Diabetic Macular Edema at the time of Cataract Surgery trial: a prospective, randomized clinical trial of intravitreous bevacizumab versus triamcinolone in patients with diabetic macular oedema at the time of cataract surgery – preliminary 6 month results
Abstract
Background
To compare visual and anatomical outcomes between intravitreous bevacizumab (BVB, Avastin) and triamcinolone (TA, Triesence) when administered at the time of cataract surgery in patients with diabetic macular oedema (DME).
Design
Prospective, single-masked, randomized clinical trial at The Royal Victorian Eye and Ear Hospital, Melbourne.
Participants
Patients with clinically significant cataract and either centre-involving DME or DME treated within the previous 24 months.
Methods
Participants were randomized 1:1 to receive intravitreous BVB 1.25 mg or TA 4 mg during cataract surgery, and at subsequent review if required over 6 months.
Main Outcome Measures
Change in central macular thickness (CMT) and best corrected visual acuity at 6 months.
Results
Forty-one patients (mean age 66.4 years, 73.2% male) were recruited. Visual acuity and CMT were similar between groups at baseline (P > 0.2).After six months, both groups gained vision (mean +21.4 letters in TA group P < 0.0001, +12.5 letters in BVB, P = 0.002), with no significant difference between groups (P = 0.085). In addition, 60.9% of eyes receiving TA achieved a VA of ≥6/12 compared to 73.3% in the BVB group (P = 0.501). However, only TA was associated with a sustained reduction in CMT (−43.8-µm reduction TA vs. +37.3-µm increase BVB, P = 0.006 over 6 months). Following surgery, additional injections were required in 70.6% of participants in the BVB group, compared to 16.7% in the TA group (P < 0.0001). Three patients in the TA group experienced a rise of IOP over 21 mmHg (12.5%) during the 6-month follow-up; BVB had no cases (P = 0.130). There were no cases of endophthalmitis in either group.
Conclusions
When administered at the time of cataract surgery in patients with DME, at 6 months both TA and BVB improve visual acuity; however, only TA results in a sustained reduction in CMT. Further follow-up will determine whether this translates into better long-term visual outcomes in the TA group.
Introduction
Diabetic retinopathy (DR) is the leading cause of legal blindness in the working age population in developed countries such as Australia.1 As the prevalence of diabetes increases, the number of people with sight-threatening DR is estimated to triple in the United States by 2050, from 1.2 million in 2005 to 3.4 million, with similar predictions for Australia.2 Diabetic macular oedema (DME) is the most common cause of visual loss in DR.3, 4
Cataract also occurs more frequently in people with diabetes, and at an earlier age.5, 6 Although the removal of the cataract is usually a quick and effective means of restoring vision, the outcomes of cataract surgery in people with DR are worse compared to people without diabetes due to the associated development or acceleration of DME post-operatively.7-11 Indeed, patients with co-existent DME at the time of cataract surgery are most at risk of a poor outcome, with studies showing that only 53% of these patients achieve a post-operative visual acuity of ≥6/12, in comparison to >95% in those without DR.9, 12, 13
Recent large randomized controlled trials have shown that anti-vascular endothelial growth factor (anti-VEGF) agents such as ranibizumab,14, 15 aflibercept16, 17 or bevacizumab (BVB)18 given as intravitreous injections are an effective treatment for DME with better visual results than the previous standard treatment of laser photocoagulation10, 11, 17. However, recent studies have indicated that these agents may not be as effective for DME aggravated or induced by cataract surgery.19-21
One of the key mechanisms in the development of DME is the breakdown of the blood–retinal barrier, resulting in the egress of fluid into the retina (macula).22 Cataract surgery is an inflammatory insult to the eye,23 resulting in the release of soluble inflammatory mediators that lead to further breakdown of this already compromised blood retinal barrier, and could be the key factor in the causation, or worsening, of DME postoperatively.
The use of intravitreous depot-steroid injections such as triamcinolone acetonide (TA), at the time of surgery, may therefore be a better option for treatment in this group, given that TA has both anti-VEGF and anti-inflammatory effects.24
Despite several studies comparing the use of intravitreous injections of anti-VEGF agents and TA in the treatment of laser-resistant DME,25-28 none have directly compared these two agents in the setting of cataract surgery in an eye with pre-existing DME.
This paper reports the study methodology and 6-month preliminary follow-up data of the Diabetic Macular Edema at the time of Cataract Surgery (DiMECat) study, a prospective, single-masked, randomized clinical trial comparing the outcomes of cataract surgery in patients with current or past DME who receive either intravitreous bevacizumab (BVB, Avastin, Genentech Inc., San Francisco, CA, USA; 1.25 mg) or triamcinolone (TA, Triesence; Alcon Pharmaceuticals, Ft. Worth, TX; 4 mg) at the time of surgery.
Methods
Study design, inclusion and exclusion criteria
This was a prospective, single masked, randomized clinical trial to compare visual and anatomical outcomes when either intravitreous BVB or TA was administered at the time of cataract surgery and at subsequent review if required, in patients with DME.
Results presented here include data from all participants who were enrolled in the 6-month pilot study (n = 25) and preliminary results for those who have reached the 6-month time-point in the extended 12-month study (n = 16).
The study protocol followed the tenets of the Declaration of Helsinki and was approved by the Human Research Ethics Committee of the Royal Victorian Eye and Ear Hospital, Melbourne, Australia. The study trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12611000888965). Written informed consent was obtained by a study investigator from all participants prior to enrolment in the study.
Patients with diabetes and refractory DME, or a recent history of treated DME, with visually significant age-related cataract who met the inclusion and exclusion criteria (Table 1), and were willing to comply with the protocol and provide informed consent were recruited from the Royal Victorian Eye and Ear Hospital Medical Retina Clinic (Melbourne, Australia).
| Inclusion criteria |
| Over 18 years of age |
|
Clinically significant macular oedema (CSME) involving the fovea in the study eye at baseline OR CSME in the study eye within 24 months of study entry OR Microaneurysms at the edge of the foveal avascular zone of the study eye, which are not amenable to treatment with laser (≤500 µm from the foveal centre) |
| Exclusion criteria |
| Uncontrolled glaucoma or glaucomatous visual field defects |
| Past history of intraocular pressure (IOP) rise >35 mmHg following steroid treatment |
| Loss of vision due to other causes (e.g. age-related macular degeneration, myopic macular degeneration) |
| Significant macular ischaemia (determined by most recent high resolution digital fluorescein angiogram) |
| Visual acuity of <6/60 in the fellow eye |
| Known allergies to TA or BVB or steroids or components of BVB |
| Systemic treatment with >5 mg prednisolone (or equivalent) daily |
| Presence of intercurrent severe disease such as septicaemia or intercurrent ocular infection |
| Intravitreous TA within 10 weeks of baseline visit |
| Intravitreous ranibizumab or BVB within three weeks of the baseline visit |
| Argon laser photocoagulation within three months prior to baseline visit |
| Women of childbearing potential not using adequate contraception and lactating mothers |
Randomization lists were created using Stata IC 12.1 for Windows (StataCorp LP, College Station, TX) and involved first stratifying by DME presence/absence and then block randomized in blocks of size 4, 6 or 8 subjects. Prior to cataract surgery patients were randomly assigned by the aforementioned method to receive either intravitreous injection of TA or BVB at the time of cataract surgery. Each eye was randomized separately if both eyes were eligible to be enrolled, with at least one month scheduled between the two cataract operations. The trial medications were discontinued prior to the completion of the study if there were adverse events, protocol violations, lack of efficacy or personal reasons.
Surgery
Before cataract surgery, all patients underwent standard ophthalmic examinations by the investigators. Biometry was performed before the operation with the use of the IOL Master (Zeiss, Carl Zeiss Meditec Ltd, Jena, Germany) or an A-scan. All patients underwent phacoemulsification and IOL implantation using topical or local anaesthesia and a standard surgical technique.
The AcrySof SA60AT (Alcon, Inc, Fort Worth, TX) intraocular lens (IOL) was used for all surgeries. A 2.8-mm corneal incision was used for insertion of the AcrySof IOL (Monarch injector systems, Alcon, Fort Worth, TX). The wounds were not sutured unless a leak was evident after hydration. After surgery, prednisolone acetate 1% (Prednefrin Forte, Allergan) and chloramphenicol 0.5% (Chlorsig, Sigma Pharmaceuticals, Australia) eye drops were prescribed 4 times daily for 1 week. Antibiotic eye drops were stopped after 1 week, and corticosteroids were tapered over a period of 4 weeks after surgery.
In the event of complicated cataract surgery where there was vitreous loss, the patient was withdrawn from the study with no study medication administered (with any other treatment given at the time of surgery being at the surgeon's discretion).
Patient evaluation
Patients were followed up and examined at baseline (up to two weeks prior to surgery), one week post-surgery and then monthly for six months following surgery. Participant characteristics including age, gender, type and duration of diabetes, haemoglobin A1c, medication use and ophthalmic history were recorded. History of previous laser photocoagulation and intravitreous injections were documented from the patient's history.
At each follow-up, routine assessment included best-corrected logarithm of the minimum angle of resolution (logMAR) visual acuity (BCVA) testing with Early Treatment Diabetic Retinopathy Study (ETDRS) charts using standardized procedures, intraocular pressure (IOP) measured using a Goldmann applanation tonometer, slit lamp examination and fundus evaluation. Fast macular thickness scans and 6-mm cross hair scans were obtained using Spectralis optical coherence tomography (OCT) (Heidelberg Engineering, Heidelberg, Germany). Central macular thickness (CMT, µm) was an automated measurement performed automatically by the instrument's software analysis without manual operator adjustment.
Fundus photographs (Topcon TRC-50EX, Topcon) were performed at the baseline and month six visits, and fluorescein angiography (HRA2, Heidelberg Engineering, Heidelberg, Germany) was performed at six months. Best-corrected visual acuity and all study imaging procedures were performed by trained, certified technicians who were masked to treatment assignment. Patients were also masked to the type of the injection they received throughout the study.
Significant elevation of IOP was defined as an IOP greater than 21 mmHg. Patients with an IOP measured between 22 and 30 mmHg on two consecutive occasions were commenced on first line topical glaucoma medication. Those that had persistent IOP despite first line therapy, or who had an elevation > 30 mmHg at any time were referred to the specialist glaucoma unit at the RVEEH for further management. Patients that developed an IOP > 30 mmHg did not receive any further intravitreous TA injections (due to the risk of developing greater IOP rises with subsequent injections).
Any other adverse events were recorded at each examination. If the patient did not respond to the assigned treatment (no clinical response to the medication or continued worsening of BCVA and CMT), they were considered a treatment failure, study medications were discontinued and alternative treatments could be introduced. Treatment failures were not classified as adverse events. It should also be noted that progression to proliferative DR was not considered in itself to be a treatment failure, despite being an adverse event requiring panretinal photocoagulation.
Retreatment criteria
The response to treatment post-surgery was monitored by measuring CMT and BCVA.
Treatment with macular laser, pan-retinal photocoagulation or additional injections was allowed from 28 days post cataract surgery (or 77 days for those requiring re-injection in the TA group).
Retreatment consisted of focal laser (following ETDRS guidelines), as clinically required, or further intravitreous injections. When re-injection was required, patients received the same study medication and dose they received at the time of their cataract surgery.
- Increase in CMT by 50 µm or more compared to the best recorded CMT in the study or
- Decrease of five letters or more compared to best recorded BCVA score in the study and at least 28 days had passed since previous BVB injection in the BVB treated group or at least 77 days had passed since TA injection in the TA treated group. This was based on the difference in their half-life in the eye. Injections were withheld if there were any adverse events that would deem retreatment inadvisable. There could be a maximum of five retreatments in the BVB group (month one to month five) and a maximum of one retreatment in the TA group (from month 3). All intravitreous injections were given via a 30-guage needle (BVB) or 27-guage needle (TA) in accordance to the Royal Australian and New Zealand College of Ophthalmologists Guidelines for Performing Intravitreous Therapy.29
Sample size
Sample size calculations were based on showing a difference of eight letters in mean BCVA change over three measurements between BVB and TA. Adjusting for an estimated loss to follow up of 15% of eyes, 46 eyes per group was required for 90% power of detecting this difference as significant at the two-sided 5% level.
Statistical methods
Data from all participants who completed at least 6 months of follow-up has been included in this intention-to-treat analysis. Two subjects were lost-to-follow-up (one from each treatment group) but are included in these analyses as described below. All analyses were undertaken using Stata IC 12.1 for Windows (StataCorp LP, College Station, TX).
Continuous baseline characteristic variables were summarized as means with 95% confidence intervals (95%CI), or as medians (interquartile range, IQR) if they did not appear to be normally distributed. The variables were then compared across treatment groups using two-sample t-test (with or without unequal variance as needed), and the two-sample Wilcoxon rank-sum (Mann–Whitney) test, respectively. Categorical variables were summarized as percentages and compared across treatment groups using Fisher's exact test.
As such, subjects with missing data (either intermittent or sustained through loss-to-follow-up) were included in the calculations of post-baseline means and post-baseline change variables by providing the average of all available data divided by the number of available data points.
A linear regression model was then constructed with this summary mean and the baseline measurement and treatment group. The Stata margins command was utilized to test for a significant difference between treatment groups and to calculate the adjusted mean of summary means (adjusted for baseline measurement). The interaction term between treatment and baseline measurement was included in the model if it was found to be significant. The interaction term was significant for CMT (P = 0.007) but not for VA (P > 0.05).
The change from baseline was also calculated as measurement at time less baseline measurement. These change variables were then summarized into per-person means as above. The regression models for mean change included only the mean change and treatment group.
- Excluded from analysis.
- Included and all missing values were assumed to be ‘retreated’ to a maximum of two retreatments for TA group and to a maximum of five for BVB group (the maximum that each drug's protocol would allow).
- Included and all missing values were assumed to be ‘no treatment’.
The per-subject number retreatments were summarized as median (IQR) or percentages and compared between treatment groups using to a two-sample Wilcoxon rank-sum (Mann–Whitney) test and a Fisher's exact test, respectively.
Adverse events were defined as any IOP measurement greater than 21, 25 and 30 mmHg at any visit over the 6 months. Subjects with missing values for IOP were included only for those visits where there IOP data was recorded; in effect, a last-observation-carried-forward approach. The number of subjects who had one or more instances of raised IOP were compared between treatment groups using the Fisher's exact test.
Results
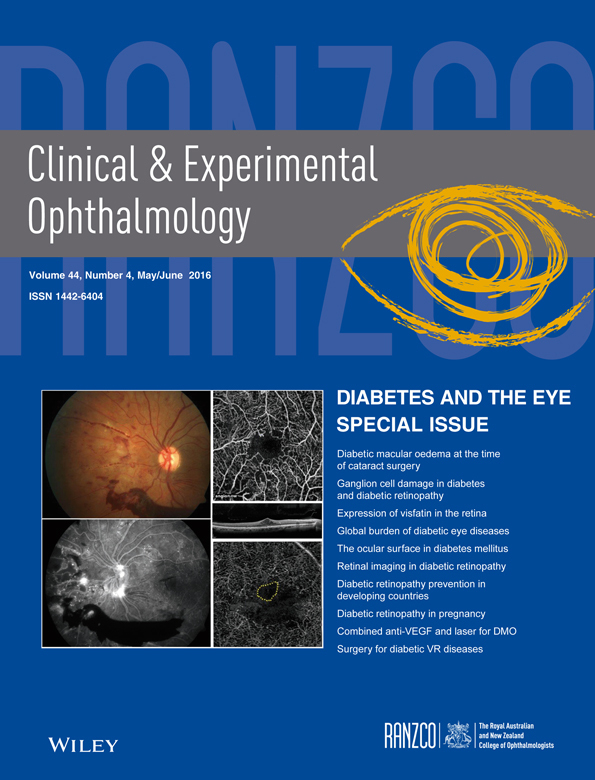
Seventy patients were enrolled between June 2012 and December 2014. Eighteen patients are yet to reach 6 months follow-up, and 11 patients were excluded due to concurrent medical illnesses or intraoperative complications that necessitated withdrawal from the trial (Fig. 1). Results presented here are for 41 patients enrolled prior to April 2014 who were randomized, treated and eligible to have reached 6 month follow-up. There were no statistically significant differences between the treatment groups for any of the demographic or baseline characteristics (Table 2). The mean age of all patients was 66.4 (95% CI 63.7 – 69.1), 73.2% were male and the majority of patients had Type II diabetes requiring insulin (65.0%). Most participants had mild to moderate DR (53.7%), with 17.1% having severe DR and 29.3% treated PDR. Most participants (82.9%) had macular oedema present at baseline. The average BCVA was 51.4 (95% CI 46.5 – 56.2) letters, a Snellen equivalent of approximately 6/30. Twenty-two eyes (53.7%; 10 in BVB group, 12 in TA group) had received at least one macular laser treatment prior to study enrolment,12 eyes had received prior PRP (29.3%; 6 in BVB group, 6 in TA group) and five (12.2%) having prior injection treatment (range one to five). Two participants had both eyes enrolled in the study.
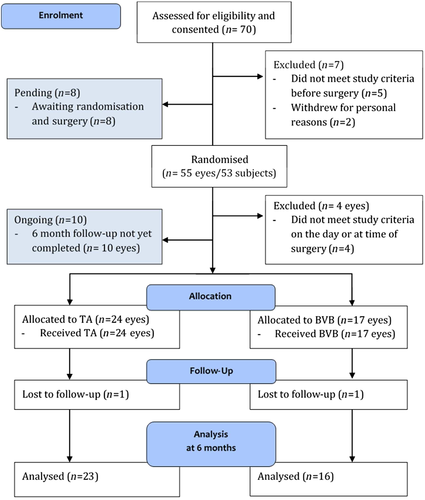
| BVB (n = 17) | TA (n = 24) | P | |
|---|---|---|---|
| Pilot study, n (%) | 11 (64.7) | 14 (58.3) | 0.753 |
| Age at time of surgery, years, mean (95%CI) | 69.0 (65.3 – 72.8) | 64.5 (60.7 – 68.3) | 0.082† |
| Gender, % male | 58.8 | 83.3 | 0.151 |
| Baseline HbA1c %, median (IQR) | 7.8 (7.2, 8.8) | 7.3 (6.3, 8.4) | 0.214 |
| Diabetes, %: | 0.851 | ||
| Type 1 | 0 | 4.4 | |
| Type 2, requiring insulin | 70.6 | 60.8 | |
| Type 2, not requiring insulin | 29.4 | 34.8 | |
| Study eye | |||
| Left eye, n (%) | 7 (41.2) | 13 (54.2) | 0.530 |
| CMT (µm), mean (95% CI) | 365.2 (308.1 – 422.2) | 366.6 (309.9 – 423.3) | 0.971‡ |
| CMT > 300 µm at baseline | 12 (64.7) | 14 (58.3) | 0.753 |
| BCVA, mean (95% CI) | 54.6 (46.9 – 62.3) | 49.1 (42.5 – 55.7) | 0.265† |
| DR severity %: | 0.326 | ||
| Mild | 0 | 16.7 | |
| Moderate | 52.9 | 41.6 | |
| Severe | 11.8 | 16.7 | |
| Inactive/treated PDR | 35.3 | 25 | |
| Active PDR | 0 | 0 | |
| DME at baseline, n(%) | 15 (88.2) | 19 (79.2) | 0.679 |
| Any previous treatment (TA, BVB or laser§), n(%) | 12 (70.6) | 17 (70.8) | 1.00 |
| Previous treatment with macular laser, n(%) | 10 (58.8) | 12 (50) | 0.752 |
| Previous treatment with BVB, n(%) | 1 (5.9) | 3 (12.5) | 0.629 |
| Previous treatment with TA, n(%) | 1 (5.9) | 0 | 0.415 |
- P value relates to difference between treatment groups determined by Fisher's exact test for categorical variables and Two-sample Wilcoxon rank-sum (Mann–Whitney) test for continuous variables.
- † P value relates to two sample t-test.
- ‡ P value relates to two sample t-test with unequal variance.
- § Laser treatment denotes either macular laser and/or panretinal photocoagulation.
- BVB, bevacizumab; TA, Triamcinolone; HbA1c, haemoglobin A1c; CMT, central macular thickness; BCVA, best corrected visual acuity; DR, diabetic retinopathy; PDR, proliferative diabetic retinopathy; DME, diabetic macular oedema.
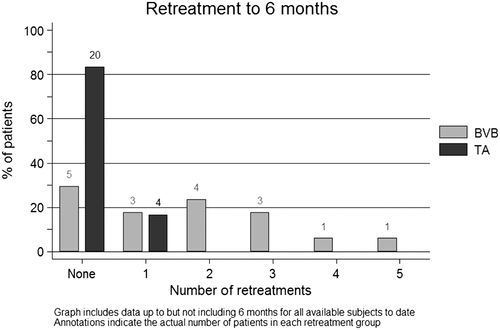
Following the initial intravitreous injection given at the time of cataract surgery, 70.6% of participants in the BVB group required further injections, compared to 16.7% in the TA group (P < 0.0001; refer Fig. 2). The means for number of retreatment injections were 1.71 and 0.17, respectively. No participants met the treatment failure guidelines or required macular laser during follow-up.
Over time, vision improved significantly from baseline in both treatment groups (mean +21.4 (95% CI [14.9, 27.9]) letters in TA group P < 0.0001, +12.5 (95%CI[4.8, 20.2]) letters in BVB, P = 0.002), with the overall mean Snellen equivalent VA at six months improving to between 6/12 and 6/15. However, the mean of all post-baseline letter score measurements did not differ significantly between the groups (P = 0.085; refer Fig. 3).
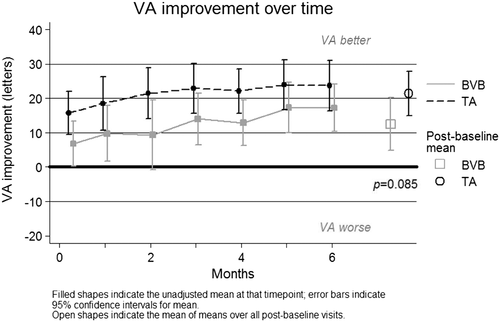
At baseline, one subject in each treatment group had vision in their study eye which would allow them to drive (≥69 letters, 6/12); they both retained this VA at 6 months. At 6 months, 60.9% of eyes receiving TA achieved driving vision, compared to 73.3% in the BVB group (P = 0.501).
The proportion of patients who achieved a visual acuity improvement of 15 letters or more at six months was 69.6% in the TA group and 60.0% in the BVB group (P = 0.728). The proportions for a 10-letter improvement were 82.6% and 73.3%, respectively (P = 0.687).
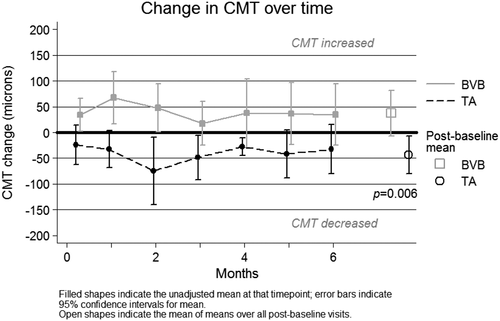
There was a significant difference in mean post-baseline change in CMT between the two groups over six months. The BVB group experienced a mean post-baseline increase of +37.3 microns (95% CI [−6.6, +81.3]), while the TA group experienced a mean post-baseline decrease of −43.8 microns (95% CI [−6.8, −80.8]), P = 0.006 (refer Fig. 4).
Previous treatments and treatment-naïve patients
There were no statistically significant differences between subjects who had previously received any treatment (BVB, TA or laser) compared to treatment naïve subjects in age, gender, baseline VA, CMT, DME status, macular severity or DR severity.
There were no statistically significant differences in change in VA or change in CMT in previously treated versus treatment naïve eyes (P = 0.875 for VA change; P = 0.995 for CMT change).
Need for retreatment during the study did not differ significantly in those previously treated eyes compared to treatment naive eyes (overall P = 0.647; in BVB study group P = 0.666; in TA study group P = 0.844). In addition, no eyes required any form of retinal laser during the course of the study.
Adverse events
Three patients experienced a rise of IOP over 21 mmHg (12.5%) during the six-month follow-up in the TA group; BVB had no cases (P = 0.130). In all cases the IOP rose to 25 mmHg or more and occurred at month four. One participant went on to have an IOP of 29 mmHg at month six and therefore did not receive any further TA injections. The IOP normalized with the use of two topical agents (timolol and brimonidine) that were then ceased over the following month without further rises in IOP.
There were no cases of endophthalmitis in either group, and no serious adverse events attributable to the study treatments were reported.
Discussion
With the projected increasing prevalence of diabetes, the co-existence of cataract and DR is set to increase over the next few decades. Our preliminary results have demonstrated that either intravitreous TA or BVB administered at the time of cataract surgery resulted in improved visual acuity at six months, with a trend towards greater improvement in the TA group. The TA group also experienced a significantly better mean CMT than the BVB group with a reduction in CMT (−43.8 µm) while the BVB group experienced slight thickening from baseline (+37.3 µm).
In contrast, prior studies have shown that visual outcomes after cataract surgery in patients with co-existing DME are poor, with one recent large study finding that only 53% of this group achieved a post-operative BCVA of 6/12 or better, with more than half either having no meaningful improvement in vision, or worse, loss of vision.9 This is in stark contrast to the visual results seen in individuals undergoing cataract surgery who do not have diabetes, where greater than 95% achieve a BCVA of 6/12 or better.12, 13
The development, or worsening, of DME after cataract surgery is the major cause for the observed poorer visual outcomes post cataract surgery.10, 31-33 Despite recent advances in the treatment of DME with anti-VEGF agents,17, 26, 34 the efficacy of these agents with treating or preventing post-operative DME are more mixed. Although some studies in patients with DR but no DME at the time of surgery have shown a short term benefit of a reduced CMT and BCVA at one to three months post-surgery in comparison to controls with no adjunctive treatment, this benefit was then largely lost at six months post operatively.20, 21, 35
In those with DME at the time of surgery, the results are even worse. In a prospective, non-comparative study of standard of care of individuals with DME undergoing cataract surgery, Bressler and colleagues found only 32% of patients had a significant improvement in vision (at least four lines), with 10% losing at least two lines of vision.9 This study also highlighted the lack of a consensus of how best to manage this group of patients, as only 7% of study eyes received intraoperative treatment, while 35% of all eyes did not receive any treatment for their DME throughout the entire study period. This variability in treatment may also account for the markedly different results achieved in comparison with our current study.
Outcomes with the use of anti-VEGF agents are also variable. Although one prospective study found a benefit with BVB treatment with reduced CMT and improved BCVA in comparison to controls at up to three months post operatively,36 two further prospective studies found that concurrent treatment with BVB or ranibizumab only prevented the worsening of DME post operatively in comparison to controls, with the actual CMT largely remaining unchanged from baseline, indicating that the anti-VEGF treatment failed to reduce the severity of the pre-existing DME post operatively.19, 37 Our pilot data supports these findings, as the CMT in our BVB group tended to increase slightly over the 6 month post-operative period, rather than improve, despite multiple re-treatments.
As increased inflammation induced by cataract surgery has been postulated to be the cause of DR and DME progression, the use of intravitreous TA (IVTA) at the time of cataract surgery has been advocated as potentially the best treatment for those with high risk DR or pre-existing DME undergoing surgery.38
The mechanism of action of IVTA in DR is thought to be due to its ability to both inhibit the actions of VEGF and to its anti-inflammatory effects.24 It has been found to reduce the breakdown of the blood-retinal barrier,39 inhibit the production of pro-inflammatory prostaglandins and also down regulate the production of VEGF.39-42 This theory is further supported by preliminary studies that have shown a beneficial effect on DME with a single ocular steroid injection administered in patients with diabetes undergoing cataract surgery.40, 43, 44
Our preliminary 6-month results support this hypothesis, as only the TA group maintained a sustained reduction in CMT in the 6-month post-operative period. Although not statistically significant, we also demonstrated a trend towards a greater improvement in BCVA post-surgery in the TA group in comparison to the BVB treated group.
Various adverse events have been reported with IVTA injections, with the most significant being endophthalmitis and raised intraocular pressure (IOP).45 Although complications such as endophthalmitis may occur with any intraocular procedure, raised IOP, which has been reported to occur in up to 23.5%,38, 40 is unique to TA injections and is not seen with BVB. Thus far, only 12.5% of our TA group have experienced an increased IOP rise after treatment – however time will tell as to whether this will increase with repeated treatments over an extended duration of follow up.
It is clear that the coexistence of visually significant cataract and DME will become increasingly common. Given our trial design, and the high proportion of patients who had DME at the time of surgery (89%) our results relate best to patients with concurrent DME undergoing cataract surgery. A significant proportion of our population were treatment naïve, and therefore these results may not be generalizable to patients who undergo intravitreous treatment in the lead up to surgery. Although such a treatment approach may also result in better outcomes, this approach has not yet been formally tested in a trial and may be logistically more difficult in a large, public hospital setting. A potential future study could incorporate this pre-operative treatment approach compared with, or in combination with, intra-operative treatment.
In conclusion, our preliminary findings have shown that treatment with either BVB or TA will result in a significant improvement in BCVA post cataract surgery. However, only the TA group maintained a sustained reduction in CMT through to 6 months, whereas the CMT tended to increase in the BVB group despite repeated postoperative injections. Despite these differences in CMT, no significant difference in BCVA or number of letters gained was found; however, longer term outcomes in a larger number of patients, as we intend to do over the next 6 months, will determine whether the differences in CMT will translate into better BCVA gains in the TA group.
Acknowledgments
The authors would like to extend thanks to Tanya Pejnovic and Carly Parfett for their coordination efforts in the study.