Postoperative biochemical remission of serum calcitonin is the best predictive factor for recurrence-free survival of medullary thyroid cancer: a large-scale retrospective analysis over 30 years
Summary
Context
The increase in thyroid screening in the general population may lead to earlier detection of medullary thyroid carcinoma (MTC).
Objective
We aimed to evaluate secular trends in clinicopathological characteristics and long-term prognosis of MTC and its prognostic factors.
Design
This was a retrospective analysis from 1982 to 2012.
Patients
Three hundred and thirty-one patients with MTC were included and grouped based on the year of diagnosis (1982–2000, 2001–2005, 2006–2010 and 2011–2012).
Measurements
These included recurrence and mortality as well as biochemical remission (BCR) of serum calcitonin.
Results
Mean tumour size (from 2·5 cm to 1·7 cm, P < 0·001) and percentage of extrathyroidal extension (from 52·0% to 26·0%, P = 0·026) decreased. The percentage of patients achieving BCR within six postoperative months (po-BCR) increased with time (from 39·6% to 76·1%, P < 0·001). The 5-year overall recurrence rate significantly decreased in 2006–2012 compared to 1982–2005 (10% vs 18%, respectively, P = 0·031), although the 5-year survival rate did not improve (92% vs 92%, P = 0·929). Failure to achieve po-BCR was the strongest predictive factor associated with recurrence (hazard ratio [HR] = 58·04, 95% CI 7·14–472·11; P < 0·001). Male gender (HR = 3·18, 95% CI 1·18–8·56; P = 0·022), tumour size >2 cm (HR = 18·33, 95% CI 2·35–143·06; P = 0·006) and distant metastasis (HR = 4·00, 95% CI 1·31–12·21; P = 0·015) were significant prognostic factors for mortality.
Conclusions
Clinicopathological characteristics and recurrence of MTC improved with time. Po-BCR was the best predictive factor for recurrence-free survival.
Introduction
Medullary thyroid carcinoma (MTC) is accounting for 3–10% of all thyroid cancers.1, 2 The clinical presentation of MTC varies from asymptomatic cases that are diagnosed by familial screening to advanced cases with distant metastasis. The reported 5-year survival of MTC is 78–91%, and 10-year survival is 61–88%.1, 3 Distant metastasis, which was observed at initial presentation in 7–23% of patients, is known to be the main cause of MTC-related mortality.4 In addition to distant metastasis, advanced age at diagnosis and the extent of primary tumour or nodal disease have been proposed as prognostic factors for adverse outcomes.5, 6
In recent years, the incidence of small thyroid cancer has increased globally mainly as a result of thyroid ultrasound screening.7 Consistent with this, changes over time in clinicopathological characteristics, outcomes6-9 and prognostic factors10, 11 for differentiated thyroid cancer (DTC) have been reported. In a similar manner, increases in thyroid screening with ultrasound and measurement of serum calcitonin in subjects with thyroid nodules might have led to earlier detection of smaller MTCs. Therefore, the clinicopathological characteristics and prognosis of MTC might also change over time. However, the reported prognosis or predictive factors for MTC were mainly based on patients diagnosed before early 2000,9, 12-14 and changes in clinicopathological features or prognosis over time have been rarely reported.9
In this study, we evaluated the clinicopathological characteristics of a relatively large number of patients with MTC from two tertiary referral hospitals and evaluated how these characteristics changed over the past 30 years. We also identified changes in long-term prognosis and its prognostic factors over time.
Subjects and methods
Patient selection
We retrospectively reviewed the medical records of 331 patients who were diagnosed with MTC and followed in the years 1982–2012 at Seoul National University (SNU) Hospital (n = 165) or Severance Hospitals (n = 166) in Seoul, Korea. Patients consisted of 122 males and 209 females with a mean age of 47·3 ± 14·5 years (median, 48·0 years; range, 7–81 years). The median follow-up period was 4·6 years (range, 0·2–30·8 years). Pathology data, such as tumour size, extrathyroidal extension (ETE) and lymph node (LN) metastasis, based on the World Health Organization's International Histological Classification of Tumors,15 were obtained. Tumour size was categorized into the following groups: tumours ≤1 cm, tumours 1·1–2 cm and tumours >2 cm. LNs were also assessed based on positive or negative LNs, and nonresected LNs were categorized as negative LNs. Genetic tests for RET proto-oncogene mutation (exons 8, 10, 11, 13, 14, 15, 16), which was start to implement frequently since 2008, were performed in 172 patients, and family history of MTC or the presence of phenotypes of multiple endocrine neoplasia type 2 (MEN2) was obtained by reviewing the medical records for 310 patients. To evaluate changes in pathological findings over time, the patients were classified into four groups based on the year of diagnosis: 1982–2000 (n = 71), 2001–2005 (n = 55), 2006–2010 (n = 132) and 2011–2012 (n = 73). For comparison of prognosis, we regrouped the time periods based on the similarity of clinocopathological characteristics presented (1982–2005 vs 2006–2012).
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of SNU Hospital (IRB No. H-1301-068-459) and Severance Hospitals (IRB No. 3-2013-0306).
Follow-up protocol and definition of disease status
Measurement of preoperative serum calcitonin levels and neck ultrasound or computed tomography (CT) were carried out for most patients. Neck ultrasonography was regularly performed at the beginning of 2000. During postsurgical follow-up, the measurement of serum calcitonin levels and neck ultrasound were carried out between 2 and 6 months after initial surgery and then checked annually. For patients with calcitonin levels >10 pg/ml or those showing an increase in calcitonin levels, the presence of recurrence or distant metastasis was evaluated by imaging modalities including abdominal or chest CT or 18F FDG PET CT. Serum calcitonin measurement was started since 1994. At the SNU Hospital, it was measured using immunoradiometric assay (IRMA; CIS-Biointernational, Gif-Sur-Yvette, France) from 1994 to the present, while at the Severance Hospital, the measurement of serum calcitonin was performed using immunoradiometric assay (CT-USA-IRMA; Biosource, Nivelles, Belgium) from 1994 to 2009; then, it was replaced by a chemiluminescence DPC immunoassay (Immulite 2000; Siemens, Gwynedd, UK). A cut-off for calcitonin levels was 0–10 pg/ml for immunoradiometric assay and 0–8·4 pg/ml (male) or 0–5 pg/ml (female) for chemiluminescence DPC immunoassay. Functional sensitivity and reference interval of each kit were similar.16 Preoperative calcitonin levels were available in 254 patients, and we could not obtain them in 77 patients.
We assessed postoperative disease status within 6 months after surgery (po) and at the last follow-up. The patient was classified as being in biochemical remission (BCR) when the serum calcitonin level was <10 pg/ml and no structural disease was found by neck ultrasound or other available imaging modalities. Biochemical disease (BCD) was defined as when serum calcitonin level was ≥10 pg/ml with no evidence of structural disease, whereas clinical disease (CD) was defined as when there was structural evidence of residual disease with any imaging modalities. Serum calcitonin level was >10 pg/ml in all patients with CD.
At the last follow-up, CD included a status of either structural recurrence or persistence. The term ‘CD/structural recurrence’ was defined as newly identified structural disease in a patient previously classified as postoperative-BCR (po-BCR) or po-BCD. The other patients with CD, who showed persistent structural disease during follow-up, were classified as ‘CD/persistence’.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) or n (%) for descriptive statistics. All data were analysed with IBM SPSS statistics software (version 20.0; SPSS Inc., Chicago IL, USA). A t-test or one-way anova was used for continuous variables. Pearson's chi-square test or logistic regression analysis was used for categorical variables. Kaplan–Meier survival analysis was used to estimate the overall recurrence and survival rate. The Cox proportional hazard (Cox PH) regression model was used for the identification of prognostic factors for overall recurrence and mortality. In all cases, a P value <0·05 was considered statistically significant.
Results
Secular trends in clinicopathological characteristics of MTC
The changes in baseline clinicopathological characteristics according to the time periods are shown in Table 1. The mean age of subjects increased over time, but the percentages of males remained similar. Although the RET proto-oncogene tended to be tested more frequently with time (from 35·2 to 54·8%, P = 0·004), the frequency of positive RET mutation (from 68·0 to 27·5%, P = 0·001) or hereditary MTC (from 33·9 to 15·1%, P = 0·020) decreased. The 12 subjects, 21·1% of patients with positive RET mutation, were diagnosed initially by family screening using genetic studies, and these percentages remained constant over time (from 17·6 to 27·3%, P = 0·778). The mean tumour size (from 2·5 cm to 1·7 cm, P < 0·001) and the percentage of subjects with ETE (from 52·0 to 26·0%, P = 0·026) also decreased. However, the percentage of LN metastasis and distant metastasis remained constant over time with overall percentages of 45·8% and 8·0%, respectively. On the other hand, prophylactic central LN dissection was performed consistently over 30 years in Severance Hospital, while it began in 20038 in SNU Hospital. When we separately analysed those results in each institutes, there was significant decrease in LN metastasis as well as the proportion of positive LN in Severance Hospital, but not in SNU Hospital (Table 1).
| Total (n = 331) | 1982–2000 (n = 71) | 2001–2005 (n = 55) | 2006–2010 (n = 132) | 2011–2012 (n = 73) | P | |
|---|---|---|---|---|---|---|
| Male, n (%) | 122 (36·9) | 28 (39·4) | 19 (34·5) | 48 (36·4) | 27 (37·0) | 0·952 |
| Age, mean ± SD (years) | 47·3 ± 14·5 | 40·5 ± 13·4 | 45·6 ± 13·3** | 49·8 ± 14·0** | 50·5 ± 15·2** | <0·001 |
| RET mutation tested subjects, n (%) | 172 (52·0) | 25 (35·2) | 26 (47·3) | 81 (61·4) | 40 (54·8) | 0·004 |
| RET (+), n (%) | 57 (33·1) | 17 (68·0) | 9 (34·6) | 20 (24·7) | 11 (27·5) | 0·001 |
| Detected by screening, n (%) | 12 (21·1) | 3 (17·6) | 1 (11·1) | 5 (25·0) | 3 (27·3) | 0·778 |
| RET mutation tested families, n | 151 | 17 | 25 | 75 | 35 | |
| RET (+), n (%) | 36 (23·8) | 9 (52·9) | 8 (32·0) | 14 (18·7) | 5 (14·7) | 0·009 |
| Hereditary MTC, n (%)a | 62/310 (20·0) | 21/62 (33·9) | 10/50 (20·0) | 20/125 (16·0) | 11/73 (15·1) | 0·020 |
| Basal calcitonin (pg/ml)b | 1183·8 ± 2448·6 | 1121·4 ± 1345·2 | 1149·3 ± 1692·9 | 1110·0 ± 2907·7 | 1353·2 ± 2310·6 | 0·929 |
| Tumour ≤1 cm | 228·0 ± 743·9 | 391·3 ± 464·5 | 214·4 ± 258·9 | 136·9 ± 522·0 | 327·2 ± 1087·1 | 0·682 |
| Tumour >1 cm | 1582·9 ± 2465·4 | 1318·3 ± 1410·3 | 1390·5 ± 1821·5 | 1415·1 ± 2767·2 | 2163·3 ± 2685·9 | 0·425 |
| Tumour size, nc | 305 | 60 | 50 | 122 | 73 | |
| Mean ± SD (cm) | 1·9 ± 1·5 | 2·5 ± 1·3 | 2·4 ± 1·6 | 1·6 ± 1·3** | 1·7 ± 1·6** | <0·001 |
| ≤1/1–2/>2 cm, n (%) | 95/108/102 (31·1/35·4/33·4) | 8/23/29 (13·3/38·3/48·3) | 10/15/25 (20·0/30·0/50·0) | 46/48/28 (37·7/39·3/23·0) | 31/22/20 (42·5/30·1/27·4) | <0·001 |
| LN dissectionc, nd | 315 | 64 | 52 | 126 | 73 | |
| None/CND/LND, nd (%) | 32/122/161 (10·2/38·7/51·1) | 16/13/35 (25·0/20·3/54·7) | 10/14/28(19·2/26·9/53·8) | 5/56/65 (4·0/44·4/51·6) | 1/39/33 (1·4/53·4/45·2) | <0·001 |
| At Severance Hospital | 0/73/92 (0/44·2/55·8) | 0/10/16 (0/38·5/61·5) | 0/8/17 (0/32·0/68·0) | 0/31/41 (0/43·1/56·9) | 0/24/18 (0/57·1/42·9) | 0·191 |
| At SNU Hospital | 32/49/69 (21·3/32·7/46·0) | 16/3/19 (42·1/7·9/50·0) | 10/6/11 (37·0/22·2/40·7) | 5/25/24 (9·3/46·3/44·4) | 1/15/15 (3·2/48·4/48·4) | <0·001 |
| LN metastasis, nc (%) | 143/312 (45·8) | 35/63 (55·6) | 24/50 (48·0) | 54/127 (42·5) | 30/72 (41·7) | 0·313 |
| At Severance Hospital, n (%) | 69/161 (42·9) | 17/23 (73·9) | 14/24 (58·3) | 23/72 (31·9) | 15/42 (35·7) | 0·001 |
| Dissected LN, n | 34·2 ± 33·6 | 26·9 ± 20·4 | 31·4 ± 26·1 | 36·8 ± 34·9 | 35·4 ± 40·3 | 0·643 |
| Positive LN, n | 6·4 ± 14·1 | 10·0 ± 13·1 | 8·0 ± 11·6 | 5·2 ± 15·6 | 5·7 ± 13·1 | 0·496 |
| Positive/dissected LN, (%) | 15·1 ± 25·5 | 33·6 ± 31·9 | 27·1 ± 33·8 | 8·8 ± 18·5 | 8·9 ± 19·4 | <0·001 |
| At SNU Hospital, n (%) | 74/151 (49·0) | 18/40 (45·0) | 10/26 (38·5) | 31/55 (56·4) | 15/30 (50·0) | 0·455 |
| Dissected LN, n | 19·7 ± 20·6 | 14·2 ± 15·5 | 17·5 ± 17·9 | 18·2 ± 18·7 | 26·7 ± 26·2 | 0·181 |
| Positive LN, n | 6·0 ± 8·7 | 5·4 ± 5·9 | 5·5 ± 7·6 | 4·8 ± 6·9 | 8·5 ± 12·6 | 0·357 |
| Positive/dissected LN, (%) | 26·5 ± 30·2 | 40·2 ± 35·8 | 25·8 ± 30·5 | 20·9 ± 24·4 | 27·2 ± 33·2 | 0·150 |
| ETE, nc (%) | 102/290 (35·2) | 26/50 (52·0) | 18/48 (37·5) | 39/119 (32·8) | 19/73 (26·0) | 0·026 |
| Distant metastasis, nc (%) | 25/312 (8·0) | 7/62 (11·3) | 3/49 (6·1) | 9/128 (7·0) | 6/73 (8·2) | 0·726 |
| Po-BCR, n (%)b,** | 188/291 (64·6) | 19/48 (39·6) | 25/51 (49·0) | 90/121 (74·4) | 54/71 (76·1) | <0·001 |
| Tumour ≤1 cm | 82/92 (89·1) | 5/7 (71·4) | 6/10 (60·0) | 42/45 (93·3) | 29/30 (96·7) | 0·003 |
| Tumour >1 cm | 104/189 (55·0) | 13/36 (36·1) | 18/39 (46·2) | 48/73 (65·8) | 25/41 (61·0) | 0·015 |
- CND, central node dissection; ETE, extrathyroidal extension; LN, lymph node; MTC, medullary thyroid carcinoma; ND, node dissection; Po-, postoperative status within 6 months after surgery; Po-BCR, postoperative biochemical remission; SD, standard deviation; SNU, Seoul National University. For continuous data, P values are based on one-way anova, and for categorical data, P values are based on Pearson's chi-square test.
- a Hereditary MTC was considered if the patient showed any of the following characteristics: RET proto-oncogene mutation, a family history of MTC or phenotype of multiple endocrine neoplasia type 2 (MEN2). This information was available in 310 patients.
- b Basal calcitonin levels and po-BCR rates were only available in subjects diagnosed with MTC after 1994.
- c All subjects were performed total thyroidectomy.
- d Only subjects known their extent of LND were included.
- e Po-BCR was defined as when the serum calcitonin level was <10 pg/ml, and no structural disease was found by neck ultrasound or other available imaging modalities within 6 months after surgery.
- **P < 0·001, compared to 1982–2000.
Then, we compared the long-term outcomes using Kaplan–Meier analysis (Fig. 1). The 5-year overall recurrence rate was 14% (Fig. 1a) and significantly decreased (10% vs 18%, P = 0·031, Fig. 1c) in the 2006–2012 group than in the 1982–2005 group. However, the 5-year survival rate was 92% (Fig. 1b) and did not differ between the groups (92% vs 92%, P = 0·929, Fig. 1d). The 10-year overall recurrence rate and survival rate were 35% and 87%.
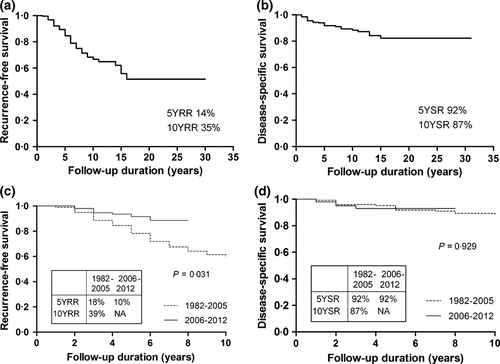
Long-term outcome of MTC and its related clinicopathological characteristics
We assessed the disease status at the last follow-up according to the postoperative disease status within 6 months after surgery (Fig. S1). Among 188 subjects with po-BCR state, 94·7% maintained BCR status. However, 2·7% have progressed to CD, in which 20·0% re-entered to BCR status after reoperation and 80·0% maintained CD status irrespective of further treatment received (operation n = 3). Among 56 patients with po-BCD, 51·8% maintained BCD status and 48·2% have progressed to CD. Among them, 7·4% achieved final BCR status, 33·3% re-entered to BCD status and maintained after operation, but 59·3% progressed CD status irrespective of further treatment received (operation n = 12). In 47 subjects with po-CD status, 2·1% or 6·4% re-entered to BCR or BCD status after operation, respectively. 91·5% maintained CD status regardless of the treatment applied (operation n = 25).
To evaluate prognostic factors for the long-term outcome of MTC, we compared the clinicopathological features of BCD, CD/structural recurrence or CD/persistence groups with the BCR group at the last follow-up (Table 2). The percentages of males and frequencies of ETE, LN metastasis and distant metastasis were higher in the other three groups than those in the BCR group. Interestingly, the po-BCR rate of the BCD or the CD/structural recurrence group was significantly lower than the BCR group (10·9% or 20·0%, respectively, vs 98·4%, both P < 0·001). No patients in the CD/persistence group achieved po-BCR. The death group showed similar clinicopathological features to the CD/persistence group; the percentages of distant metastasis, LN metastasis and ETE, and preoperative calcitonin level were significantly higher, and mean tumour size was larger than that in the BCR group (Table 2).
| The disease status at the last follow-up | BCR | BCD | CD | Death | |
|---|---|---|---|---|---|
| Structural recurrence | Persistence | ||||
| N | 194 | 51 | 35 | 51 | 31 |
| Male, n (%) | 50 (25·9) | 21 (42·0)** | 14 (40·0)** | 36 (72·0)** | 22 (71·0)** |
| Age, mean ± SD (years) | 47·8 ± 14·9 | 45·1 ± 12·1 | 46·0 ± 15·8 | 48·3 ± 14·3 | 50·0 ± 14·7 |
| Median follow-up duration (years) | 3·9 (0·3–29·5) | 7·5 (0·2–25·9) | 12·8 (3·1–30·8) | 2·9 (0·3–20·7) | 3·1 (0·6–14·8) |
| Hereditary MTC, n (%)a | 33/192 (17·2) | 13/48 (27·1) | 7/28 (25·0) | 9/42 (21·4) | 4/24 (16·4) |
| Basal calcitonin (pg/ml)b | 618·5 ± 1871·2 | 1795·0 ± 2059·4** | 1137·2 ± 1145·5 | 3316·9 ± 4094·5** | 2468·3 ± 3127·4* |
| Tumour size, n | 190 | 47 | 27 | 41 | 25 |
| Mean ± SD (cm) | 1·5 ± 1·0 | 2·1 ± 1·1** | 2·9 ± 2·0** | 3·4 ± 2·0** | 4·0 ± 1·7** |
| ≤1/1–2/>2 cm, n (%) | 82/70/38 (43·2/36·8/20·0) | 6/21/20 (12·8/44·7/42·6)** | 4/8/15 (14·8/29·6/55·6)** | 3/9/29 (7·3/22·0/70·7)** | 0/2/23 (0/8·0/92·0)** |
| ETE, n (%) | 30/182 (16·5) | 25/45 (55·6)** | 17/23 (73·9)** | 30/40 (75·0)** | 20/25 (80·0)** |
| LN metastasis, n (%) | 43/188 (22·9) | 36/47 (76·6)** | 22/28 (78·6)** | 42/49 (85·7)** | 24/29 (82·8)** |
| Distant metastasis, n (%) | 0/190 (0) | 2/48 (4·2)** | 1/26 (3·8)* | 22/48 (45·8)** | 14/29 (48·3)** |
| Po-BCR, n (%)b,c | 179/182 (98·4) | 5/46 (10·9)** | 4/20 (20·0)** | 0/43 (0)** | 0/22 (0)** |
- BCR, biochemical remission; BCD, biochemical disease; CD, clinical disease; ETE, extrathyroidal extension; LN, lymph node; MTC, medullary thyroid carcinoma; Po-, postoperative status within 6 months after surgery; Po-BCR, postoperative biochemical remission; SD, standard deviation.
- For continuous data, P values are based on one-way anova. For categorical data, P values are based on the linear association.
- a Hereditary MTC was considered if the patient showed any of the following characteristics: RET proto-oncogene mutation, a family history of MTC or phenotype of multiple endocrine neoplasia type 2 (MEN2). This information was available in 310 patients.
- b Basal calcitonin levels and po-BCR rates were only available in subjects diagnosed with MTC after 1994.
- c Po-BCR was defined as when the serum calcitonin level was <10 pg/ml and no structural disease was found by neck ultrasound or other available imaging modalities within 6 months after surgery.
- *P-value <0·05, **P-value <0·001, compared to the BCR group.
Prognostic factors related to long-term prognosis in MTC patients
We evaluated prognostic factors for overall recurrence and mortality using Cox PH models in subjects diagnosed after 1994, since regular measurement of serum calcitonin levels was started in 1994 (Table 3). Using univariate analysis, male gender, tumour size >2 cm, LN metastasis, ETE and failure to achieve po-BCR (no po-BCR) were poor prognostic factors for overall recurrence. However, after multivariate analysis, only no po-BCR (HR = 58·04, 95% CI 7·14–472·11; P < 0·001) remained a significant and strong variable affecting overall recurrence (Table 3, Model 1). Multivariate analysis excluding no po-BCR showed that LN metastasis (HR = 7·18, 95% CI 2·56–20·12; P < 0·001) and ETE (HR = 3·09, 95% CI 1·34–7·12; P = 0·008) were the risk factor for overall recurrence (Table 3, Model 2). When we included all subjects from 1982, the risk effects of those factors were not different (data not shown).
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall recurrence | Overall mortality | Overall recurrence | Overall mortality | |||||
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |
| Gender (Male, %) | 1·87 (1·02–3·44)* | 1·09 (0·49–2·41) | 4·22 (1·85–9·65)** | 3·18 (1·18–8·56)* | 1·87 (1·02–3·44)* | 1·22 (0·56–2·63) | 4·22 (1·85–9·65)** | 3·18 (1·18–8·56)* |
| Age (years) | 1·01 (0·98–1·03) | 0·99 (0·96–1·02) | 1·03 (1·00–1·06)* | 1·03 (0·99–1·07) | 1·01 (0·98–1·03) | 1·00 (0·97–1·03) | 1·03 (1·00–1·06)* | 1·03 (0·99–1·07) |
| Tumour size (>1 cm) | 2·23 (0·86–5·81) | – | Not applicablea | Not applicablea | 2·23 (0·86–5·81) | – | Not applicableb | Not applicableb |
| Tumour size (>2 cm) | 2·69 (1·35–5·38)* | 0·84 (0·36–1·95) | 36·04 (4·83–269·15)** | 18·33 (2·35–143·06)* | 2·69 (1·35–5·38)* | 1·11 (0·51–2·45) | 36·04 (4·83–269·15)** | 18·33 (2·35–143·06)* |
| LN metastasis (%) | 5·39 (2·50–11·61)** | 3·12 (0·99–9·82) | 4·66 (1·75–12·42)* | 1·44 (0·42–4·92) | 5·39 (2·50–11·61)** | 7·18 (2·56–20·12)** | 4·66 (1·75–12·42)* | 1·44 (0·42–4·92) |
| ETE (%) | 4·78 (2·17–10·52)** | 0·94 (0·38–2·30) | 5·54 (2·03–15·15)** | 1·59 (0·53–4·77) | 4·78 (2·17–10·52)** | 3·09 (1·34–7·12)* | 5·54 (2·03–15·15)** | 1·59 (0·53–4·77) |
| Distant metastasis (%) | 2·31 (0·31–16·99) | – | 16·27 (7·27–36·38)** | 4·00 (1·31–12·21)* | 2·31 (0·31–16·99) | – | 16·27 (7·27–36·38)** | 4·00 (1·31–12·21)* |
| No po-BCR (%) | 19·14 (7·29–50·17)** | 58·04 (7·14–472·11)** | Not applicableb | Not applicableb | ||||
- BCR, biochemical remission; ETE, extrathyroidal extension; LN, lymph node; Po-, postoperative status within 6 months after surgery; Po-BCR, postoperative biochemical remission.
- Model 1. Adjusted for age, gender and significant factors in univariate.
- Model 2. Adjusted for age, gender and significant factors in univariate except for no po-BCR.
- Hazard ratios were calculated by unadjusted Cox hazard regression and adjusted for all risk factors.
- a There was no disease-related mortality in subjects mortality in patients with tumour <1 cm, so the risk effects of tumour>1 cm on mortality could not be calculated.
- b There was no disease-related mortality in subjects who achieved po-BCR, so the risk effects of no po-BCR on mortality could not be calculated.
- *P-value <0·05, **P-value <0·001.
Most patients (95·3%) with po-BCR maintained BCR status except for 9 patients (Table 4). Nine patients with po-BCR showing a progression to BCD (n = 5) or CD (n = 4) are characterized in Table S1. Of the four patients who progressed to CD, two showed RET gene mutation with American Thyroid Association (ATA) risk B or C,17 and the other two patients had tumours larger than 2 cm.
| Po-BCR(n = 188) | No po-BCR (n = 103) | P | |
|---|---|---|---|
| Male, n (%) | 50 (26·6) | 54 (52·4) | <0·001 |
| Age, mean ± SD (years) | 47·6 ± 15·0 | 47·0 ± 13·8 | 0·722 |
| RET mutation tested subjects, n (%) | 105 (55·9) | 52 (50·5) | 0·380 |
| RET (+), n (%) | 31 (29·5) | 18 (34·6) | 0·517 |
| Hereditary MTC, n (%)a | 34/190 (17·9) | 22/101 (21·8) | 0·423 |
| Basal calcitonin (pg/ml) | 656·5 ± 1890·8 | 2325·3 ± 3114·5 | <0·001 |
| Tumour size, n | 192 | 104 | |
| Mean ± SD (cm) | 1·5 ± 1·2 | 2·7 ± 1·7 | <0·001 |
| ≤1/1–2/>2 cm, n (%) | 82/67/37 (44·1/36·0/19·9) | 10/31/54 (10·5/32·6/56·8) | <0·001 |
| ETE, n (%) | 28/178 (15·7) | 64/92 (69·6) | <0·001 |
| LN metastasis, n (%) | 44/184 (23·9) | 81/99 (81·8) | <0·001 |
| Distant metastasis, n (%) | 0/185 (0) | 18/99 (18·2) | <0·001 |
| At the last follow-up | |||
| BCR, n (%) | 179 (95·2) | 3 (2·9) | <0·001 |
| BCD, n (%) | 5 (2·7) | 41 (39·8) | |
| CD, n (%) | |||
| Structural recurrence, n (%) | 4 (2·1) | 16 (15·5) | |
| Persistence, n (%) | 0 (0) | 43 (41·8) | |
| Disease-related mortality, n (%) | 0 (0) | 22 (21·4) | <0·001 |
- BCR, biochemical remission; BCD, biochemical disease; CD, clinical disease; ETE, extrathyroidal extension; LN, lymph node; Po-, postoperative status within 6 months after surgery; Po-BCR, postoperative biochemical remission; SD, standard deviation.
- For continuous data, P values are based on t-test. For categorical data, P values are Pearson's chi-square test.
- a Hereditary MTC was considered if the patient showed any of the following characteristics: RET proto-oncogene mutation, a family history of MTC or phenotype of multiple endocrine neoplasia type 2 (MEN2).
For overall mortality, male gender, increasing age, tumour size >2 cm, LN metastasis, ETE and distant metastasis were significant poor prognostic factors using univariate analysis (Table 3). Using multivariate analysis, male gender (HR = 3·18, 95% CI 1·18–8·56; P = 0·022), tumour size >2 cm (HR = 18·33, 95% CI 2·35–143·06; P = 0·006) and distant metastasis (HR = 4·00, 95% CI 1·31–12·21; P = 0·015) remained as prognostic factors for overall mortality.
There was no disease-related mortality in subjects who achieved po-BCR, so the risk effects of no po-BCR on mortality could not be calculated. There was no mortality in all 95 subjects with tumours <1 cm, and only 2 of 108 (1·9%) patients with tumour size 1–2 cm died, while 23 of 102 (22·6%) patients with tumour size >2 cm died from MTC. Of the 25 patients showing initial distant metastasis at diagnosis, only two patients achieved BCD at last follow-up, whereas the others maintained CD/structural recurrence or CD/persistence and 14 of them were dead. The sites of distant metastasis in the two cured patients were all para-aortic LN, and both patients achieved BCD after mediastinal dissection (Table S1).
To show whether the prognostic factors have been changed over time, we did the same analysis in each period. Prognostic factors for overall recurrence or mortality in each period (1994–2005 or 2006–2012, Table S2) were similar for the entire study periods (1994–2012, Table 3). No po-BCR was associated with the strongest risk of recurrence in the 1994–2005 group; however, recurrence was not observed in patients who achieved po-BCR in 2006–2012, so no po-BCR as the risk factor for recurrence could not be evaluated in the 2006–2012 group. Other than no po-BCR, LN metastasis was the only significant factor correlated with overall recurrence (HR = 11·14, 95% CI 1·25–99·19; P = 0·031) in 2006–2012. In the same manner, multivariate analysis excluding no po-BCR in 1994–2005 also showed that LN metastasis was the risk factor for overall recurrence (data not shown; HR = 7·19, 95% CI 2·18–23·74, P = 0·001).
Failure to achieve postoperative BCR (no po-BCR) was the strongest predictive factor for recurrence
Although overall mortality was unaltered, overall recurrence improved with time, and the patients who did not achieved po-BCR showed greatest risk of recurrence. When we subanalysed the subjects according to the po-BCR status, overall recurrence was also not significantly different between the periods (Fig. 2a) as well as overall mortality (Fig. 2b). These findings differ from the results shown in Fig. 1a, suggesting the importance of the increased percent of po-BCR rate itself on the improvement of recurrence of recent period. Then, we compared the rates of po-BCR achieved according to pathological characteristics between 1994–2005 and 2006–2012 (Fig. 2c–e). The rate of po-BCR was higher in 2006–2012 in all patients, regardless of pathological aggressiveness. However, for patients with tumour size <0·5 cm or >2 cm, the rates were similar for the two time periods.
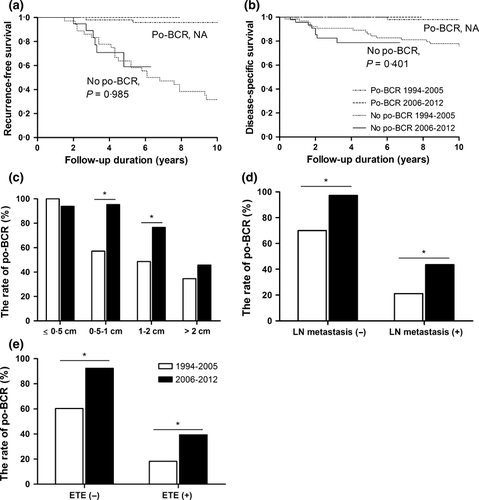
Interestingly, the long-term outcome was very different according to the postoperative status of serum calcitonin levels; patients who achieved po-BCR were very likely to remain BCR (95·2%) and showed no disease-related mortality, while patients who did not achieve po-BCR resulted CD in 57·3% and showed 21·4% of a disease-related mortality at the last follow-up (Table 4). In addition, the tumour size was larger, the preoperative calcitonin level was higher, and the percentage of LN metastasis, ETE or distant metastasis was higher in patients who did not achieve po-BCR. In multivariate analysis, tumour size >2 cm (odds ratios (OR) 3·86, P = 0·008), LN metastasis (OR 12·98, P < 0·001) and ETE (OR 8·12, P < 0·001), which showed prognostic effects on recurrence in univariated analysis (Table 3), showed significant negative predictive factors for po-BCR (data not shown).
Discussion
In the present study, we investigated the changes in clinicopathological characteristics and prognosis of MTC over the last 30 years and showed improvements of most pathological features and recurrence with time. Although the overall percentage of LN metastasis remained constant over time, it was decreased in the institute where the rates of central LN dissection were not changed over time. The number of total dissected nodes or the percentage of positive LN per total dissected nodes was also different between institutions. Those results suggest the percentage of LN metastasis can be largely changed by the surgical extents or by the expertness of the surgeon; thus, it is hard to conclude about the secular changes of LN metastasis in this study. Interestingly, the po-BCR rates improved in recent time periods, and the po-BCR rate was the most important predictive factor for recurrence-free survival. However, frequency of distant metastasis, the strongest risk factor for mortality, was unchanged over time, resulting in no improvement in disease-specific survival. Male gender and large tumour size were also significant risk factors for mortality.
Similar to secular trends of DTC,8, 10, 11 the mean age of patients with MTC increased with time, and the pathological features of initial presentations became less aggressive. These results suggested the effect of early detection of MTC in recent periods, and it is consistent with previous reports.9, 18, 19 Alevizaki et al.9 reported that stage was more advanced in the sporadic MTC group diagnosed before 2001, when calcitonin measurements and genetic screening were not routinely performed. It is true that more frequent use of ultrasound as a screening test or serum calcitonin level measurements on subjects with thyroid nodules might have increased the early detection of MTC.2 In our study, the application of serum calcitonin measurements, neck ultrasonography or RET genetic testing was changed over time, and these changes of standard diagnostic procedure could influence on the detection rate of initial MTCs or recurrent diseases. The increasing mean age and smaller tumour size are consistent with this possibility. Although the genetic screening of inherited MTCs could result in increment of early detection of MTCs,9, 20 and screening for the RET mutation has been used more frequently in recent years in our study, the percentages of positive RET mutation and hereditary MTC decreased over time. We could not explain the reason at this time; only suppose that the main proportion of recently diagnosed MTC might be related factors other than genetic causes.
Although pathologic characteristics and recurrence-free survival improved during recent periods, mortality was unchanged over time. MTC survival could have been affected by several prognostic factors such as age,21 male gender, invasive histological characteristics,22 the adequacy of primary surgery2, 23 and the calcitonin doubling time.2, 24, 25 In agreement with other studies, we found that male gender, tumour size >2 cm and distant metastasis were significant prognostic factors for survival. In the present study, although there was a decrease in tumour size, the percentage of male and distant metastasis remained similar over time, and the proportion of tumour >2 cm remained about 25% even in 2011–2012. It could be a reason for the unchanged mortality over time. On the other hand, the small number of death events which occurred in the early could cause similar mortality between two periods. Distant metastasis was associated with the strongest risk for mortality, and all patients with distant metastasis died, with the exception of two patients with para-aortic LN metastasis who were cured. If we consider the facts that there was no mortality or no distant metastasis in patients with tumours <1 cm, and only 1·9% of mortality in the patients with tumours of 1–2 cm, the importance of early detection of MTC should be emphasized even at present.
Importantly, we demonstrated that no po-BCR was the only significant and strong factor for recurrence after multivariated analysis. The analysis of disease status according to the risk stratification26 based on po-BCR status also proved the prognostic roles of postoperative calcitonin level. There exist a few reports supporting our results. Two recent studies suggested postoperative calcitonin level was an important predictive factor for disease progression and persistence,24, 27 so postoperative calcitonin levels could be a reliable index of disease status and complete resection of tumours.28 Saltiki et al. suggested that postoperative calcitonin level ≥14·5 pg/ml might predict disease progression and was the only predictor associated with the 10-year progression of the disease.24
The improvement of po-BCR over time could be related to a reduction in the extent of the disease by early detection, that is improved pathological characteristics. In addition, the po-BCR rate after 2006 was increased regardless of the presence of LN metastasis and ETE in this study, and it could be explained by improvements in surgical skills for complete resection, as previously suggested.9, 21, 29 Therefore, it could be postulated that po-BCR might be the best predictive factor for recurrence-free survival, reflecting both pathologic features and surgical outcomes.
As for the predictive factors for no po-BCR, tumour size >2 cm, LN metastasis and ETE were determined after multivariated analysis. Regarding the tumour size, it was demonstrated that tumour size >2 cm made it difficult to achieve po-BCR, regardless of the periods diagnosed (35% during 1994–2005; 46% during 2006–2012), whereas tumours <0·5 cm showed high po-BCR rates during both periods (100% and 94%, respectively). Furthermore, 71% of CD/persistence patients and 92% of the death group had tumour size >2 cm. These results suggested that tumours sized over 2 cm are not easy to remove completely by surgery. Thus, the minimization of the extent of disease by early detection, as well as complete resection, was important to improve the prognosis.
The present study had some limitations. We could not obtain adequate information in pathological characteristics (8% of patients), or pre- and postoperative calcitonin levels (23%) in some patients. In addition, we arbitarily subgrouped the periods as before and after 2005–2006, according to similar pathological characteristcs. Although the incidence rates for thyroid cancers have increased rapidly since 2005–2006 because of improved sensitivity of diagnostic techniques, such as the advent of ultrasound and fine needle aspiration,30 the subgrouping was not based on objective criteria and comparison of prognosis in different periods was limitation of our study. However, despite the limitations, our study has a strength that we analysed a relatively large number of patients with MTC from different hospitals, and followed them for a long-term period.
In conclusion, there has been improvement in clinicopathological characteristics and the recurrence of MTC over time. Po-BCR was the best predictive factor for recurrence-free survival, reflecting both improved pathological characteristics and surgical completeness over time. However, the percentages of distant metastasis and mortality remain unchanged over time, suggesting the importance of earlier detection of MTCs.
Funding
This work was supported by Research Grant Number CB-2011-03-01 from the Korea Foundation for Cancer Research Fund.
Disclosure statement
Nothing to disclose.




