly because there is a chance of error at every cell division 5, and is noted by the presence of mixed cell populations in one or more tissues (somatic mosaicism). As mosaicism can arise through any type of post-zygotic alteration, it is not surprising that everyone has at least one cell in their body that differs from the others and thus every individual is really a mosaic 6, 7. A recent study showed that somatic mutations can be detected in healthy pairs of monozygotic twins, supporting the concept that somatic mutations occur from early life and as such we are likely to accumulate our share of somatic mutations across our lifespan, increasing our own genetic variation 8. Similarly, all females can be considered mosaics due to X inactivation 9, 10.
Somatic mosaicism affects different tissues at variable levels, depending on: the timing at which the alteration occurred, the gene affected and whether it is expressed in that particular tissue, and the survival capacity of the mutant cells. If the germline of an individual contains mutant cells (germline mosaicism), these may be passed on to the next generation. Germline mosaicism is suspected if unaffected parents have several offspring manifesting a particular phenotype of dominant inheritance 11, which in turn also rules out somatic mosaicism in the offspring. For the purpose of this article, we will focus solely on somatic mosaicism.
Clinically, somatic mosaicism can be overlooked due to subtle physical signs often attributed to normal variation between individuals 12. It can contribute to phenotypic discrepancies in monozygotic twins, as well as variable expressivity in monogenic disorders 13. Moreover, it has been long postulated that certain disorders could only survive in a mosaic form 14. Somatic mosaicism is suspected when an individual presents with major discrepancies in body symmetry, or altered pigmentation in only certain parts of their body not previously reported in their family 11, 15. Mild manifestations of such discrepancies are more likely caused by stochastic developmental events or unequal environmental exposures, such as skin color changing due to ultraviolet (UV) light exposure. The most well-studied example of somatic mosaicism is cancer; all tumors represent a subset of cells within an individual that have accumulated mutations resulting in uncontrolled cell growth and survival advantage 16. Both benign and malignant tumors constitute evidence of how common somatic mosaicism is in the human body, and allow for research on the survival mechanisms of mosaic cell lines.
Identifying mosaicism in the general population would contribute to our knowledge of normal human development. However, this is challenging due to the ethical considerations of performing invasive tests on healthy individuals. For this reason, research on healthy controls tends to be carried out on tissues more readily available such as blood and/or saliva, which means somatic mosaicism will often be missed. As such, our knowledge comes primarily from investigating somatic mosaicism in human disease. Technologies routinely used for clinical investigation, such as fluorescence in situ hybridization (FISH), karyotype and Sanger sequencing have limited resolution to detect low levels of mosaicism. However, the emergence of next-generation sequencing (NGS) has revolutionized detection methods 17. This new technological era is promoting the detection of somatic mosaicism down to 1 bp (base pair) changes as well as levels as low as 1% 18-20, which will lead to a better understanding of its role in human disease.
The main mechanisms involved in somatic mosaicism will be briefly described using examples of clinically defined disorders. Additionally, we will discuss how high-throughput technologies have improved the detection rate of somatic mosaicism, as well as the implications and relevance of identifying somatic mosaicism in the clinic.
Molecular mechanisms leading to mosaicism
Somatic mosaicism can arise by any type of mutation, ranging from chromosomal to single nucleotide alterations. Chromosomal mosaicism encompasses both numerical and structural anomalies and is usually detectable by standard cytogenetic techniques, whereas molecular changes require higher resolution screening for detection. Different molecular mechanisms result in different types of somatic mosaicism. Figure 1 shows a schematic representation of how each mechanism overall leads to somatic mosaicism.
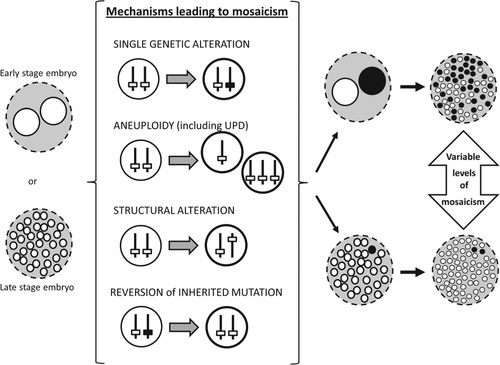
Mosaicism caused by a single genetic alteration
Somatic mosaicism, by definition, can be caused by a single somatic alteration. Neurofibromatosis 1 (NF1) (MIM # 162200) and neurofibromatosis 2 (NF2) (MIM # 101000) are caused by dominant mutations in the NF1 and NF2 tumor suppressor genes, respectively 21. Features include Lisch nodules 15, fibromatous tumors of the skin, and cancer susceptibility. Most cases of NF1 and NF2 are constitutional, but individuals with somatic mutations are also described 21-23. Such mutations are only present in a subset of cells, giving rise to a patch-like pattern of skin discolouration and lesions such as café-au-lait spots 21. Similarly, single mutations in the PIK3CA gene have been recently shown to cause overgrowth only in the somatic tissues containing the mutation 24, 25. These localized observable phenotypic marks remain the best predictors of somatic mosaicism.
Mechanisms that result in mosaic aneuploidy
Aneuploidy is defined as ‘a condition in which there are more or fewer chromosomes that is an exact multiple of the haploid number’ and also includes ‘duplications and deletions of parts of chromosomes’ that overall produce a genetic numerical imbalance 26. Most cases are due to meiotic errors in the parental germline; however, segregation errors can occur post-zygotically, resulting in somatic mosaicism 12, 27. Studies have found that aneuploid cells have reduced cell proliferation and altered metabolic pathways which may explain why most trisomic embryos fail to develop 28. Mechanisms leading to aneuploidy have been thoroughly reviewed 29-31.
Occasionally, a later somatic event can occur to restore chromosome number, this is known as chromosomal rescue. The mixture of both aneuploid and rescued cells results in a mosaic karyotype 32. Chromosomal rescue is random, and as such leads to an increased risk for uniparental disomy (UPD), whereby both copies of a chromosome pair derive from the same parent 33, 34. UPD has its own clinical consequences, increasing the chance of homozygosity for a recessive mutation, and interfering with genomic imprinting, differential markings between maternal and paternal alleles where one allele is expressed and the other is silenced 35. Somatic mosaicism involving UPD thus adds another layer of variability in observed phenotypes.
Mechanisms that result in mosaicism for structural anomalies
Structural mosaicism can occur during mitosis due to mechanisms such as non-allelic homologous recombination (NAHR) and non-homologous end joining (NHEJ). Both will result in a mixture of cells containing original or repaired sequence. Further insights into these mechanisms, including clinical examples, can be found in other reviews 36, 37.
Mechanisms of reversion in chromosomal instability disorders
Chromosomal instability (CI) refers to the gain or loss of genetic material due to mis-segregation of chromosomes 38. CI often results in hypermutation which predisposes individuals to cancer 38, 39. Bloom syndrome (BS) (MIM # 210900) is a well-studied CI disorder. It is inherited in an autosomal recessive manner, requiring mutations in both alleles of BLM 29, 31. BS is characterized by short stature, facial dysmorphism and increased risk of cancer, thought to be due to the abnormally high rates of sister chromatid exchange (SCE) in lymphocytes 40, that in turn result in CI and subsequent accumulation of somatic mutations. In rare cases, high SCE can actually cause some of the cells to revert back to the normal phenotype; this is referred to as reversion. BS patients in which reversion has occurred contain cell lines with high SCE and others with low SCE, thus becoming mosaics, which reduces expressivity of the disease 41. Ellis et al. suggested that reversion may take place due to somatic intragenic recombination, where crossing over between maternal and paternal alleles that contain differentially mutated sites results in a normal BLM allele with low SCE phenotype 40. In 2001, Ellis et al. suggested another mechanism: a back mutation would occur when one strand of the sister chromatid is inaccurately replicated, reverting back to the non-deleterious sequence 41. Other mechanisms that have been proposed in BS and other CI disorders such as Fanconi anemia are: reverse mutation, unprompted correction of a mutation during mitosis, or a second mutation in the gene restoring the reading frame 42-44.
Mosaicism: a survival mechanism?
Many disorders have only ever been described in mosaic forms and survival is thought to be possible only when some normal cells are present 14. Classical examples include McCune–Albright syndrome (MIM # 174800), Proteus syndrome (MIM # 176920) and Sturge–Weber syndrome (SWS, MIM # 185300).
When considering autosomal aneuploidy, only three full trisomies are compatible with life: trisomy 13, 18, and 21 27, 45. Rare cases of full trisomy 22 have been described, but these infants died shortly after birth 46. Constitutional autosomal monosomy is also extremely rare, suggesting embryonic lethality 47. Monosomy 21 appears to be viable, with only three cases described in live-born infants, although these individuals have serious health complications 48. Burgess et al. suggest that these cases are unlikely to represent true monosomy 21 but rather a lack of depth in analysis methods 49. The recent development in technologies available for molecular testing (discussed later) is likely to shed a light on this statement. Nevertheless, other autosomal aneuploidies are viable in a mosaic form 50. For example, mosaic trisomy 8, also known as Warkany syndrome, is a rare condition characterized by mental retardation, bone malformations, facial dysmorphisms, delayed development, cancer predisposition and cardiac anomalies 51. Other mosaic trisomies reported include trisomy 14, 16 and 17 52-54.
Mosaicism causing milder phenotype of disease
Milder phenotype in Mendelian disorders
Mosaicism in human Mendelian diseases was discovered through observation of milder clinical phenotypes 55. The true frequency of mosaicism in monogenic disorders is hard to predict due to the variability of phenotypes observed even with germline mutations and low-level mosaicism often leading to very mild effects which are likely to remain undetected.
One example of a Mendelian disorder seen in mosaic form is hereditary tyrosinemia type I (MIM # 276700), caused by inherited mutations in the fumarylacetoacetate hydrolase (FAH) gene. The disorder is transmitted in an autosomal recessive pattern. Clinical features involve liver and renal dysfunction, neurological deterioration and hepatoma. Patients with this metabolic disorder can have mosaic livers with mutant and revertant populations of hepatocytes 56. The revertant hepatocytes form liver nodules and have growth advantage over mutant cells 56.
Adenosine deaminase deficient severe combined immunodeficiency (ADA-SCID) (MIM # 102700) is another autosomal recessive disorder. The disease phenotype encompasses multiple viral, fungal and bacterial infections early in life and failure to thrive 57. The ADA gene encodes for adenosine deaminase which eliminates the deoxyadenosine generated when DNA is broken down 58. The mutation reduces enzymatic activity, which leads to accumulation of deoxyadenosine to toxic levels 58. Revertant lymphocytes have growth advantage, leading to milder phenotypes 59.
Milder phenotype in chromosomal syndromes
Similarly, somatic mosaicism can contribute to the phenotypic spectrum of a syndrome, particularly involving aneuploidies. Aneuploidies of the sex chromosomes are more commonly seen in live births than autosomal aneuploidies 23, and the post-zygotic gain or loss of an X or Y chromosome results in somatic mosaicism.
Klinefelter syndrome (KS), characterized by an XXY karyotype 60, is the most common sex chromosome aneuploidy found in males, affecting 1 in 500 births 61. KS patients present with enlarged breasts, minimal body hair, small testes, and often have language deficits 60, 62. Mosaic KS patients are very difficult to diagnose clinically as their phenotype ranges from normal intelligence to mental retardation as well as fertile to complete infertility, a clinical indication that would not be tested until adulthood.
Turner syndrome (TS) is a genetic disorder that affects females and is caused by partial or complete absence of the second X chromosome 63. Individuals with TS are typically short in stature and often fail to mature reproductively. TS has been described with karyotypes such as 45X, 45X/46XX, and 45X/47XXX 62, 63. It is important to note that some TS patients may carry a Y chromosome, which results in mosaic 45X/46XY or 45X/46XX/46XY karyotypes. These individuals, despite being mosaics, often have more complications involving sex and gonadal development than non-mosaic TS individuals 64.
High-throughput technology and detection of mosaicism
Cytogenetic analysis is routinely used in the clinic and allows examination of the whole genome, but only at the chromosomal level 65; low resolution prevents it from detecting sub-microscopic alterations. Sanger sequencing allows for single nucleotide examination, and is useful for screening a limited number of candidate genes 66. However, when searching for new mutations and/or genes for diseases without leading candidates, it is more time-efficient to examine all genes at once 66. The nature of high-throughput technology, which includes both chromosomal microarray analysis (CMA) and NGS, matches the need for detection of mosaicism as it provides the opportunity to sample, analyze and compare the whole exome or genome of multiple tissues with high coverage.
Chromosomal microarray analysis
Conventional cytogenetic analysis, such as karyotyping and FISH, has the potential to identify mosaicism at the chromosomal level if enough cells from the right tissue are tested 65. Analysis is carried out in single cells at metaphase and, by looking at the chromosomes as a whole rather than at the DNA sequence, aneuploidy and mosaic aneuploidy can be detected. These techniques are still used in the clinic but are slowly being replaced by CMA. Unlike the conventional cytogenetic techniques, CMA can detect copy number variation (CNV) and does not require cells to be in a specific cell cycle stage. CMA is generally divided into comparative genomic hybridization arrays (aCGH) and single nucleotide polymorphism (SNP) arrays. Probes are designed to allow both types of platforms to detect SNPs and CNVs 67. While aCGH compares two independent samples, SNP array requires only one sample, which is compared with an in silico reference, based on hundreds of control samples 67. Previous studies show that aCGH can detect mosaicism levels of 10–20% while SNP array is more sensitive and can detect levels below 5% 68. However, balanced rearrangements are not uniformly detectable by CMA 65. Furthermore, only selective SNP markers approximately 1 kb apart are included in CMA platforms, thus single nucleotide variants (SNV) and indels lying outside of the markers are not detected. The advantages of using CMA for mosaicism detection are further discussed in a recent review 1.
Next-generation sequencing
Since the first successful application of NGS in discovering the genetic cause of Miller syndrome in 2009 69, many more disease/gene associations have been established 18-20, 24, 70-72. This modern technique allows for detection of small genetic changes such as SNVs and indels within a whole exome or genome and with much higher sequencing depth. NGS can be used to identify somatic mosaicism as it allows for manual inspection of each individual read in a digital format. By lowering the threshold in the variant calling procedure, small percentages of alternative calls can be obtained at specific positions in the gene(s) known or expected to be associated with the observed phenotype 20. This strategy is not as easily applicable when the gene in question is unknown because lowering the thresholds for alternate reads also increases the rate of false positives identified. A series of prioritizing strategies such as cross-referencing against common variants in public or private databases 20, looking at the evolutionary conservation of residues and protein prediction models, and hypothesizing on gene function based on the network or pathway it belongs to 73-75 can help reduce the number of variants of interest, but the workload remains substantial and the detection of low level somatic mosaicism continues to be a challenge.
Somatic mosaicism, particularly at low levels, is often missed by Sanger sequencing, which still remains the standard sequencing technology for screening one or few genes. This is because, unlike the digital output from NGS technologies, Sanger results are represented on an electropherogram as the average of signals, and mosaicism, if visible, will show as a reduced peak that can be hard to distinguish from background noise 76.
NGS is particularly useful for interrogating exomes or genomes of individuals with a defined clinical diagnosis but no identifiable molecular cause, especially when known genes for the disorder have already been tested. Somatic mosaicism is considered one of the main contributors to this observation. A recent example illustrating this was published on tuberous sclerosis complex (TSC) (MIM # 191100). TSC is an autosomal dominant disorder caused by known mutations in TSC1 and TSC2; however 10–15% of patients reported did not have such mutations. With 95% captured regions and more than 200 reads, a NGS study identified two TSC2 somatic mutations in 5% of reads in unrelated patients, supporting the role of mosaicism in TSC 77.
Furthermore, NGS is also useful in identifying mutations underlying cancers 78. Cancer is a condition of mosaicism in two folds: first, mosaicism is achieved by accumulation of mutations that confer survival advantage to the mutant cells and give rise to tumors 16; second, each mutant cell accumulates additional genetic alterations, due to lack of cell cycle checkpoints and DNA repair mechanisms, creating mosaicism within tumors (tumor heterogeneity) 79. Multiple NGS studies have successfully identified these two types of mosaicism, leading to a better understanding of different molecular subtypes of cancer and allowing for targeted therapy for each specific tumor and/or patient 80.
Overall, the digital nature of NGS analysis allows for more sensitive and accurate detection of mosaicism, especially at low levels, while Sanger sequencing remains a cost-effective method for validation 76.
Discoveries of somatic mosaicism and associated genes using NGS
As mentioned previously, NGS is commonly used for establishing associations of disease with mutations in genes previously unknown to be related to that particular phenotype. Successful examples of ‘gene discoveries’ in mosaic disorders are summarized in Table 1. Such examples include the identification of genes associated with disorders which had been postulated as necessary mosaics by Rudolf Happle in 1987 14, and for which the identification of the causal gene had been delayed due to the lack of resolution from earlier molecular techniques. Examples include SWS and Proteus syndrome (PS), both described in Table 1. Whole-genome sequencing of paired affected and normal tissues of SWS patients identified a mutation in GNAQ at frequencies ranging from 1% to 18% 71. The same GNAQ mutation was found in skin tissue of 92% of non-syndromic port-wine stains, suggesting that the same underlying mechanism occurring at different developmental stages can lead to different phenotypes 71. In PS, exome sequencing identified a somatic activating mutation in the oncogene AKT1. Mutant cells made up 1–50% of tested tissues and cell lines from patients 18.
| Disease | Mechanism of disease | Phenotypic characteristics | Genesa | Identification of somatic mosaicism | OMIM | References |
|---|---|---|---|---|---|---|
| Proteus syndrome | Post-zygotic activating mutation | Asymmetric and disproportionate overgrowth of body parts, cerebriform connective tissue nevi, epidermal nevi, dysregulated adipose tissue, and vascular malformations | AKT1 | Whole-exome sequencing in affected tissue vs normal tissue | 176920 | Lindhurst et al. 18 |
| Metaphyseal chondromatosis with D-2-hydroxyglutaric aciduria (MC-HGA) | Change in enzymatic specificity | Severe chondrodysplasia, organic aciduria, and variable cerebral involvement | IDH1 | Whole-exome sequencing in four unrelated affected individuals | 614875 | Vissers et al. 90 |
| CLOVE syndrome (congenital lipomatous overgrowth, vascular malformations and epidermal nevi) | Post-zygotic activating mutation | Progressive, complex, and mixed primarily truncal vascular malformations, dysregulated adipose tissue, varying degrees of scoliosis, and enlarged, but not severely distorted, bony structures | PIK3CA | Whole-exome sequencing in affected tissue vs normal tissue and targeted-capture NGS array | 612918 | Kurek et al. 72 |
| Fibroadipose hyperplasia | Post-zygotic activating mutation | Segmental progressive overgrowth of subcutaneous, muscular, and visceral fibroadipose tissue with skeletal overgrowth | PIK3CA | Whole-exome sequencing in affected tissue vs normal tissue | — | Lindhurst et al. 24 |
| Megalencephaly-capillary malformation syndrome (MCAP) | Post-zygotic mutation, likely activating | Large brain, asymmetric overgrowth, patchy skin vascular malformations, cortical brain malformations: polymicrogyria | PIK3CA | Whole-exome sequencing in trio (affected individual and unaffected parents) followed by Sanger and ultra-deep sequencing. | 602501 | Rivière et al. 20 |
| Megalencephaly, polymicrogyria, polydactyly, hydrocephalus syndrome (MPPH) | Post-zygotic activating mutation | Megalencephaly, hydrocephalus, and polymicrogyria; polydactyly may also be seen | PIK3CA | Sanger sequencing (based on findings from the MCAP trio) | 603387 | Rivière et al. 20 |
| Hemimegalencephaly (HME) | Post-zygotic mutation, likely activating | Overgrowth of either one of the two cerebral hemispheres | PIK3CA, AKT3 MTOR | Whole-exome sequencing in affected tissue vs normal tissue | — | Lee et al. 19 |
| Lipofibromatous hamartoma of nerve or macrodactyly type I | Post-zygotic activating mutation | Congenital anomaly consisting of enlargement of all tissues localized to the terminal portions of a limb, typically within a ‘nerve territory’ | PIK3CA | Whole-exome sequencing in affected tissue vs normal tissue | — | Rios et al. 91 |
| Sturge–Weber syndrome | Post-zygotic activating mutation | Port-wine stains and disrupted vascular development, seizures, and glaucoma | GNAQ | Whole-exome sequencing in affected tissue vs normal tissue in three individuals | 185300 | Shirley et al. 71 |
- a Only genes that have been shown in mosaic patients are listed for each disorder.
In addition, the genetic causes of overgrowth syndromes have also been identified. Several of these syndromes involve the PI3K-AKT3-mTOR pathway as mutations in several members of this pathway were found in megalencephaly-capillary malformation syndrome (MCAP) (MIM # 602501), hemimegalencephaly and CLOVE (congenital lipomatous overgrowth, vascular malformations and epidermal nevi) syndrome (MIM # 612918) 19, 20, 72. In a similar fashion, NGS has allowed researchers to group individuals with similar phenotypes, now found to have the same genetic cause, into newly defined syndromes, such as fibroadipose hyperplasia 24.
Clinical implications
Technical limitations
Although the application of high-throughput technology in mosaicism detection is fruitful, there are still some limitations. Mosaicism detection requires deep sequencing, often with over 100× coverage compared with average 30× coverage used for routine whole-exome sequencing 24, 71. In fact, high-throughput targeted panels are becoming more popular as they can reach a basepair coverage of over 1000×, which allows for detection of lower levels of mosaicism 25. These methods are not applicable for clinical diagnosis just yet due to high costs and ethical concerns (discussed below).
Another issue to consider is ascertainment bias. The percentage of mosaicism is likely to vary between tissues and, in current practice, usually blood and/or saliva samples are tested simply because they are more easily accessible. However, the level of mosaicism in blood, if any, will probably not reflect that in other tissues 81 and can lead to false negative results 19, 20. A good example of this is trisomy 21: at birth, leukocytes possessing the extra chromosome 21 are observed at a higher abundance than later in life 82. This is due to aneuploid cells having difficulty making it through mitotic cell checkpoints; these cells often undergo apoptosis and due to the high turnover rate in blood cells it results in almost 100% normal leukocytes in adulthood 28. However, in other tissues where the turnover rate is not as high, the abundance of trisomy 21 stays relatively the same as it was at birth, thus the presence of trisomy 21 in these tissues is more likely causal of the observed phenotype 81, 82. It is therefore important to test more than one tissue, and in particular any affected tissues whenever possible, in order to get a better overview of mosaicism levels 83.
Difficulty in relating levels of mosaicism to observed phenotype
With mosaicism becoming an increasingly frequent observation, there are many issues that clinicians need to be aware of in order to treat and counsel patients properly. First and foremost, mosaicism has been underestimated within the population. Individuals accumulate mutations over their lifetime that may not have a phenotypic effect; as such, we would expect to find mosaicism in phenotypically normal individuals 84. Similarly, loss of the X chromosome is seen in normal females as they age 85 which means that having some 45X cells does not mean the patient has TS. It is therefore important to understand that finding mosaicism does not make it causal of the phenotype under investigation. Furthermore, the percentage of mosaicism does not necessarily correlate to the severity of the phenotype as mosaicism may have a greater impact on the phenotype when expressed in one tissue over another 83. In addition, if the mutant cells do not have growth advantage over the normal cells, as in the trisomy 21 example, you may see a reduced percentage of detectable mosaicism associated with a more severe phenotype 81, 82. This links back to the fact that a somatic mutation occurring earlier during development is likely to have a greater impact on the observed phenotype 71. Therefore, unless we sampled every cell from every tissue in the body, it is impossible to accurately quantify the percentage of mosaicism in an individual, and even if we could, it would not be sufficient to infer the consequential phenotype.
Genetic counseling
In addition to the difficulty in explaining the concepts and limitations of somatic mosaicism discussed above to patients, further counseling is required when it comes to calculating recurrence risks and explaining uninformative results. The main concern is that, despite the technological advances, somatic mosaicism can never be completely ruled out. A negative result does not necessarily mean the individual is not mosaic: it could be because the researcher or physician is testing the wrong tissue (or even too small of a sample from that tissue) or because the mutation level is too low to detect by current technologies.
When it comes to addressing recurrence risks of mosaicism within a family, detailed family history and all molecular testing must be taken into account, just like for non-mosaic genetic disorders 12. As a general rule, a family in which more than one individual is affected indicates a higher recurrence risk. If an unaffected individual has several affected offspring, the most likely cause of disease is germline mosaicism in this individual, in which case the offspring themselves are not mosaic and their risk of transmitting the disease allele to their offspring should be calculated as for other constitutional mutations. These clinically unaffected individuals are not likely to seek prenatal testing and/or counseling unless they have an affected child and wish to have more children. If mosaicism is found in more than one generation, as well as in more than one offspring, the most likely explanation for disease recurrence is that this family is affected by a CI disorder which should be confirmed by genetic testing 11, 39. However, we still expect most cases of somatic mosaicism associated with disease to be sporadic, and thus counseling will be similar to that provided for constitutional de novo mutations.
Individuals with known somatic mosaicism who want to know their risk of having an affected child can choose to have their germline tested. However, this is an impractical and highly invasive procedure for women, and often not very informative for men due to high variation in semen parameters between samples 86. For such cases, women may opt for in vitro fertilization with prenatal genetic diagnosis to ensure the fertilized embryos do not carry the mutation(s), or extended prenatal testing. However, further complications arise due to the possibility of false positive prenatal test results which cannot inform us on the extent of the mosaicism detected and thus may lead to the termination of phenotypically normal fetuses. Indeed, when mosaicism arises early in development, the mutant cell line can be confined to the placental tissue, which is known as confined placental mosaicism (CPM), or can be present in both the placenta and the fetus, resulting in generalized mosaicism 33, 87. CPM is estimated to arise in approximately 1–2% of pregnancies 88. A finding of mosaicism from a chorionic villus sampling (CVS), most useful in detecting chromosomal abnormalities, could therefore be either CPM or somatic mosaicism in the fetus, and it is recommended to follow up the CVS with amniocentesis where the cells being tested are fetal in origin 89. However, even with all precautions taken to provide the appropriate prenatal testing, a true case of fetal mosaicism does not necessarily mean that the individual will have any phenotypic consequences because amniocentesis cannot predict the origin or proportion of cells carrying the anomaly. Likewise, false negatives are also likely to occur in a small but not negligible number of cases, which would require further counseling. For example, mosaicism arising later in development could be missed during prenatal testing as it may not have yet arisen or may be confined to only tissues not tested.
In summary, detecting somatic mosaicism during the prenatal period represents a true challenge. It is limited by the technologies available, the tissues sampled and the time at which the testing is carried out. However, it is most often limited simply by the lack of reason for carrying out such detailed testing, as it most often involves clinically unaffected parents. This is further complicated by the fact that we cannot accurately predict the phenotype of the fetus based on a prenatal finding of somatic mosaicism. Genetic counselors will have to convey these limitations very clearly to expecting parents.
Conclusion
Somatic mosaicism has become an important consideration in the clinic. Healthy humans are in fact mosaic, but somatic mutations can also lead to disease or even allow for survival, depending on the gene or chromosome involved. This is further complicated by the fact that the level of mosaicism does not necessarily correlate with the severity of clinical manifestation, and may even not have any visible effects. With the recent technological advances which are detecting mosaicism at a higher rate than ever before, the idea that somatic mosaicism necessarily leads to disease is shifting in the scientific field; however, it certainly remains a default association for most patients. We will need to redefine somatic mosaicism as a genetic mechanism rather than the cause for a particular phenotype in order to change the perception of the general public. Thresholds concerning levels of mosaicism and tissues affected will need to be established to distinguish somatic mosaicism from normal genetic variation. The hope is that, as NGS becomes more widely used in the clinic, new findings will be translated into improved counseling and clinical management of somatic mosaicism.
Acknowledgements
We would like to thank Dr Wendy P. Robinson and Dr Jan M. Friedman from the Medical Genetics Department at the University of British Columbia (UBC) for the valuable comments on the manuscript. A. S. A. C. holds a Doctoral Grant from the Fundação para a Ciência e a Tecnologia (Portugal/EU); S. L. W. is funded by a CFRI Graduate Studentship 2014-2015 and CIHR (operating grant to her supervisor W. P. R.). J. T. holds a CIHR Canada Graduate Scholarship, UBC 4YF and LEEF award; X. C. Y. was supported by the UBC Medical Genetics Graduate Support Initiative Award 2012–2013 and the CFRI Graduate Studentship 2013–2014.




