Contemporary retinal imaging techniques in diabetic retinopathy: a review
Abstract
Over the last decade, there has been an expansion of imaging modalities available to clinicians to diagnose and monitor the treatment and progression of diabetic retinopathy. Recently, advances in image technologies related to OCT and OCT angiography have enabled improved visualization and understanding of this disease. In this review, we will describe the use of imaging techniques such as colour fundus photography, fundus autofluorescence, fluorescein angiography, infrared reflectance imaging, OCT, OCT-Angiography and techniques in adaptive optics and hyperspectral imaging in the diagnosis and management of diabetic retinopathy.
Introduction
Diabetes is a major cause of blindness in the developing and developed world. The total number of people with diabetes is expected to rise to an estimated 300 million cases by the year 2025, with the most significant increases in developing countries.1Early detection of diabetic retinopathy (DR) can reduce the loss of visual acuity, blindness and associated morbidity. Proliferative diabetic retinopathy (PDR) is the most common vision-threatening lesion in type 1 diabetes, and diabetic macular oedema (DME) is more common in type 2 diabetes and is the primary cause of moderate visual loss.2
Fundus examination enables direct visualization of the retinal vasculature and associated pathology. The pathogenesis and clinical features of DR are primarily attributed to retinal vasculature damage.3 There are multiple imaging modalities that can acquire both two-demensional and three-dimensional images using invasive and non-invasive imaging techniques. Imaging technologies have changed rapidly in recent years with the advent of optical coherence tomography (OCT) and its clinical applications.3-5
A wide range of imaging techniques, including fundus photography, fluorescein angiography (FA), OCT and OCT-Angiography (OCTA), are used not only for the diagnosis and classification of disease but also to monitor disease progression over time. Additionally, large clinical trials such as the RISE, RIDE, VIVID, VISTA and DRCR.net studies have utilized OCT to analyse anatomical changes and to guide treatment decisions in the clinical setting. OCTA is a novel application of OCT that enables direct, non-invasive imaging of the retinal vasculature and is a promising area for future development in the imaging of DR. Techniques in image processing and segmentation of retinal vasculature are necessary for the accurate interpretation of acquired images. Adaptive optics (AO) techniques have been applied to scanning laser ophthalmoscopy and used to visualize microvascular structures of the retina, but have few clinical uses at this time (Figs 1-4).

In this review, we will describe the use of imaging techniques such as colour fundus photography, FA, OCT, OCTA and techniques in AO in the diagnosis and management of DR.
Fundus photography
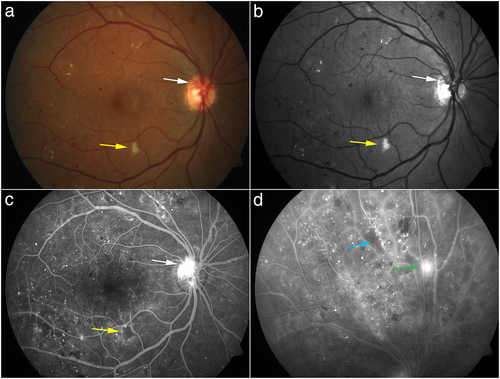
Fundus photography is an imaging technique commonly used in the clinical setting and in clinical trials to document the extent of disease. There are three types of fundus photography: colour fundus photography, red-free imaging and stereo fundus photography.6 Seven standard field colour fundus photographs have been used to identify features of DR such as microaneurysms, intraretinal haemorrhages, cotton-wool spots, venous beading and intraretinal microvascular abnormalities (IRMA).7 In current clinical practice, colour fundus photography can be used to document the status of DR, and especially useful in recording the distribution of hard exudates, retinal alterations in severe non-proliferative DR and the appearance of laser photocoagulation burns. Figure 1 is an example of multimodal imaging of a patient with proliferative diabetic retinopathy with findings on color fundus photography, red-free imaging, and fluorescein angiography.
Fundus photography has an adjunct role in detecting DR and grading DME, alongside clinical examination. Although it is rarely clinically necessary to make the diagnosis, it is used primarily for documentation and clinical trial purposes. It has also been used in a telemedicine setting where a physician may not be present to evaluate the patient but can evaluate the acquired images.
Digital imaging is currently widely used in the clinical and trial setting and has a high agreement with film photography.8 Digital fundus photography has been shown to be equivalent to stereoscopic film imaging in grading DME, although neither is as sensitive as an OCT.7 Widefield colour fundus photography used in conjunction with OCT is able to detect more DR than clinical examination alone and clinical examination combined with non-widefield imaging.9 New models of ultra-widefield imaging can even better image the periphery using colour fundus photography. Nonmydriatic ultra-widefield scanning laser ophthalmoscopy (Optomap) showed moderate correlation with two-field digital fundus photography when grading DR and DME.10 However, even with the detection advantage with widefield imaging, it is not quite clear what clinical advantage wide field imaging confers.
Digital fundus photography is a highly accessible, non-invasive and well-tolerated, rapid imaging procedure. Photographers can assess the quality of the image in real time. Nonmydriatic imaging is even better tolerated by patients because of less photosensitivity during imaging. However, the ability to interpret the image is limited by its quality, as well as the difficulty of assessing the three-dimensional cysts of DME with a two-dimensional image. Seven standard field colour fundus photographs are primarily used for research purposes because they are difficult and time consuming to obtain in actual clinical practice.
Future directions for this imaging modality include the expansion of nonmydriatic digital imaging as a tool for DR screening in telemedicine. In the US, despite recommendations for annual dilated eye exams in diabetics, less than 50% undergo a dilated retinal exam.11
A systematic review of the literature for both dilated and nonmydriatic single field fundus photography as a screening tool demonstrated that there is level 1 evidence that this imaging modality with interpretation by trained readers is overall very effective.12 A telemedicine screening programme targeting minority populations in an urban area utilizing nonmydriatic colour fundus images found that one in five people had DR, which represents an early point at which ophthalmologists can work alongside primary care physicians to monitor and prevent progression of DR with lifestyle modifications and lipid control. Colour fundus photography also identified non-DR ocular pathology in 44% of the participants.13
With the expansion of screening programmes for DR, there is increasing interest in automated algorithms to decrease the burden of manual DR screening. A review by Fleming et al. of the current literature on automated grading of DR found that automated grading, operating as a disease/no disease grader, is effective and could be used as a first step prior to manual review of the imaging. There is high sensitivity in disease/no disease grading, and there are several existing software algorithms that have successfully detected all DR with proliferative and pre-proliferative DR.14 Currently, no published algorithms for the automated detection of DR have been validated in large populations to be recommended for clinical use.6, 15 Most focus individually on a few findings such as microaneurysms, haemorrhages and exudates; an effective algorithm would integrate multiple findings. A recent study by Brady et al. investigated crowdsourcing as a novel technique for retinal fundus classification of DR, and while further studies are needed to validate this, it represents an interesting future direction in the analysis of colour fundus photography.16
Fundus autofluorescence and infrared imaging
Fundus autofluorescence (FAF) is an imaging modality that relies on the visualization of lipofuscin pigment in the retinal pigmented epithelium, which occurs in disease of the outer retina and is a hallmark of ageing cells.17 However, the origin and significance of increased FAF in DME is still unclear. The accumulation of lipofuscin, as associated with age, does not appear to be the pathogenic mechanism of DME, and more studies are needed to understand this mechanism.18, 19 Pece et al. described patterns of FAF patients with cystoid DME, including a multicystic pattern with increased FAF, a single cystic pattern with increased FAF and a combined single- and multi-cystic pattern.20 FAF may play a role in the diagnosis in the cystoid macular oedema. McBain et al. found that the diagnosis of CME based on FAF imaging had 81% sensitivity and 69% specificity when compared with the reference standard FA; however, the study population did not include those with DME.21
Scanning laser ophthalmoscopy with infrared reflectance imaging uses infrared light to illuminate the retina. It is useful in imaging through cloudy media, imaging in small pupils, and can be used to image through blood, retinal hyperpigmentation, heavy exudate or subretinal fluid. It can be used as an imaging modality to image diabetic patients with cystoid macular oedema, enabling quantification and localization of these cystic structures.22-25
Increased FAF has been shown to be associated with functional and structural impairment of the retina in DME. Chung et al. showed an association between visual acuity, spectral domain (SD)-OCT markers of photoreceptor integrity, and the amount of FAF in the foveal region in patients treated with intravitreal bevacizumab.26 In patients with diabetes with clinically significant macular oedema, increased FAF was noted to correlate with OCT patterns and central field microperimetry.27 In the clinical setting, however, fundus autofluorescence is not commonly used in the diagnosis or management of DME.
Fluorescein angiography
Fluorescein angiography (FA) is the current gold standard for visualizing retinal vasculature in vivo that has been used for over 30 years to assess chorioretinal disorders.28 It enables the staging of DR, visualization of PDR, areas of non-perfusion, IRMA, macular oedema, ischaemia and microaneurysms. It can also identify the source of fluorescein leakage for macular laser treatment and to monitor treatment response to anti-VEGF therapy.29 Widefield imaging has a larger field of view compared with the 7 standard view and is defined by a 200 degree view of the posterior pole that can detect more retinal pathology and especially areas of non-perfusion in patients with DR30 The ETDRS describes the use of FA to assess the severity of DR, particularly the focal and diffuse classification of leakage, which is becoming less relevant in the era of anti-VEGF treatment.31, 32
In current clinical practice, FA can be used as a tool to assess DR severity, DME and response to treatment. Microaneurysms manifest as areas of hyperfluorescence, and patchy areas of hypofluorescence represent ischaemia. It can also visualize expansion of the foveal avascular zone (FAZ) in DR, neovascularization of the disc and neovascularization elsewhere in PDR. Figure 2 shows multimodal imaging of a patient with proliferative diabetic retinopathy with examples of the findings outlined above.
Fluorescein angiographic findings in DR have been compared with OCT measurements of retinal thickness using data from the Diabetic Retinopathy Clinical Research Network. This study found that there were no FA variables that had a stronger association with visual acuity suggesting that FA does not offer a significant advantage over OCT in assessing the clinical significance of imaging findings. Simultaneous FA and SD-OCT showed that increased FA leakage of diabetic microaneurysms was correlated with perianeurysmal fluid and retinal thickness on OCT33, 34 Widefield FA was shown to detect additional peripheral retinal pathology in DR that would have been missed by ETDRS 7 standard field photography.35 However, it is unclear what the clinical significance of the additional peripheral pathology represents. Several studies have utilized FA to investigate the correlation between peripheral nonperfusion and the development of DME, but additional studies are required to further elucidate this relationship.36, 37
The major advantage of FA is that it is the only imaging modality commonly used in DR that provides information on vascular flow and vessel permeability over time by visualizing leakage and pooling. However, it is an invasive study that involves the administration of intravenous dye. This test should be performed carefully especially in patients with severe DR and associated systemic vascular complications such as severe renal disease and clinical or subclinical cardiovascular disease.38-40 The most common adverse reactions are nausea and vomiting, but more serious side effects include localized reactions, urticaria, seizures and, very rarely, anaphylaxis.41 Bearelly et al. reported anaphylaxis in only 4 of 1400 consecutive cases, and only 21 cases and 7 associated deaths have been reported in the literature in the last 55 years.42 In high-risk cases, prophylactic measures such as fluorescein desensitization can be used. However, prior to performing FA one must carefully consider whether the information provided is necessary to make therapeutic decisions and whether the same or equivalent information can be provided by a non-invasive method of imaging such as OCT.29
Future directions for the application of FA in DR include the automated software analysis of FA images. Widefield images have been used for automated calculation of ischaemia index in patients with DME, which demonstrated that recalcitrant DME had larger areas of retinal nonperfusion and required more macular photocoagulation treatments.43 In order to more reliably and reproducibly quantify the area and location of leakage in DME, Rabbani et al. report developing an automated segmentation algorithm that was reliable for different DME subtypes and congruent with expert manual segmentation.44
Optical coherence tomography
OCT has been one of the most widely used tools in clinical and research practice over the past two decades. It enables high-resolution, non-invasive imaging of the posterior segment and can assess retinal thickness and morphology, DME and choroidal morphology in patients with DR.45 OCT is primarily used in the management of patients with DME to monitor treatment response to anti-VEGF, but has other applications in visualizing other retinal pathology secondary to diabetes, as discussed in the succeeding texts.46 There are three types of OCT: time domain (TD), spectral-domain (SD) and swept-source (SS). Spectral domain devices are currently the most commonly used commercial OCT devices.
OCT performs well in diagnosing DME when compared with fundus stereo photography and biomicroscopy,47 is more sensitive than colour fundus photography48 and is currently the gold standard for the diagnosis and monitoring of DME. In DME, it can be used to visualize details of retinal anatomy, thickness profiles associated with different types of DME, and to determine whether it is centre -sparing or centre -involving; an important criterion in determining treatment.49 OCT is most useful in measuring and quantifying macular oedema; however, it can also identify other features such as hard exudates typically found in the outer plexiform layer, associated epiretinal and fibrovascular membranes, vitreomacular adhesion or traction, RPE hyperplasia, integrity of retinal layers and atrophy of the retina.50-52
OCT can be used to differentiate the four subtypes of DME: early, simple, cystoid and serous macular detachment, and can determine the presence of macular traction.53 It can assess the integrity of the IS/OS and ELM, which may assist in visual prognostication and can also determine the size of intraretinal cysts.53, 54 More recently, Byeon et al. investigated the OCT morphology of focal and diffuse DME, as identified on FA and found that the main difference between focal and diffuse was in the location of the pathology within the layers of the neurosensory retina. Diffuse leakage patterns showed fluid accumulation in the inner nuclear layer and outer plexiform layer, and focal leakage was noted to have fluid collections mostly in the outer plexiform layer and outer nuclear layer with minimal involvement of the inner nuclear layer.55 Although these may be indicators of prognosis classification of DME is rarely clinically useful in the era of anti-vascular endothelial growth factor (anti-VEGF) treatment.
Most importantly, OCT is used to monitor treatment response to anti-VEGF. In the RESTORE study, the improvements in visual acuity in response to ranizumab were accompanied by improvements in anatomical end points, central retinal thickness on OCT and resolution of leakage on fluorescein. In the RISE/RIDE study, ranibizumab-treated patients showed significant improvement in macular oedema, as noted on OCT as well as gains in visual acuity. The READ-2 study used OCT markers of subfoveal thickness as a secondary outcome, and a reduction in excess foveal thickness was noted to correlate with improvements in visual acuity.56-59
Patients with diabetes have altered choroidal morphology on SD-OCT. Subfoveal choroidal thickness, subfoveal medium choroidal vessel layer thickness and choriocapillaris thickness are all reduced in patients with PDR and DME, but not in less advanced disease.60, 61 Enhanced depth imaging can be used to better visualize the choroid; using this technique, Querques et al. found that macular choroidal thickness was less in patients with diabetes compared with age and gender matched controls.62 These findings are corroborated by previous findings on indocyanine green angiography and laser Doppler flowmetry that found reduced choroidal blood flow.63 This suggests choroidal thickness on OCT could be used as a marker for the progression of disease, although this is not a common parameter used in the clinical setting.
Vascular findings of DR such as PDR and microaneurysms are more commonly imaged in the clinical setting with colour fundus photography, FA and now OCTA However, OCT findings of these features have been described in the literature, even though this imaging modality is not often used to visualize microaneurysms and PDR in the clinical xsetting. OCT can be used to identify macular traction secondary to PDR and to look for the presence of epiretinal membrane in these patients. Cho et al. described findings on SD-OCT associated with retinal neovascularization. In neovascularization of the disc, connections between the optic disc and new vessels can be visualized. In IRMA, OCT shows a focal loss of retinal architecture at the location of the lesion. Additionally, SD-OCT enables visualization of features differentiating IRMA from NVE, which include hyperreflective dots in the superficial inner retina and vitreous as well as changes in the ILM such as outpouching and disruption.64, 65 OCT was able to assess structural differences in leaking versus non-leaking microaneurysms and the subsequent development of CSME. Leaking microaneurysms were found to have a capsular structure with the presence of hyperreflective dots on OCT.65
Overall, OCT offers many advantages as a part of routine clinical imaging. It is a rapid, non-invasive study that can be easily performed at each visit. Multiple OCT B-scans can be merged to form an en face image for further analysis. Novel applications of OCT technology that are applicable to DR include OCTA and Doppler OCT, which measures total retinal and choroidal blood flow and may provide a quantitative analysis of disease progression.
OCT angiography
OCT-Angiography (OCTA) is a relatively new imaging technique that uses SD-OCT for three-dimensional visualization of the retinal and choroidal vessels without the injection of a contrast dye. The image is generated through the analysis of decorrelation signals, which are produced by the movement of erythrocytes between individual scans. The split-spectrum amplitude decorrelation algorithm is then applied to reduce the signal to noise ratio and generate a cleaner image.66
Changes in retinal vasculature associated with DR have been observed on OCTA In DR, areas of ischaemia can be observed at the posterior pole or the mid-periphery. As with FA, OCTA can visualize the increase in the size of the FAZ and perifoveal intercapillary area (Salz et al., data in press). As the ischaemia progresses, interruptions in the macular capillary arcades have also been shown to appear on OCTA, as well as microvascular features such as fine vessel tortuosity and capillary loops.67, 68
OCTA is able to detect the majority of microaneurysyms seen on FA which appear as capillary loops, dilated capillary segments or focal dilations instead of areas of pinpoint hyperfluorescence as visualized on FA, (Salz et al., data in press) and may show some microaneurysms that FA fails to detect. Ishibazawa et al. noted some dot-like capillaries on OCT angiogram not picked up by FA.69 Regardless, OCTA enables the reader to pinpoint the exact intraretinal depth of the microaneurysm because segmentation software allows the reader to differentiate between the superficial and deep vascular plexuses. Recently, Huang et al. found that some areas of focal leakage on FA thought to be microaneurysms were found to be small tufts of neovascularization that extended above the ILM as visualized on OCTA70
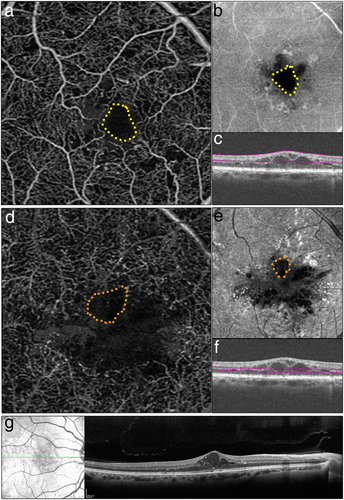
It is also possible to visualize cystoid spaces associated with DME and to differentiate between these spaces and areas of capillary non-perfusion (Chin et al., data in submission), based on the pattern of the surrounding vasculature. In DME, cystoid spaces are smooth, oblong areas and areas of capillary non-perfusion have irregular borders. Figure 3 is an example of DME as viewed on OCTA, the corresponding OCT-B scan, and enhanced depth imaging (EDI) OCT.
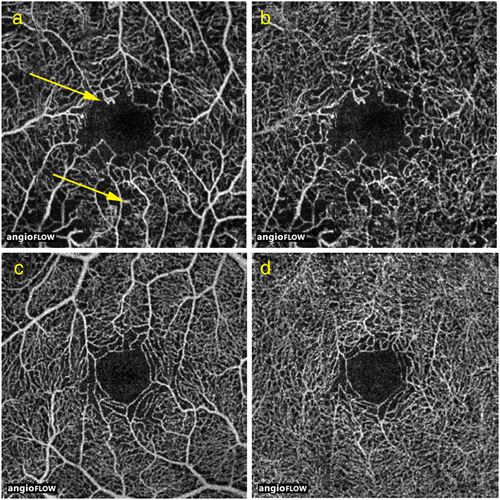
While FA is still considered the gold standard for imaging retinal vasculature in vivo, OCTA has the advantage of being a non-invasive, relatively quick imaging study that can be performed alongside routine OCT imaging.67 A structural image is acquired simultaneously, which allows the reader to make a direct correlation between retinal OCT B-scans and the OCT angiogram. FA and ICG are dynamic studies that can show leakage, staining and pooling over time, while OCTA shows vascular flow at a given cross section in time. While it cannot visualize alterations in vascular permeability, fine vascular structure that would otherwise be obscured by hyperfluorescence in FA and ICG studies can be more clearly seen with OCTA However, FA does not image radial peripapillary or deep capillary networks well compared with OCTA, as leakage can obscure fine vascular structures.71 OCTA images are depth-resolved, which enables clinicians to scroll through an en face image of the retinal and choroidal vasculature or view automated segmentation at the levels of the superficial and deep plexuses, outer retina, choriocapillaris and choroidal vasculature. In the clinical setting, FA images are typically viewed two-dimensionally; however, these images can be viewed with stereo visualization, which enables a qualitative assessment of depth. Figure 4 shows examples of microvascular changes in a patient with proliferative diabetic retinopathy on OCTA, including microaneurysms, vessel tortuosity, and areas of ischemia.
A major limitation of OCTA is the scan size, with the greatest resolution achieved at smaller scanning sizes such as the commonly used 3 × 3 mm scan. Since the same number of A-scans are used regardless of the scanning size, a larger scanning area will yield a less dense image with reduced resolution. Additionally, OCTA is subject to projection artefacts, in which vasculature from outer layers is projected onto the deep plexuses and choriocapillaris, which can affect the accurate interpretation of vascular pathology in the deeper layers. It is also prone to movement artefact; patient movement presents as horizontal white lines, and artefact because of blinking appears as black lines across the image.68
OCTA represents a promising area of retinal imaging technology that can potentially be applied in the screening, monitoring and treatment of DR. It can be used to monitor expansion of the FAZ and response to treatments such as anti-VEGF and focal laser photocoagulation, as well as to visualize choriocapillaris changes underlying areas of laser photocoagulation. Most of the current literature describing OCTA of DR has been small studies that are cross sectional in nature, and larger, prospective studies represent future directions for OCTA research. Additionally, since a limitation of current OCTA is scan size, the development of widefield OCTA would enable better evaluation of peripheral vasculature.
Adaptive optics
Adaptive optics (AO) is a tool to correct ocular aberrations that can be added to any ophthalmic imaging technology, which thus far has been adapted to scanning laser ophthalmoscopy and OCT. AO employs a wavefront sensor to measure the eye's aberrations and a wavefront corrector to compensate for them72, 73 Currently, there is only one commercial AO–scanning laser ophthalmoscopy model with limited clinical use. The confocal imaging allows for high-resolution imaging of retinal microvasculature. Real-time imaging can look at blow flow dynamically and can measure erythryocyte velocity at different points in retinal vasculature.74 A limitation of AO systems is the small field of view, which in most systems is 1–2°. To overcome this issue, Burns et al. developed a steerable AOSLO combined with a wide-field line scanning ophthalmoscope to guide the clinician to the region of interest.75
Given the high-contrast and high-resolution imaging of microvasculature, this method of retinal imaging is useful in visualizing microvascular changes that are not visible on other imaging methods. It can be used to measure vessel lumen and perform arteriole wall measurements. In diabetic eyes, changes in the photoreceptor layer showed areas of dark regions that matched areas of overlying vascular remodelling. Microvascular changes that could be visualized with AO–SLO include irregular branching of retinal blood vessels, the presence of shunt vessels, capillary sprouts and variable diameter of vessel branches.76 Lombardo et al. noted that perifoveal capillaries had a narrowed diameter in diabetics compared with controls.77
Currently, AO can be used to further investigate the subclinical microvascular changes that occur in diabetic eyes. Further applications of this imaging technique to the clinical setting is limited because of the small size of the field view.
Hyperspectral imaging and retinal oximetry
Hyperspectral imaging utilizes the different spectral properties of oxygenated and deoxygenated haemoglobin. Arterio-venous differences in oxygen saturation can be mapped in disease states. Multiple experimental approaches to retinal oximetry have been developed but are not commonly employed in the clinical setting. A major limitation of this imaging modality at the present time is the time-intensive image acquisition and image processing, as well as signal to noise ratio limitations and difficulty in reproducibly measuring areas with poor illumination.78-81
In patients with DR, Tiedeman et al. showed that subjects with hyperglycaemia had increased retinal O2 consumption.82 A Fourier transform spectral imaging system has been used to map ischaemia in central retinal vein occlusions with oxygen saturation maps.83 This could be applied to mapping areas of ischaemia in DR.
Summary for clinicians
Over the last decade, there has been an expansion of imaging modalities available to clinicians to diagnose and monitor the treatment and progression of DR. Particularly, OCT in combination with anti-VEGF treatment has helped revolutionize the detection and treatment of DME. This review highlights the unique advantages and disadvantages of each and presents a brief review of the current literature. Colour fundus photographs and FA are primarily used for the initial documentation, diagnosis and staging of DR. They can be used to evaluate and identify areas of macular ischaemia, leaking microaneurysms for focal laser photocoagulation and retinal neovascularization. OCT can be used to evaluate and monitor DME, macular traction or detachment because of fibrovascular or epiretinal membranes that can often develop in patients with diabetes, as well as the response to treatment. Techniques in AO are limited by the small scan area and require further development to be applicable in clinical practice. Hyperspectral imaging and retinal oximetry are largely experimental techniques that have not been widely applied in the clinical setting. OCT angiography allows clinicians to view a depth-resolved angiogram of the retinal and choroidal vasculature as well as corresponding B-scans and an en face image to correlate angiographic findings. In the future, it may become an important imaging modality in the everyday clinical setting to better assess retinal and choroidal vasculature as we learn how to better interpret this novel technology. These improvements in ancillary exam technology alongside routine clinical examination allows for more precise treatment and monitoring of DR with the goal of improving visual outcomes for patients with diabetes.