Muscle magnetic resonance imaging findings in patients with idiopathic inflammatory myopathies
Abstract
Background
Idiopathic inflammatory myopathies (IIMs) are disorders that cause chronic muscle inflammation and weakness due to an autoimmune pathogenesis. Dermatomyositis (DM) is a typical IIM disorder, along with others including antisynthetic syndrome (ASS), immune-mediated necrotizing myopathy (IMNM), overlap myositis, inclusion body myositis (IBM), and anti-mitochondrial M2 antibody-positive myositis (AMA-myositis). Noninvasive muscle magnetic resonance imaging (MRI) is useful for determining the distribution, nature, and extent of lesions in affected muscles. T1-weighted MRI is useful for observing morphological changes, including muscle atrophy. Short tau inversion recovery images or T2-weighted images are useful for detecting muscle inflammation and edema and are suitable for selecting optimal biopsy sites. Muscle MRI is also useful for follow-up studies.
Results
On muscle MRI, patients with DM show a symmetric pattern with prominent fasciitis. The MRI findings in ASS are similar to those in DM. In IMNM, MRI findings are asymmetric, present a rapid and severe course, and fasciitis is less prominent. In IBM, atrophy is more severe at presentation than in other IIMs, and fasciitis is absent. In AMA-myositis, fasciitis is severe and atrophy is mild.
Conclusion
Muscle MRI can help differentiate between IIMs along with using other laboratory findings, including myositis-specific antibodies.
1 INTRODUCTION
Idiopathic inflammatory myopathies (IIMs) or myositis are disorders that cause muscular inflammation and muscle weakness due to an autoimmune pathogenesis. Other organs, such as the skin, joints, lungs, gastrointestinal tract, and heart, are also often affected in IIMs, contributing to morbidity and mortality. Dermatomyositis (DM) is one of the representative diseases of IIMs. In addition, anti-synthetase syndrome (ASS), immune-mediated necrotizing myopathy (IMNM), overlap myositis, inclusion body myositis (IBM), and anti-mitochondrial M2 antibody(AMA)-positive myositis (AMA-myositis) are included in IIMs. Although the degree of muscle atrophy and weakness can be estimated by surface and manual muscle strength tests (MMTs), obtaining detailed information on them is difficult. Needle electromyography (EMG) is useful for differentiating between neurogenic and myogenic changes; however, identification of deep muscles is not easy. Although muscle biopsy is one of the most valuable examinations in the differential diagnosis of neuromuscular disorders (NMDs) owing to its invasive nature, it is permitted to be performed in a single region and once alone. Furthermore, biopsies cannot be used to estimate the spread of the disturbed muscles.
Muscle computed tomography (CT) and muscle T1-weighted magnetic resonance images (MRI) are useful for obtaining information on the muscle size. Muscle MRI, short-tau inversion recovery (STIR) images,1 or T2 weighted images are useful for assessing muscle inflammation and edema and are powerful tools for selecting muscle biopsy sites, while muscle MRI is also useful for follow-up of IIMs.
2 MUSCLE CT AND MUSCLE MRI
The advantages of muscle CT are as follows: changes in muscle morphology, atrophy, or hypertrophy can be observed easily and quickly (within 5 min) and the images are suitable for investigating fatty degeneration and calcification. However, muscle CT cannot be used to determine the site of inflammation and is therefore not suitable for selecting optimal muscle biopsy sites. In addition, muscle CT exposes patients to radiation and the boundaries between muscles are unclear, especially in young men. In contrast, among older adults, especially women, the boundaries are clearer (Figure 1).2 In muscular dystrophy, fat infiltrates the affected muscles and the muscle CT value decreases more significantly than the reduction in muscle size.3
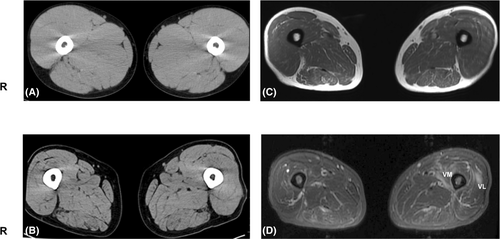
Muscle MRI has recently become a popular diagnostic tool. In particular, T2-weighted images reveal sharp changes in fat and water in the skeletal muscles (Figure 1). To estimate fatty infiltration, MRI T1-weighted imaging is as useful as muscle CT, Moreover, it has excellent resolution and is useful for observing morphological change.4-6 T2-weighted imaging is also a useful modality because both increased water and fat infiltration are detected as high signals. Further, T2 weighted imaging detects the distribution of inflammation in the muscles. In the STIR images, only edema and inflammation show high-intensity signals, and fatty degeneration shows isointensity. Therefore, T2-weighted and STIR images are useful for selecting optimal biopsy sites for IIMs. Muscle MRI is non-invasive in contrast to EMG or muscle biopsy and can also determine the nature of deep muscles that are not estimated by EMG and MMTs. Differences between coworking muscles cannot be easily evaluated using MMTs. Muscle MRI is useful for monitoring therapeutic efficacy and natural disease progression of IIMs. MRI involves no radiation exposure and information can be obtained regardless of the examiner's technique. However, it is not possible to distinguish between neurogenic and myogenic changes using muscle MRI alone. Therefore, nerve conduction tests, EMG, and muscle biopsies are important for the diagnosis of NMDs. Usually, a single MRI test can provide a limited range of muscle information, typically including only the upper extremities, trunk, and neck, or only the lower extremities and trunk. Therefore, muscle MRI is not suitable for whole-body screening. However, recent advances in MRI techniques have increased the speed of image acquisition, making whole-body examinations increasingly feasible.7 Moreover, special consideration is needed to test patients with pacemakers or metal implantations. In summary, MRI is a frequently used imaging modality in cases of suspected IIMs because it is sensitive in detecting the disease, pinpointing its anatomical location, and displaying its pattern in both the acute and chronic stages. The schematic anatomy of the cross-sectional views of the upper and lower extremities is shown in Figure 2.8
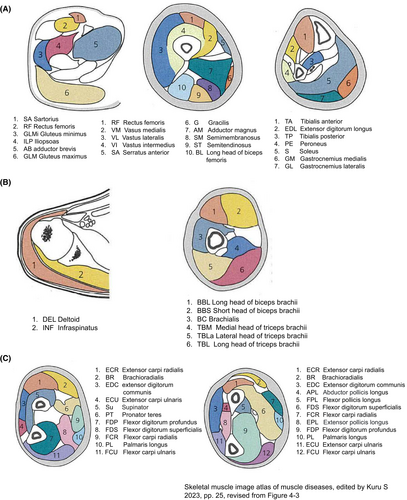
3 IDIOPATHIC INFLAMMATORY MYOPATHIES
IIMs include DM, ASS, IMNM, overlap syndrome, AMA-positive myositis, and IBM. The classification criteria for IIMs were updated by the American College of Rheumatology and European League against Rheumatisms in 2017.9 In 2023, consensus recommendations of imaging indications and MRI protocols in patients with suspected IIMs were established by experts form Europe and United States.10 Baseline imaging is highly recommended in all patients with suspected IIMs and MRI is the preferred imaging modality. The generally recommended minimum MRI protocol includes T1-weighted and STIR or fat-saturated sequences for the thighs or other affected body parts (e.g., pelvis, lower leg). Intravenous MRI contrast agents are not recommended.10, 11 The affected muscles exhibit hyperintense signals on STIR MRI, reflecting an increase in tissue water content. Edema can be found in muscles without muscle weakness, and subclinical changes can be captured using muscle MRI. Muscle atrophy and fat degeneration may occur during the chronic stages of IIMs. Muscles with fatty degeneration show T1 or T2 hyperintense signals and isointensity on STIR images (Table 1).12 In IIMs, average diagnostic delay is 2.3 years (95% CI 1.3–3.4) with frequent reports of initial misdiagnosis (normal aging, infection) that may induce inappropriate treatment (antibiotics) and even increase mortality.13 Recently, consensus-updated guidelines for IIM-associated cancer screening have been published14 and highlight the increased risk of malignancy in patients with adult-onset IIMs, except IBM, including lung, ovarian, corolectal, lymphoma, breast and nasopharyngeal cancers.14
| Histology | T1-WI | T2-WI | STIR |
|---|---|---|---|
| Edema・inflammation | Iso | High | High |
| Fatty degeneration | High | High | Iso |
| Fibrosis | Low | Low | Low |
- Note: Revised from12
- Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; WI, weighted imaging.
3.1 Dermatomyositis
Dermatomyositis is a representative disorder of IIMs and typically has a subacute course. Systemic symptoms include fever, general malaise, fatigue, and weight loss; however, their specificity is low. Muscle symptoms include weakness of the trunk and proximal muscles of the extremities, cervical muscles, and pharyngeal muscles. The cutaneous symptoms of Gottron's sign and heliotrope rashes have high disease characteristics and are referred to as typical rashes. To date, five DM-specific autoantibodies (DMSAs) have been identified: Mi-2, MDA5, NXP2 (nuclear matrix protein-2), TIF1-γ, and SAE, and approximately 80% of patients with DM are positive for DMSAs.15 On MRI, the quadriceps (vastus lateralis (VL) and VI muscles) and gluteal muscles are most commonly affected. The edema pattern is often patchy and may be characterized by a preferential perimysial and epimysial pattern of inflammation. MRI often reveals edema and fluid signals, involving the fascial and subcutaneous soft tissues that precede the edema in the muscle itself. In the chronic setting, atrophy and fatty infiltration of the involved muscle are best demonstrated on T1-weighted sequences but are generally mild. Chronic fasciitis may be seen as low-signal fascial ticking.1, 16
3.1.1 Case presentation
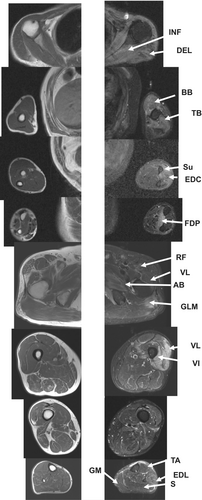
A 67-year-old man was hospitalized because of proximal muscle weakness. One year ago, he had elevated serum creatine kinase (CK) levels (2000 IU/L) with a decline in grip power; however, he had no difficulties in performing activities of daily living. At the time of hospitalization, he had sun sensitivity and Gotton signs. Neurological findings revealed upper extremity predominant muscle weakness as follows: grip power of 15/9 kg and MMTs (Medical Research Council Scale: MRC) of the deltoid (DEL) 3/3, first dorsal interossei 2/2, iliopsoas (ILP) 5/4, quadriceps 5/4, and tibialis anterior (TA) muscle 5/4. Laboratory examination revealed high CRP (1.54 mg/dL), and high CK (2446 IU/L) levels, with anti-Mi-2 antibody positive, and TIF-1γ antibody negative. Muscle MRI STIR imaging revealed high-intensity signals in the infraspinatus (INF), DEL, biceps brachii (BB), triceps brachii (TB), supinator (Su), extensor digitorum communis (EDC), and flexor digitorum profundus (FDP) muscles. On STIR imaging, the gluteus maximus (GLM), rectus femoris (RF), adductor brevis (AB), vastus intermedius (VI), and extensor digitorum longus (EDL) muscles of the lower limbs show high-intensity signals (Figure 3).8
3.2 Anti-synthetase syndrome (ASS)
Anti-aminoacyl-tRNA synthetase (ARS)-positive myositis is referred to as ASS and the age of onset is usually 34–59 years. In ASS, a variety of extramuscular symptoms, such as interstitial pulmonary disorders, mechanic's hand, Raynaud's phenomenon, arthritis, and fever are observed.17 In ASS patients with muscle-predominant symptoms, anti-Jo-1 or anti-OJ antibodies were positive, and CK levels were also elevated. In patients with anti-EJ, PL-7, and PL-12 antibodies, interstitial pneumonia was predominant and CK levels were normal or mildly elevated.17 In ASS, skeletal muscle MRI shows several edematous changes, mainly in the fascia and edema under the skin, similar to findings observed in DM. In ASS, thigh MRI most frequently shows myofascial edema (90%), followed by fatty muscle infiltration (69%), muscle edema (63%), and subcutaneous edema (61%).18 The pattern of edema on MRI is similar to that of DM and is characteristically bilaterally, with a predilection for the tensor fascia latae and relative sparing of the adductor muscles18 (Table 2). The most representative MRI findings in ASS are bilateral asymmetry, relative sparing of the adductor muscle, and significant myofascial edema of the tensor fascia latae.18
| Disease | Epidemiology | Clinical signs | Autoantibodies |
|---|---|---|---|
| DM | Bimodal (5–15, 40–50 years old) M:F 1:2 |
|
Anti-MDA5 Anti-Mi2 Anti-TIF-1 Anti-NXP-2 Anti-SAE1 |
| ASS | Adult onset (48 ± 15 years old) M:F = 3:7 |
|
Anti-Jo-1 Anti-OJ Anti-EJ |
| IMNM | Older age of onset (>50 years old) |
|
Anti-SRP Anti-HMGCR |
| IBM | >45 years old M:F 2:1 |
|
Anti-cN1A |
| Overlap syndrome | Female predominance Adult onset (30–59 years old) |
Anti-Ko Anti-PM/Scl Anti-Ro(SSA) Anti-La (SSB) Antiu1-RNP Anti-U3RNP/fibrillarin |
|
| AMA myositis | 40 to 65 years old Female predominant |
|
Anti-mitochondrial M2 |
3.2.1 Case presentation
A 77-year-old-man with pulmonary fibrosis complicated by emphysema was admitted to our hospital with generalized muscle weakness. He first complained of shoulder pain during exercise and presented with a heliotrope rash and shawl sign. The MMTs were as follows: proximal muscles of the lower limbs, 3/3 and upper limbs, 4–5/4–5. Laboratory examination revealed an aldolase level of 53.8 IU/L, positive antinuclear antibodies, and positive anti-Jo-1 antibodies. In the upper extremities, MRI STIR images revealed high-intensity signals in the INF, DEL, BB, medial head of the triceps brachii (TBM), and flexor pollicis longus (FPL) muscles. High-intensity signals were observed in the fascia of the extensor carpi ulnaris (ECU) muscle. T1 weighted imaging showed mild atrophy of the forearm and upper limbs. STIR imaging revealed high-intensity signals in the GLM, AB, and RF muscles of the lower extremities. T1 weighted imaging revealed proximal atrophy in the GLM, RF, BL, semitendinosus (ST), VL, and VI muscles. High-intensity signals were observed for the VI, semimembranosus (SM), TA, vastus medialis (VM), BL, gastrocnemius medialis (GM), and tibialis posterior (TP) muscles, and the fascia. Subcutaneous edema was observed on the buttocks and shoulders (Figure 4).19

3.3 Immune-mediated necrotizing myopathy
The average age at onset of patients with IMNM is 36–52 years, with the disorder being slightly more common in women. Muscle symptoms are usually more severe and steroid-resistant in recurrent myositis than in other IIMs. In IMNM, symptoms are usually limited to muscles and are not accompanied by lung symptoms; however, they are sometimes complicated by malignant tumors. Muscle pathology is characterized by marked necrosis, regeneration, and poor lymphocyte infiltration. Two specific autoantibodies, SRP (signal recognition particle) and HMGCR (3-hydroxy methyl-glutaryl[HMG]-CoA-reductase) were identified.20 IMNM cases negative for both antibodies have also been reported.20 General atrophy, especially in the proximal portion of the upper extremities, was observed in the patients with SRP-positive IMNMs (Table 2). The STIR images revealed high-intensity signals in the INF, BB, TB, BR, and S muscles. T1 weighted imaging demonstrated general proximal atrophy. STIR images revealed high-intensity signals in the AB, IL, GLM, GLMi, AM, BL, VI, VL, SM, and GM as well. In general, the IMNM has a predilection for muscles in the hip region (e.g., glutei and obturator externus) and thigh regions (e.g., anterior and posterior compartments), sometimes sparing the VI. IMNM tends to cause more prominent and diffuse muscle edema on MRI in acute settings. Fascial edema is minimal (Table 3).21, 22
| Disease | Distribution and pattern | Other features | Fasciitis |
|---|---|---|---|
| DM |
|
|
|
| ASS |
|
|
|
| IMNM |
|
|
|
| IBM |
|
|
|
| Overlap myositis |
|
- | |
| AMA-myositis |
|
|
|
- Note: Revised from 1, 7, 10, 16, 18, 27
- Abbreviations: AB, adductor brevis; AM, adductor magnus; AMA, anti-mitochondrial M2 antibody-positive myositis; ASS, antisynthetic syndrome; BL, long head of the biceps femoris; DM, dermatomyositis; EDL, extensor digitorum longus; GL, gastrocnemius lateralis; GLM, gluteus maximus; GM, gastrocnemius medialis; IBM, inclusion body myositis; IIMs, idiopathic inflammatory myopathies; ILP, iliopsoas; IMNM, immune-mediated necrotizing myopathy; RF, rectus femoris; S, soleus; SM, semimembranosus; ST, semitendinosus; STIR, short-tau inversion recovery; TA, tibialis anterior; TP, tibialis posterior; VI, vastus intermedius; VL, vastus lateralis; VM, vastus medialis; WI, weighted imaging.
3.3.1 Case presentation
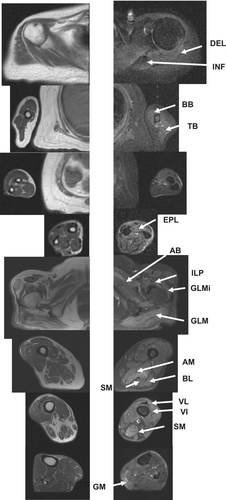
A 52-year-old woman was admitted to the authors' hospital. She complained of hand stiffness for the past 2 years. She had difficulty opening the cap of a plastic bottle. On admission, her grip power was 8/7 kg. The MMT (MRC scale) was as follows: DEL, 4/4; EDC, 4/4; and ILP, 3/3. Laboratory examination revealed a CK level of 1928 IU/L, and anti-SRP antibody positivity. Muscle MRI STIR images detected diffuse high-intensity signals in the DEL, INF, BB, TB, and EPL muscles. T1 weighted imaging detected atrophy of BB and TB muscles. The GLM, AB, IL, GLMi, VL, VI, AM, BL, and SM muscles of the lower extremities showed high-intensity signals in STIR images, and atrophy in T1 weighted image (Figure 5).23
3.4 Overlap myositis
Overlap myositis (also known as connective tissue disease overlap syndrome) is a subtype of IIMs, in which autoimmune diseases coexist. Overlapping myositis is diagnosed when a patient fulfills the criteria for both IIMs and an underlying autoimmune connective tissue disease (SS, SLE, RA, SS).24 MRI features vary depending on the underlying disease process but include bilateral systemic edema involving the gluteal ant thigh muscles.25
3.5 Anti-mitochondrial M2 antibody positive myositis
Anti-mitochondrial M2 antibodies are autoantibodies associated with primary biliary cholangitis (PBC). It develops in middle- and older-aged adults, is more common in women, and presents as progressive muscle weakness and atrophy. It frequently causes heart problems, including arrhythmia and heart failure and is not necessarily associated with PBC.26, 27 In our institute, among 63 IIMs, five patients tested AMA positive.27
3.5.1 Case presentation
A 53-year-old woman with atrial flattening and left ventricular hypofunction showed elevated CK levels on laboratory examination. The patient was then referred to a neurologist by cardiologist. Upon admission, the patient had mild weakness in the ILP muscle. Laboratory examination revealed high CK (1687 IU/L) and KL6 (124 U/mL) levels, and anti-mitochondrial M2 antibody positivity. T1-weighted MRI revealed no apparent muscle atrophy. The STIR images detected high-intensity signals in the INF, TB, and FDP muscles. In the lower extremities, the ILP, GLM, VI, VM, VL, SM, BL, and AM exhibited high-intensity signals. The lower leg muscles were only mildly affected. Fascial edematous high-intensity signals were detected in the ILP, VM, VL, GM, and GL muscles (Figure 6).
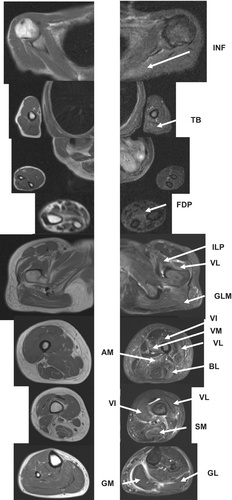
3.6 Inclusion body myositis
Inclusion body myositis is a gradually progressive myositis that occurs in patients older than 50 years, with an expected occurrence of 5–10 cases per 1 million people.28 Typical initial symptoms include muscle weakness and atrophy of the deep finger flexors and quadriceps muscles in muscle pathology. The MRI findings corresponded well with this, showing a characteristic pattern of impairment with signal changes in the flexors of the deep fingers and quadriceps muscles. As this disease is rare, patients and clinicians are not aware of it, and diagnostic difficulties contribute to a considerable delay between the initiation of symptoms and diagnosis.29 Although IBM is traditionally included in the group of IIMs, recent reports have revealed a peculiar progression of muscle degeneration and characteristic abnormal accumulation of aggregated proteins, including TDP43, amyloid β, tau, and p62.30 As the myositis specific antibody, cytosolic 5′-nucleotide 1A (cN1A) antibody is identified.31 Although MRI may show muscle edema in one-third of patients, the indolent nature of the disease usually results in fatty infiltration (up to 100%) and atrophy (>90%) in a typically bilateral asymmetric pattern. The FDP is the most affected muscle in the forearm owing to fat infiltration.32 Advanced fatty infiltration and muscle atrophy can result in wavy contours of the fascia. The sartorius muscle, which is not usually affected in other adult myopathies, is commonly involved. Severe fat infiltration of the VL muscle and significantly less severe effect on the RF muscle are obsreved.2 Fascial edema was typically absent.16
3.6.1 Case presentation
A 52-year-old man presented to our hospital with lower muscle weakness at the age of 50 years. Laboratory examination revealed mildly elevated CK levels (814 IU/L). Muscle MRI T1 weighted imaging revealed fatty degeneration in the BB, FDP, and Su muscles of in the upper extremities. On STIR imaging, high-intensity signals were observed in the TB, INF, and FDP muscles. On T1-weighted imaging, the GLMi, GLM, VL, VI, BL, PE, and GM muscles of the lower extremities showed fatty degeneration. STIR imaging detected high-intensity signals in the GL and AM muscles, while the lower limb muscles showed mild high-intensity signals (Figure 7).33
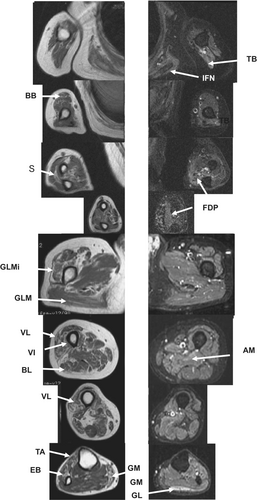
4 LIMITATIONS OF MUSCLE MRI
In routine clinical practice, MRI diagnosis is made qualitatively, and the determination of hyperintensity on STIR or T2-weighted imaging is mainly subjective. The muscle hyperintensity signal is relative to the muscles that are considered normal. Therefore, it is the basis for the fact that reliability is not perfect. In practice, however, there was good agreement among observers when it came to detecting myoedema with STIR or gadolinium-enhanced fat suppression T1-weighted imaging.34 For example, the concordance rate was moderate in the axial muscles, but the concordance rate was almost perfect in the proximal muscles, including the gluteus muscles.34
Muscle pathological changes in IIMs are considered specific. However, the hyperintensity signals muscle MRI cannot be said to be specific. Hyperintensity in STIR or T2-weighted imaging can also occur in other conditions. First of all, a moderately hyperintense signal is always noted in the distal part of the GM muscle, which is located at the attachment on the soleus muscle. The hyperintensity signals in STIR or T2-weighted images are also induced by exercise.35 In normal muscles, signal intensity increases during and after exercise on T2-weighted or STIR images. The distribution of exercise-induced signal changes appears to be very similar to that of hyperintensity observed in IIMs patients. Therefore, exercise is one of the factors that can be misdiagnosed as IIMs.
On STIR or T2-weighted imaging, various diseases such as traumatic, vascular, infectious, and inflammatory disorders also show high-intensity signals. Statin myopathy, or hypokalemic myopathy, also shows high intensity signals in the impaired muscles. Even in a variety of neurogenic diseases, including amyotrophic lateral sclerosis,36 acute motor axonal neuropathy, or lumbar neuropathy,37 muscles show a high intensity range only for 4 days when denervation occurs on STIR or T2-weighted imaging.
5 MUSCLE MRI AND DISEASE ACTIVITY ASSESSMENT
Muscle MRI is an important method for the assessment of disease activity in IIMs. Past studies have shown a good correlation between the degree of inflammation in the biopsied muscle and the degree of STIR hyperintensity signals.38, 39 In patients with high disease activity, especially those with high CK values, the hyperintensity range (semi-quantitative assessment) on STIR imaging is significantly higher than in patients with low disease activity.34, 40 In most IIMs patients with low disease activity, MRI signals were normalized.34, 40 In DM, the sensitivity of femoral muscle MRI to muscle disease activity was estimated to reach 90%.40 IMNM found a correlation between high intensity on STIR images and favorable therapeutic responses22, 41; Therefore, muscle MRI, along with CK values, proved to be a very good marker of IIMs disease activity.
6 CONCLUSIONS
The DEL, INF, and GLM muscles are easily damaged during IIMs and therefore should be carefully monitored. Muscle MRI is useful not only for the selection of optimal biopsy sites but also for the follow-up of patients with IIMs. In addition, characteristics of the muscles affected by IIMs have gradually been clarified.1, 16, 18, 21, 22, 27 Muscle MRI can be used together with other laboratory findings, including myositis-specific autoantibodies, EMG, and muscle biopsy, for the differential diagnosis of NMDs, including IIMs. Muscle MRI also can be a good marker for disease activity and therapeutic responses.
ACKNOWLEDGMENTS
A part of this study was supported by the JSPS KAKEN Grant Number JP (22K07392) and Research Grant of University of Fukui (LS124105). We are grateful to the doctors in the Department of Neurology at the University of Fukui and to Dr. Yasutaka Kawamura and Dr. Hirohiko Kimura of the Department of Radiology, University of Fukui, and Dr. Minoru Hasegawa of the Department of Dermatology. We are grateful to Dr. Ichizo Nishino of the National Center of Neurology and Psychiatry and Dr. Itsuro Higuchi of the Third Department of Internal Medicine at Kagoshima University.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study protocol was approved by the Review Board of the University of Fukui (No. 1007, February 27, 2014). The ethics committee waived the need for informed consent because of the retrospective nature of this study.
DISCLOSURE OF ETHICAL STATEMENTS
Studies involving human participants were reviewed and approved by the Institutional Review Board of the University of Fukui (No. 1007, February 27, 2014). The ethics committee waived the need for informed consent because of the retrospective nature of this study. Approval of the research protocol: This study was approved by the University of Fukui's institutional review board (20170130); Informed Consent: N/A; Registry and the Registration No. of the Study/Trial: N/A; Animal Studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




