Clinicopathological features and prognosis of familial papillary thyroid carcinoma – a large-scale, matched, case–control study
Summary
Objective
It remains controversial whether or not the aggressiveness of familial nonmedullary thyroid cancer (FNMTC) differs from sporadic carcinoma. The aim of this study was to determine the clinicopathological features and prognosis of FNMTC.
Design
A matched-case comparative study.
Methods
Three hundred and seventy-two patients with familial papillary thyroid carcinoma (FPTC) were enrolled as the study group, and another 372 patients with sporadic PTC were enrolled as controls and matched for gender, age, tumour/node/metastasis (TNM) staging and approximate duration of follow-up. We compared the differences in the clinicopathological features and prognosis between the subgroups.
Results
Compared with sporadic PTC, patients with FPTC were more likely to present tumour multicentricity, bilateral growth and a concomitant nodular goitre (P < 0·05). In papillary thyroid microcarcinoma (PTMC), a higher recurrence rate was noted in patients with a family history of PTC, and this remained independently predictive on multivariate analysis. The patients with FPTC in the second generation showed an earlier age of onset, more frequent Hashimoto's thyroiditis and a higher recurrence rate than the first generation, while the first-generation offspring of patients had a higher incidence of nodular goitre than the second generation.
Conclusions
The presence of familial history in PTC indicates an increase in biological aggressiveness, and patients in the second generation may exhibit the ‘genetic anticipation’ phenomenon. At present, the available data are not sufficient to support a more aggressive approach for FPTC. However, a family history of PTC is an independent risk factor for recurrence in patients with PTMC.
Introduction
Papillary thyroid carcinoma (PTC) accounts for >80% of all thyroid malignancies that arise from thyroid follicular cells, with a reported 10-year survival of >90%.1 Despite relatively mild biological behaviour and an excellent prognosis, some clinicopathological parameters tend to be associated with a worse clinical outcome, such as massive extrathyroid extension, lateral lymph node metastasis and distant metastasis at diagnosis.1 A family history of nonmedullary thyroid cancer (NMTC) is regarded as an independent risk factor for recurrence, especially in younger patients.2 Although most cases of NMTC are sporadic with no family history, familial forms have been reported, comprising approximately 5% of affected patients.3 Indeed, familial nonmedullary thyroid cancer (FNMTC) is considered to be a distinct clinical entity.4 FNMTC is characterized by two or more first-degree relatives affected by thyroid cancer of follicular cell origin without another familial syndrome or exposure to factors known to cause thyroid cancer.4 However, unlike familial medullary thyroid cancer (FMTC), caused by germline point mutations in the RET protooncogene,5 the genetic basis of FNMTC as a distinct syndrome remains poorly understood.
Several case–control studies have reported the clinicopathological features and prognosis of FNMTC, but whether or not FNMTC is more aggressive than sporadic disease is controversial, with an equal number of supporters and detractors.2, 6-9 Several studies have reported FNMTC to be more aggressive than sporadic disease, with an increased risk of tumour multicentricity, bilateral growth, nodal involvement, concomitant benign thyroid nodules and vascular invasion.2, 6-8 Thus, more aggressive treatment for patients with FNMTC has been recommended, including total thyroidectomy and central compartment/modified radical neck dissection, as well as adjuvant ablative iodine radiotherapy, followed by lifelong post-operative thyroid hormone suppressive therapy.2, 6-8 Another study has reported a similar course and outcome for FNMTC.9
In this retrospective study, we elicited detailed family histories of PTC from 8195 patients who sought evaluation at our clinic, and investigated the difference in clinical characteristics and outcomes between familial PTC and the sporadic PTC counterpart among this relatively large group of patients with PTC.
Patients and methods
Patients
A total of 8195 patients who underwent surgical treatment and/or adjuvant ablative iodine radiotherapy for PTC in the Department of Head and Neck Cancer Center of Zhejiang Cancer Hospital between January 2006 and December 2014 were enrolled in our study. Three hundred and seventy-two patients (4·54%) were classified with familial papillary thyroid carcinoma (FPTC) because the patients had one or more first-degree relatives diagnosed with PTC. Patients with a history of neck surgery for other diseases, familial cancer syndromes or prior exposure to radiation were excluded from this study; 220 patients underwent initial surgical treatment in our centre and 152 patients underwent a second surgical treatment and/or adjuvant ablative iodine radiotherapy in our centre after the initial surgical treatment. Furthermore, we selected 372 patients with sporadic papillary thyroid carcinoma (SPTC) from the 8195 patients who served as controls. The two groups were matched by number of patients, gender, age, tumour/node/metastasis (TNM) staging and approximate duration of follow-up (Table 1). In the FPTC group, 41 patients (11·02%) belonged to families with ≥3 affected members and the remaining 331 patients (88·98%) belonged to families with two affected members. One hundred and thirty-nine patients (37·37%) were of the parent/offspring type, 225 (60·48%) were of the sibling type, and 8 (2·15%) were of the parent/offspring/sibling type. Among the parent-/offspring-type patients, 23 (15·65%) were first-generation offspring and 124 (84·35%) were second-generation offspring. Furthermore, the patients with FPTC were subgrouped on the basis of tumour size (≤1·0 or >1·0 cm in largest diameter), and 217 (58·33%) patients had familial papillary thyroid microcarcinoma (FPTMC), while sporadic papillary thyroid microcarcinoma (SPTMC) patients accounted for 61·83% (230/372) of the patients with SPTC. Information regarding a family history of PTC was obtained from the hospital records and/or from the patients. This study was approved by the Ethics Committee of Zhejiang Cancer Hospital, and all of the patients signed informed consent.
| Variables | Familial PTC, n (%) | Sporadic PTC, n (%) |
|---|---|---|
| 372 (100) | 372 (100) | |
| Gender | ||
| Woman | 277 (74·46) | 277 (74·46) |
| Man | 95 (25·54) | 95 (25·54) |
| Age (years) | ||
| Mean (range) | 44·07 (16–72) | 44·07 (16–72) |
| Median | 44·00 | 44·00 |
| Follow-up time (months) | ||
| Mean (range) | 34·97 (4–108) | 34·97 (4–108) |
| Median | 29·88 | 29·93 |
| T staging | ||
| pT1 | 285 (76·61) | 295 (79·30) |
| pT2 | 23 (6·18) | 31 (8·33) |
| pT3 | 44 (11·83) | 38 (10·22) |
| pT4 | 20 (5·38) | 8 (2·15) |
| N staging | ||
| pN0/Nx | 183 (49·19) | 174 (46·77) |
| pN1a | 120 (32·26) | 129 (34·68) |
| pN1b | 69 (18·55) | 69 (18·55) |
| M staging | ||
| M0 | 367 (98·66) | 370 (99·46) |
| M1 | 5 (1·34) | 2 (0·54) |
| TNM staging | ||
| I | 280 (75·27) | 267 (71·77) |
| II | 10 (2·69) | 5 (1·34) |
| III | 49 (13·17) | 67 (18·01) |
| IV | 33 (8·87) | 33 (8·87) |
- PTC, papillary thyroid carcinoma.
Pre-operative diagnostic protocol
Ultrasonography (US) was performed in all patients in our series to determine lymph node status, tumour location, size and multicentricity. We performed fine-needle aspiration biopsy (FNAB), guided either by ultrasonography or palpation when the nodule was suspected to be thyroid carcinoma. When a lymph node in the lateral compartment was suspected to be a metastasis, we performed additional FNAB of the node to decide whether the patient required therapeutic modified radical neck dissection (MND). Tests of thyroid function, including triiodothyronine (T3), basal plasma-free T3 (FT3), thyroxine (T4), basal plasma-free T4 (FT4), thyroglobulin (Tg), thyroid-stimulating hormone (TSH), peroxidase antibody (TPOAb) and thyroglobulin antibody (TgAb), were also studied prior to surgery. Other imaging studies, such as CT scan and radioiodine scan, were also performed as necessary to evaluate pulmonary metastases and mediastinal lymph nodes.
Therapy
In our study, the therapeutic strategies for patients with PTC were made according to the guidelines of the Chinese Thyroid Association, whether or not there was a first-degree family history of PTC. The patients with bilateral PTC underwent total thyroidectomy and bilateral central compartment node dissection (CCND), while hemithyroidectomy and ipsilateral CCND were performed for unilateral patients with PTC. Total thyroidectomy might be considered if unilateral patients with PTC met one or more of following conditions: multifocal in one lobe, tumour size >4 cm, extrathyroid invasion or distant metastasis. Modified radical neck dissection (mRND) was performed only in patients with biopsy-proven lateral neck node metastases. Furthermore, post-operative radioactive iodine remnant ablation was performed in some patients, and all patients in this study received post-operative thyroid hormone suppressive therapy.
Pathology
The diagnosis of PTC and evaluation of pathological parameters, such as capsule invasion, multicentricity, bilateral growth and lymph node metastasis, were performed by pathologists who specialize in thyroid pathology. Pathology consultations were carried out by the same pathologists for all patients who underwent initial surgical treatment in other hospitals. The TNM classification was estimated according to the 2010 AJCC criteria.10
Post-operative follow-up
Follow-up was performed after completion of treatment in our centre every 3 months for the first 2 years by clinical examination, ultrasonography and blood tests, including TPOAb, TgAb, T3, FT3, T4, FT4, TSH and Tg levels, and then every 6 months. A chest radiograph or CT scan was obtained once a year. In this study, the mean post-operative follow-up periods were 34·97 months (range, 4–108 months; median, 29·88 months) and 34·97 months (range, 4–108 months; median, 29·93 months) for the FPTC and SPTC groups, respectively. A recurrence was diagnosed when suspicious lesions were apparent on imaging and confirmed by FNA after initial treatment.
Statistical analysis
Chi-square test and Fisher's exact test were used to compare the categorical parameters between the subgroups, while the Kaplan–Meier method and log-rank test were adopted to analyse time-dependent variables. Prognostic factors that were significant on univariate analysis were further evaluated using the multivariate Cox models for independent significance. These analyses were performed using the statistical package for social sciences (SPSS, Inc., Chicago, IL, USA). P values <0·05 were considered statistically significant, and P values >0·05 were considered nonsignificant (NS).
Results
Of the 8195 enrolled patients, 372 were diagnosed with FPTC and the prevalence of FPTC was 4·54%. As shown in Fig. 1, we calculated the prevalence of FPTC during different diagnostic periods (2006–2008, 2009–2011 and 2012–2014); the prevalence of FPTC gradually increased with time. We analysed the pathological data for FPTC and SPTC based on pathological reports. Based on univariate analysis, the incidences of multicentricity, bilateral growth and nodular goitre in patients with FPTC were significantly higher than in patients with SPTC (P < 0·05), as shown in Table 2. Other clinicopathological and outcome characteristics were also compared between the patients with FPTC and SPTC, but no significant difference was found between the two groups (P > 0·05; Table 2). The results of the FPTC with ≥3 affected elements were similar to FPTC with two affected elements, with the exception of bilateral growth (Table 2).
| Variables | Overall | Subgroup with family history | ||||
|---|---|---|---|---|---|---|
| Sporadic PTC (n = 372) | Familial PTC (n = 372) | P | Two affected members (n = 331) | Three or more affected members (n = 41) | P | |
| Bilaterality | ||||||
| Unilateral | 267 (71·77) | 208 (55·91) | 0·000 | 191 (57·70) | 17 (41·46) | 0·049 |
| Bilateral | 105 (28·23) | 164 (44·09) | 140 (42·30) | 24 (58·54) | ||
| Multicentricity | ||||||
| Solitary | 225 (60·48) | 168 (45·16) | 0·000 | 155 (46·83) | 13 (31·71) | 0·063 |
| Multiple | 147 (39·52) | 204 (54·84) | 176 (53·17) | 28 (68·29) | ||
| Maximal tumour diameter | ||||||
| ≤1 (cm) | 230 (61·83) | 217 (58·33) | NS | 193 (58·31) | 24 (58·54) | NS |
| >1 (cm) | 142 (38·17) | 155 (41·67) | 138 (41·69) | 17 (41·46) | ||
| Capsule invasion | ||||||
| Absent | 179 (48·12) | 180 (48·39) | NS | 153 (46·22) | 27 (65·85) | NS |
| Present | 129 (34·68) | 141 (37·90) | 131 (39·58) | 10 (24·39) | ||
| Extracapsular | 64 (17·20) | 51 (13·71) | 47 (14·20) | 4 (9·76) | ||
| Intrathyroidal dissemination | ||||||
| Present | 38 (10·22) | 34 (9·14) | NS | 28 (8·46) | 6 (14·63) | NS |
| Absent | 334 (89·78) | 338 (90·86) | 303 (91·54) | 35 (85·37) | ||
| Thyroid nodular goitre | ||||||
| Present | 134 (36·02) | 189 (50·81) | 0·000 | 167 (50·45) | 22 (53·66) | NS |
| Absent | 238 (63·98) | 183 (49·19) | 164 (49·55) | 19 (46·34) | ||
| Hashimoto's thyroiditis | ||||||
| Present | 56 (15·05) | 41 (11·02) | NS | 33 (9·97) | 8 (19·51) | NS |
| Absent | 316 (84·95) | 331 (88·98) | 298 (90·03) | 33 (80·49) | ||
| Thyroid adenoma | ||||||
| Present | 14 (3·76) | 23 (6·18) | NS | 20 (6·04) | 3 (7·32) | NS |
| Absent | 358 (96·24) | 349 (93·82) | 311 (93·96) | 38 (92·68) | ||
| Total thyroidectomy | ||||||
| Done | 154 (41·40) | 206 (55·38) | 0·000 | 178 (53·78) | 28 (68·29) | 0·074 |
| Not done | 218 (58·60) | 166 (44·62) | 153 (46·22) | 13 (31·71) | ||
| Lymph node dissection | ||||||
| None | 13 (3·50) | 22 (5·91) | NS | 20 (6·04) | 2 (4·88) | NS |
| CCND only | 277 (74·46) | 265 (71·24) | 240 (72·51) | 25 (60·98) | ||
| CCND with MRND | 82 (22·04) | 85 (22·85) | 71 (21·45) | 14 (34·15) | ||
| Iodine radiotherapy | ||||||
| Done | 90 (24·19) | 172 (46·24) | 0·000 | 152 (45·92) | 20 (48·78) | NS |
| Not done | 282 (75·81) | 200 (53·76) | 179 (54·08) | 21 (51·22) | ||
| Recurrence of disease | ||||||
| Present | 20 (5·38) | 29 (7·80) | NS | 25 (7·55) | 4 (9·76) | NS |
| Absent | 352 (94·62) | 343 (92·20) | 306 (92·45) | 37 (90·24) | ||
| Death from disease | ||||||
| Present | 1 (0·27) | 1 (0·27) | NS | 1 (0·30) | 0 (0·00) | NS |
| Absent | 371 (99·73) | 371 (99·73) | 330 (99·70) | 41 (100·00) | ||
- PTC, papillary thyroid carcinoma; CCND, central compartment node dissection; mRND, modified radical neck dissection. The bold values, less than or close to 0.05.
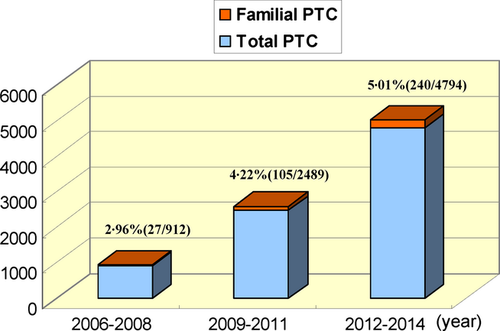
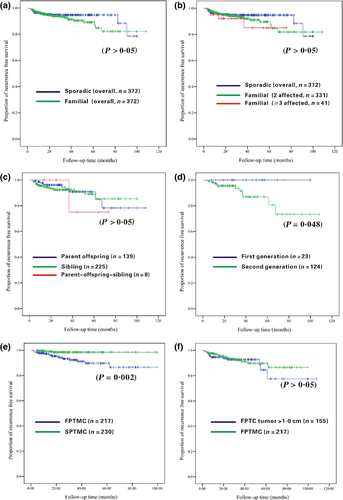
Table 3 presents the clinicopathological and outcome features of the FPTC patients with different hereditary forms; only the age of onset showed a significant difference (P < 0·05) between the parent/offspring, sibling, and parent/offspring/sibling types of patients with FPTC. The age of onset of the parent/offspring type of patients with FPTC was much younger than in the other two hereditary forms of FPTC. In addition, we performed a more thorough analysis of the clinicopathological features of the parent/offspring type of patients with FPTC in different generations. As shown in Table 3, there were significant differences in the age of onset, the incidences of concomitant nodular goitre or Hashimoto's thyroiditis, and recurrence between the first- and second-generation groups. The second-generation offspring patients generally had an earlier age of onset, more frequent Hashimoto's thyroiditis and a higher recurrence rate than the first generation, while the first-generation offspring patients had a later age of onset, but were more likely to have a nodular goitre.
| Variables | Hereditary forms | Parent/offspring type FPTC | |||||
|---|---|---|---|---|---|---|---|
| Parent/offspring type (n = 139) | Sibling type (n = 225) | Parent/offspring/sibling type (n = 8) | P | First generation (n = 23) | Second generation (n = 124) | P | |
| Age (years) | |||||||
| <45 | 93 (66·91) | 94 (41·78) | 5 (62·50) | 0·000 | 2 (8·70) | 96 (77·42) | 0·000 |
| ≥45 | 46 (33·09) | 131 (58·22) | 3 (37·50) | 21 (91·30) | 28 (22·58) | ||
| Gender | |||||||
| Men | 33 (23·74) | 57 (25·33) | 5 (62·50) | NS | 3 (13·04) | 35 (28·23) | NS |
| Women | 106 (76·26) | 168 (74·67) | 3 (37·50) | 20 (86·96) | 89 (71·77) | ||
| Bilaterality | |||||||
| Unilateral | 84 (60·43) | 121 (53·78) | 3 (37·50) | NS | 12 (52·17) | 75 (60·48) | NS |
| Bilateral | 55 (39·57) | 104 (46·22) | 5 (62·50) | 11 (47·83) | 49 (39·52) | ||
| Multicentricity | |||||||
| Solitary | 71 (51·08) | 95 (42·22) | 2 (25·00) | NS | 12 (52·17) | 61 (49·19) | NS |
| Multiple | 68 (48·92) | 130 (57·78) | 6 (75·00) | 11 (47·83) | 63 (50·81) | ||
| Maximal tumour diameter | |||||||
| ≤1 (cm) | 81 (58·27) | 130 (57·78) | 6 (75·00) | NS | 15 (65·22) | 72 (58·06) | NS |
| >1 (cm) | 58 (41·73) | 95 (42·22) | 2 (25·00) | 8 (34·78) | 52 (41·94) | ||
| Capsule invasion | |||||||
| Absent | 67 (48·20) | 106 (47·11) | 7 (87·50) | NS | 12 (52·17) | 62 (50·00) | NS |
| Present | 54 (38·85) | 86 (38·22) | 1 (12·50) | 7 (30·43) | 48 (38·71) | ||
| Extracapsular | 18 (12·95) | 33 (14·67) | 0 (0·00) | 4 (17·39) | 14 (11·29) | ||
| Intrathyroidal dissemination | |||||||
| Present | 13 (9·35) | 20 (8·89) | 1 (12·50) | NS | 1 (4·35) | 13 (10·48) | NS |
| Absent | 126 (90·65) | 205 (91·11) | 7 (87·50) | 22 (95·65) | 111 (89·52) | ||
| T staging | |||||||
| pT1 | 108 (77·70) | 179 (79·56) | 8 (100·00) | NS | 17 (73·91) | 99 (79·84) | NS |
| pT2 | 12 (8·63) | 19 (8·44) | 0 (0·00) | 2 (8·70) | 10 (8·06) | ||
| pT3 | 16 (11·51) | 22 (9·78) | 0 (0·00) | 3 (13·04) | 13 (10·48) | ||
| pT4 | 3 (2·16) | 5 (2·22) | 0 (0·00) | 1 (4·35) | 2 (1·61) | ||
| N staging | |||||||
| pN0/Nx | 60 (43·17) | 110 (48·89) | 4 (50·00) | NS | 13 (56·52) | 51 (41·13) | NS |
| pN1a | 53 (38·13) | 74 (32·89) | 2 (25·00) | 7 (30·43) | 48 (38·71) | ||
| pN1b | 26 (18·71) | 41 (18·22) | 2 (25·00) | 3 (13·04) | 25 (20·16) | ||
| M staging | |||||||
| M0 | 139 (100·00) | 223 (99·11) | 8 (100·00) | NS | 23 (100·00) | 124 (100·00) | NS |
| M1 | 0 (0·00) | 2 (0·89) | 0 (0·00) | 0 (0·00) | 0 (0·00) | ||
| Thyroid nodular goitre | |||||||
| Present | 74 (53·24) | 111 (49·33) | 4 (50·00) | NS | 19 (82·61) | 59 (47·58) | 0·001 |
| Absent | 65 (46·76) | 114 (50·67) | 4 (50·00) | 4 (17·39) | 65 (52·42) | ||
| Hashimoto's thyroiditis | |||||||
| Present | 20 (14·39) | 20 (8·89) | 1 (12·50) | NS | 1 (4·35) | 20 (16·13) | 0·006 |
| Absent | 119 (85·61) | 205 (91·11) | 7 (87·50) | 22 (95·65) | 104 (83·87) | ||
| Thyroid adenoma | |||||||
| Present | 9 (6·47) | 14 (6·22) | 0 (0·00) | NS | 3 (13·04) | 6 (4·84) | NS |
| Absent | 130 (93·53) | 211 (93·78) | 8 (100·00) | 20 (86·96) | 118 (95·16) | ||
| Total thyroidectomy | |||||||
| Done | 77 (55·40) | 122 (54·22) | 7 (87·50) | NS | 15 (65·22) | 69 (55·65) | NS |
| Not done | 62 (44·60) | 103 (45·78) | 1 (12·50) | 8 (34·78) | 55 (44·35) | ||
| Lymph node dissection | |||||||
| None | 7 (5·04) | 14 (6·22) | 1 (12·50) | NS | 1 (4·35) | 7 (5·65) | NS |
| CCND only | 101 (72·66) | 160 (71·11) | 4 (50·00) | 16 (69·57) | 89 (71·77) | ||
| CCND with MRND | 31 (22·30) | 51 (22·67) | 3 (37·50) | 6 (26·09) | 28 (22·58) | ||
| Iodine radiotherapy | |||||||
| Done | 58 (41·73) | 110 (48·89) | 4 (50·00) | NS | 10 (43·48) | 52 (41·94) | NS |
| Not done | 81 (58·27) | 115 (51·11) | 4 (50·00) | 13 (56·52) | 72 (58·06) | ||
| Recurrence of disease | |||||||
| Present | 10 (7·19) | 18 (8·00) | 1 (12·50) | NS | 0 (0·00) | 11 (8·87) | 0·048 |
| Absent | 129 (92·81) | 207 (92·00) | 7 (87·50) | 23 (100·00) | 113 (91·13) | ||
| Death from disease | |||||||
| Present | 1 (0·72) | 0 (0·00) | 0 (0·00) | NS | 0 (0·00) | 1 (0·81) | NS |
| Absent | 138 (99·28) | 225 (100·00) | 8 (100·00) | 23 (100·00) | 123 (99·19) | ||
- FPTC, familial papillary thyroid carcinoma; CCND, central compartment node dissection; mRND, modified radical neck dissection. The bold values, less than or close to 0.05.
Table 4 shows the relationship of tumour size with the clinicopathological, treatment modalities and outcome characteristics of FPTMC vs SPTMC and FPTC tumour >1·0 cm. With regard to pathological features, there was a statistically significant difference related to bilateral growth, multicentricity and concomitant nodular goitre (P < 0·05) between FPTMC and SPTMC. Moreover, the Kaplan–Meier survival analyses revealed that there was statistically significant difference in recurrence-free survival (RFS) between the two subgroups (7·31% vs 1·30%; P = 0·002; Fig. 2e). In multivariate analysis, the presence of a family history of PTC was recognized as an independent risk factor for recurrence in patients with PTMC (P = 0·004). As compared with FPTMC, there was a trend in FPTC tumour >1·0 cm to show more frequently capsule invasion and intrathyroidal dissemination (P < 0·05), but the difference in RFS was not statistically significant (P > 0·05).
| Variables | FPTMC (n = 217) | SPTMC (n = 230) | P | FPTC tumour >1·0 cm (n = 155) | P |
|---|---|---|---|---|---|
| Bilaterality | |||||
| Unilateral | 129 (59·45) | 169 (73·48) | 0·002 | 79 (50·97) | NS |
| Bilateral | 88 (40·55) | 61 (26·52) | 76 (49·03) | ||
| Multicentricity | |||||
| Solitary | 107 (49·31) | 141 (61·30) | 0·011 | 61 (39·35) | NS |
| Multiple | 110 (50·69) | 89 (38·70) | 94 (60·65) | ||
| Capsule invasion | |||||
| Absent | 148 (68·20) | 137 (59·57) | NS | 32 (20·65) | 0·000 |
| Present | 57 (26·27) | 77 (33·48) | 84 (54·19) | ||
| Extracapsular | 12 (5·53) | 16 (6·96) | 39 (25·16) | ||
| Intrathyroidal dissemination | |||||
| Present | 8 (3·69) | 16 (6·96) | NS | 26 (16·77) | 0·000 |
| Absent | 209 (96·31) | 214 (93·04) | 129 (83·23) | ||
| Thyroid nodular goitre | |||||
| Present | 118 (54·38) | 85 (36·96) | 0·000 | 71 (45·81) | NS |
| Absent | 99 (45·62) | 145 (63·04) | 84 (54·19) | ||
| Hashimoto's thyroiditis | |||||
| Present | 25 (11·52) | 35 (15·22) | NS | 16 (10·32) | NS |
| Absent | 192 (88·48) | 195 (84·78) | 139 (89·68) | ||
| Thyroid adenoma | |||||
| Present | 15 (6·91) | 11 (4·78) | NS | 8 (5·16) | NS |
| Absent | 202 (74·54) | 219 (95·22) | 147 (94·84) | ||
| Total thyroidectomy | |||||
| Done | 102 (47·00) | 83 (36·09) | 0·019 | 104 (67·10) | 0·000 |
| Not done | 115 (53·00) | 147 (63·91) | 51 (32·90) | ||
| Lymph node dissection | |||||
| None | 13 (5·99) | 12 (5·22) | NS | 9 (5·81) | 0·000 |
| CCND only | 176 (81·11) | 192 (83·48) | 89 (57·42) | ||
| CCND with MRND | 28 (12·90) | 26 (11·30) | 57 (36·77) | ||
| Iodine radiotherapy | |||||
| Done | 72 (33·18) | 37 (16·09) | 0·000 | 100 (64·52) | 0·000 |
| Not done | 145 (66·82) | 193 (83·91) | 55 (35·48) | ||
| Recurrence of disease | |||||
| Present | 16 (7·37) | 3 (1·30) | 0·001 | 13 (8·39) | NS |
| Absent | 201 (92·63) | 227 (98·70) | 142 (91·61) | ||
| Death from disease | |||||
| Present | 0 (0·00) | 1 (0·43) | NS | 1 (0·65) | NS |
| Absent | 217 (100·00) | 229 (99·57) | 154 (99·35) | ||
- FPTMC, familial papillary thyroid microcarcinoma; SPTMC, sporadic papillary thyroid microcarcinoma; PTC, papillary thyroid carcinoma; FPTC, familial papillary thyroid carcinoma; CCND, central compartment node dissection; mRND, modified radical neck dissection.
Discussion
With the increased incidence of thyroid cancer, patients with FPTC have also increased in number. Based on the data presented herein, patients with FPTC accounted for 4·54% (372/8195) of all patients with PTC treated in our centre, which is in agreement with a previous study.3 Until now, whether or not FNMTC is more aggressive than SNMTC has not been thoroughly studied, although some results have been published. Several studies have shown that FNMTC is more aggressive than SNMTC,2, 6-8 whereas another study has reported a similar course and outcome between FNMTC and SNMTC.9 In this study,the FPTC subgroup showed a significantly higher incidence of tumour multicentricity, bilateral growth and concomitant nodular goitre. Despite a more aggressive approach (total thyroidectomy and central compartment/modified radical neck dissection) in patients with FPTC, the recurrence rate was higher compared with SPTC, particularly in families with ≥3 affected members, although not reaching statistical significance, and the families with two affected members had an intermediate risk of recurrence. Thus, we thought that the number of family members affected should be considered. Previous population studies have shown that kindreds with only two affected family members with thyroid cancer probably represent a fortuitous aggregation of sporadic cases, hence diluting any possible differences.11 Kindreds with ≥3 affected members should be a focus in future studies. We suggest that if we conduct research in patients with FPTC, the aggressiveness of FPTC will become more apparent.
The issue concerning the impact of family history of PTC on RFS or overall survival is also not consensual. One study reported that the RFS in patients with FNMTC is significantly shorter than SNMTC, especially in the subset of patients with ≥3 affected members.9 However, another study reported that no significant differences existed in relation to disease recurrence between the patients with FNMTC and the SNMTC.12 In the current study, although we have matched both familial and sporadic PTC series for gender, age, TNM staging and approximate duration of follow-up, the results were similar with a previous study,9 but did not reach statistical significance.
There are three hereditary forms of FPTC (parent/offspring, sibling and parent/offspring/sibling types), and each form has unique clinical characteristics. In a classic study based on a large-sample size, Park et al.6 reported that the parent/offspring FPTC exhibited more frequent extrathyroidal invasion and a higher recurrence rate than SPTC, while sibling FPTC exhibited a higher prevalence of females, smaller tumour size and a higher incidence of Hashimoto's thyroiditis than SPTC. In the current study, despite an earlier onset of disease in the parent/offspring patients with FPTC, specifically the second-generation offspring, we did not detect other significant differences in the clinicopathological and outcome characteristics between the three hereditary forms of FPTC. When we subdivided the parent/offspring FPTC into first- and second-generation FPTC groups, we showed that the second-generation group was associated with an earlier age at diagnosis and a higher recurrence rate than the first-generation group, supporting the presence of ‘genetic anticipation,’ which is defined as the occurrence of a genetic disorder at an earlier age and increased severity in successive generations.7 Thus, FPTC diagnosed in the second generation requires more aggressive treatment than the first-generation FPTC, and screening for thyroid cancer should start earlier in the offspring of patients with thyroid cancer.
The relationship between benign thyroid disease and thyroid cancer is a topic of widespread interest. In families with FNMTC patients, other relatives often have nodular goitres. Uchino et al.13 screened family members when 2 first-degree relatives were affected with thyroid carcinoma, and 52% (77/149) of the screened family members were shown to have thyroid nodules. This rate was similar to that observed by Sadowski et al.14; specifically, 44% (20% of the second generation and 90% of the first generation) of the screened family members presented with thyroid nodules. Too much screening for the purpose of detecting early-stage thyroid carcinoma is not recommended in recent times, but screening with a focus on high-risk groups, such as first-degree relatives of patients with FNMTC, is advocated.15 FNMTC is a distinct clinical entity with a high incidence of multiple benign nodules and familial clustering of multinodular goitre.4, 16 In the current study, the prevalence of combined nodular goitre in the FPTC group was 50·81%, which was higher than the prevalence in the SPTC group. Furthermore, coexisting nodular goitre is more common in first-generation patients than second-generation cases (82·61% vs 47·58%, P < 0·05). Hashimoto's thyroiditis is another benign thyroid disease, and it has been suggested that it is commonly associated with PTC.17 In a recently published article,6 it was reported that the prevalence of Hashimoto's thyroiditis in patients with FNMTC was 27·9%, and Hashimoto's thyroiditis was particularly evident in sibling FNMTC cases. In the current study, despite a higher incidence of coexisting Hashimoto's thyroiditis in families with ≥3 affected members and parent/offspring cases, there was no statistical difference; however, the second-generation offspring had a higher incidence of Hashimoto's thyroiditis (P < 0·05), which is an autoimmune disease that most commonly exhibits familial aggregation.8 Thus, ultrasound screening is recommended in first-degree relatives of patients with FNMTC, and a careful evaluation should be performed if the patient has a thyroid nodule, especially when there are any suspicious findings.
Papillary thyroid microcarcinoma (PTMC) usually has an excellent prognosis, and some experts have even proposed lifelong follow-up in these patients, without any treatment. Recently, familial papillary thyroid microcarcinoma (FPTMC) has received more attention. Sung et al.18 showed significant differences in multifocality, extrathyroidal invasion, central lymph node metastasis, tumour stage at time of initial surgery and recurrence in patients with FPTMC, illustrating that FPTMC is less aggressive than FPTC. However, Lupoli et al.19 identified familial occurrence in 5·9% of cases of PTMC and found that FPTMC has an unfavourable outcome, so they recommended radical treatment and careful follow-up for patients with FPTMC. Our result is similar to the latter. FPTMC showed a significantly higher presence of tumour multicentricity, bilateral growth and concomitant nodular goitre. Despite a more aggressive approach in patients with FPTMC, the recurrence rate was higher compared with SPTMC, and family history of PTC is an independent risk factor for recurrence in patients with PTMC. However, FPTMC has similar prognosis to the FPTC tumours >1·0 cm. PTMC is well known to have more favourable prognostic outcomes than PTC, but when the patient with PTMC has a family history, we recommended a more aggressive treatment.
Molecular genetic studies of inherited neoplasms usually attract substantial attention because knowledge of the pathogenic factors also increases an understanding of the onset and progression of the sporadic tumour counterparts. The genetic inheritance of FNMTC, however, remains unknown, and the causative genes predisposing to FNMTC have not been yet identified. With the advent of new techniques in molecular genetics, a number of susceptibility genes have been identified: MNG1 (chromosome 14q31), which was the first locus identified to be potentially implicated in FNMTC,20 TCO (chromosome 19p132),21 fPTC/PRN (chromosome 1q21),22 NMTC1 (chromosome 2q21),23 FTEN (chromosome 8p23.1-p22),24 FOXE1 (chromosome 9q22.33),25 NKX2-1 (chromosome 14q13.3),25 DICER1 (chromosome 14q32)26 and SRGAP1 (chromosome 12q14).27 Furthermore, the role of different miRNAs28 and the effect of telomeres and telomerases29 in the genetic predisposition to FNMTC have also been investigated. However, the gene(s) responsible for FNMTC have not yet been identified, and the susceptibility genes of this familial disorder usually have not been validated by other subsequent studies. Thus, for the moment, genetic testing for FNMTC is not available.
This study has several limitations. First, we included patients who underwent initial surgical treatment in other institutions, so there may have been slight differences in the choice of surgical operation, which may have influenced the result of the research. Secondly, due to the excellent prognosis of patients with differentiated thyroid cancer, a statistical comparison of RFS between different subgroups is difficult, particularly when the duration of follow-up of our study was relatively short. Furthermore, we were not able to analyse the disease-specific survival, because patients who died from PTC were very rare.
Conclusions
FPTC should be regarded as a separate clinical entity with a higher frequency of tumour multicentricity, bilateral growth and concomitant nodular goitre. The parent/offspring-type patients with FPTC were diagnosed at an earlier age than the sibling type. Patients in the second generation may exhibit the phenomenon of ‘genetic anticipation’, with an earlier age of onset, a higher rate of recurrence and a greater likelihood of Hashimoto's thyroiditis, while the first-generation offspring patients were more likely to have a nodular goitre. Currently, the available data are not sufficient to support a more aggressive approach for FPTC. However, FPTMC outcomes differed from SPTMC, illustrating that FPTMC is more aggressive and that a more invasive surgical treatment could be considered.
Disclosure of interests
There are no conflict of interests in this study.
Acknowledgements
This research was supported by National Natural Science Foundation of China (No: 81202127), Natural Science Foundation of Zhejiang Province (No: Y14H160065). The Traditional Chinese Medicine Science and Technology Plan of Zhejiang Province (No: 2013ZA025).




