Birth and coming of age of islet autoantibodies
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Historical and new insights into pathogenesis of type 1 diabetes. Clinical and Experimental Immunology 2019, 198: 292–293.
HIPs and HIP-reactive T cells. Clinical and Experimental Immunology 2019, 198: 306–313.
Immune cell trafficking to the islets during type 1 diabetes. Clinical and Experimental Immunology 2019, 198: 314–325.
Islet-immune interactions in type 1 diabetes: the nexus of beta cell destruction. Clinical and Experimental Immunology 2019, 198: 326–340.
Summary
This review takes the reader through 45 years of islet autoantibody research, from the discovery of islet-cell antibodies in 1974 to today’s population-based screening for presymptomatic early-stage type 1 diabetes. The review emphasizes the current practical value of, and factors to be considered in, the measurement of islet autoantibodies.
Historical perspectives
Discovery of islet-cell antibodies
The concept of autoimmunity as a pathogenetic mechanism in a subgroup of patients with diabetes was first raised in the 1960s and early 1970s with the observation of insulitis 1, and the association of juvenile-onset diabetes with certain human leukocyte antigen (HLA) alleles and T cell abnormalities 2-6. The definitive autoimmune pathogenetic discovery was made in 1974, when two research groups in the United Kingdom reported the identification of islet-cell antibodies (ICA) in patients with so-called ‘multiple organ-specific autoimmunity’ 7, 8.
The first of these publications was by Gian Franco Bottazzo and Deborah Doniach. This research group had discovered thyroid autoimmunity almost 20 years earlier 9, and several other autoantibodies 10-12, and had a treasure chest of samples from patients with various and multiple endocrine autoimmune diseases. Using indirect immunofluorescence, Bottazzo et al. detected ICA in these human pancreas samples (Fig. 1a). The manuscript was published in The Lancet in November 1974 7. The abstract stated: ‘Antibodies to pancreatic islet cells were found by immunofluorescence in the sera of 13 patients with multiendocrine deficiencies associated with organ-specific autoimmunity. 10 of these patients were diabetic… The presence of organ-specific pancreatic antibodies supports the hypothesis of an autoimmune form of diabetes mellitus put forward to explain the histological ‘insulitis’ found in selected cases of this disease.’
The second publication was by William J. Irvine’s research group, which had previously reported T cell responses against pancreatic antigens in patients with diabetes 6. His group also examined a collection of samples from polyendocrine patients, and published their work in The Lancet 1 month after the study by Bottazzo et al. 8. Their abstract stated: ‘Using an indirect immunofluorescence technique, circulating antibodies to pancreatic islet cells were found in the sera of 5 patients with insulin-dependent diabetes mellitus and coexistent autoimmunity… These findings provide further direct evidence to support the hypothesis of an autoimmune form of diabetes mellitus.’
Thus, the discovery and validation of ICA were reported by two independent research groups within the space of a month. These were discovered in polyendocrine patients rather than in typical patients with type 1 diabetes. Moreover, the classification of diabetes into types 1 and 2, which had been introduced decades earlier, had not yet taken root, and terms such as ‘juvenile’, ‘adult-onset’, ‘insulin-dependent’ and ‘non-insulin-dependent’ were used to distinguish age- and therapy-related forms of the disease. In 1975, Lendrum et al. reported ICA in the sera of 51 of 105 children with recent-onset diabetes 13, revealing an autoimmune pathogenetic component in a large proportion of childhood cases of diabetes.
Prediabetes
Perhaps the most important discoveries were those that led to the notion of a ‘prediabetic’ stage of the disease. Lendrum et al. examined ICA in the diabetic twin cohort of David Pyke and in 1976, reported that the antibodies could be present years before the onset of diabetes 14. Also in 1976, Irvine et al. reported that the antibodies could precede diabetes onset by several years 15. In 1981, Gorsuch et al. measured ICA in the first-degree relatives of patients with insulin-dependent diabetes and discovered that patients who developed insulin-dependent diabetes had ICA up to 30 months before the onset of diabetes 16. These early findings eventually led to the notion that type 1 diabetes is a chronic autoimmune disease, as described by George Eisenbarth in 1986 17.
Standardization
The number of methods for detecting ICA and reports of ICA has increased rapidly since the pivotal studies described above. These reports discussed complement-fixing antibodies 18, bovine-pancreas-positive ICA 19, the two-color fluorescence detection method 20, the protein-A detection method 21 and islet-cell-surface antibodies 22, among many others. What started as a clear concept soon became complex and confused. A workshop to standardize ICA measurements was convened, and after the sobering realization of how variable these measurements could be 23, an exemplary standardization program that introduced common standards 24 and international units 25, 26 was established, and was subsequently used for antigen-specific islet autoantibody measurements 27-29. Importantly, the program gave credibility to the antibodies as markers of prediabetes 30-32 and many poorly performing detection methods became obsolete.
Islet-cell antibodies are heterogeneous and target multiple antigens
The ICA immunofluorescence test had become standard, but the identification of their target antigens became an urgent undertaking (Fig. 1a). MacCuish et al. had demonstrated T cell responses to insulin fragments in patients with and without insulin treatment in 1975 33. In 1983, Palmer et al. showed that children who developed type 1 diabetes had insulin autoantibodies (IAA) before they were treated with insulin 34. This was an important breakthrough in the field. It also signaled the presence of multiple autoantibodies, because insulin is only expressed in pancreatic islet β cells, whereas all islet cells stained for ICA 7, 8.
In 1982, Baekkeskov et al. reported autoantibodies against a 64-kDa islet protein 35, 36, and in 1990 Christie et al. described autoantibodies against 40-kDa and 37-kDa fragments of islet proteins 37. The 64-kDa target of autoantibodies was later identified as glutamate decarboxylase (GAD65) 38, a known antigenic target of autoantibodies in the neurological disorder stiff-person syndrome 39. The 40-kDa and 37-kDa fragments were identified as ICA512 (now also known as IA-2) 40 and the related protein phogrin (also known as IA-2β) 41, respectively, both of which were identified separately as the targets of autoantibodies in type 1 diabetes 42, 43. GAD65-directed autoantibodies (GADA) were shown to be part of the ICA reaction, with a β cell-specific staining pattern 44. The IA-2-directed autoantibodies (IA-2A) were shown to be part of the pan-islet-cell staining of ICA 45. Other proteins, such as ICA69, were claimed to be targets of ICA 46, but were not confirmed by other groups or in standardization workshops 47. The lipid antigens GM2-1 and sulfatides were also reported to be targeted by ICA 48, 49, but no methods have been developed for robust assessment of their validity. In contrast, the β cell zinc transporter 8 (ZnT8) protein has been confirmed to be a target of autoantibodies (ZnT8A) in more than 50% of patients with type 1 diabetes 50, 51, and tetraspanin 7 was identified as the 38-kDa target of autoantibodies against glima 52, 53. These autoantibodies were present in more than 30% of patients with type 1 diabetes 54.
Prediction of clinical disease
The notion that ICA and other islet autoantibodies precede the onset of type 1 diabetes allows the prediction of future disease. As early as 1977, Irvine’s group showed that the presence of ICA identified adult patients treated with oral hypoglycemic agents who would later require insulin treatment 55. Numerous studies, including a prominent study in triplets 56, had identified occasional cases of ICA-positive individuals who later developed diabetes, but it was not until 1988 that an analysis of the Barts–Windsor Family Study showed that it was indeed possible to estimate the risk in ICA-positive relatives of patients with type 1 diabetes 57. This was followed by the establishment of risk estimates using the standardized international units for ICA 30. The higher the titre of ICA, the higher the risk that an ICA-positive relative would develop type 1 diabetes. The risk reached 100% within 10 years in relatives who had ICA titres of > 80 Juvenile Diabetes Foundation (JDF) units/ml. These studies provided the foundation for later prevention trials in ICA-positive first-degree relatives 58.
The inclusion of IAA, GADA and IA-2A further improved our ability to stratify the risk of type 1 diabetes. The first reported use of autoantibody combinations to improve diabetes prediction was in twins in 1992, when a combination of ICA, IAA, GADA and antibodies against the 37-kDa and 40-kDa fragments was used 59. This was followed in 1994 by a study in relatives of patients with type 1 diabetes, which found that 8% of relatives with ICA only and 88% of those with ICA plus IAA, GADA or antibodies to the 37-kDa or 40-kDa fragments developed diabetes 60. It is noteworthy that not all the antibodies are useful in every situation. For example, the prediction of insulin requirement in adult-onset diabetes is made by testing for ICA 61, GADA 62 and IA-2A 63, but IAA are rare in patients in this age group 64.
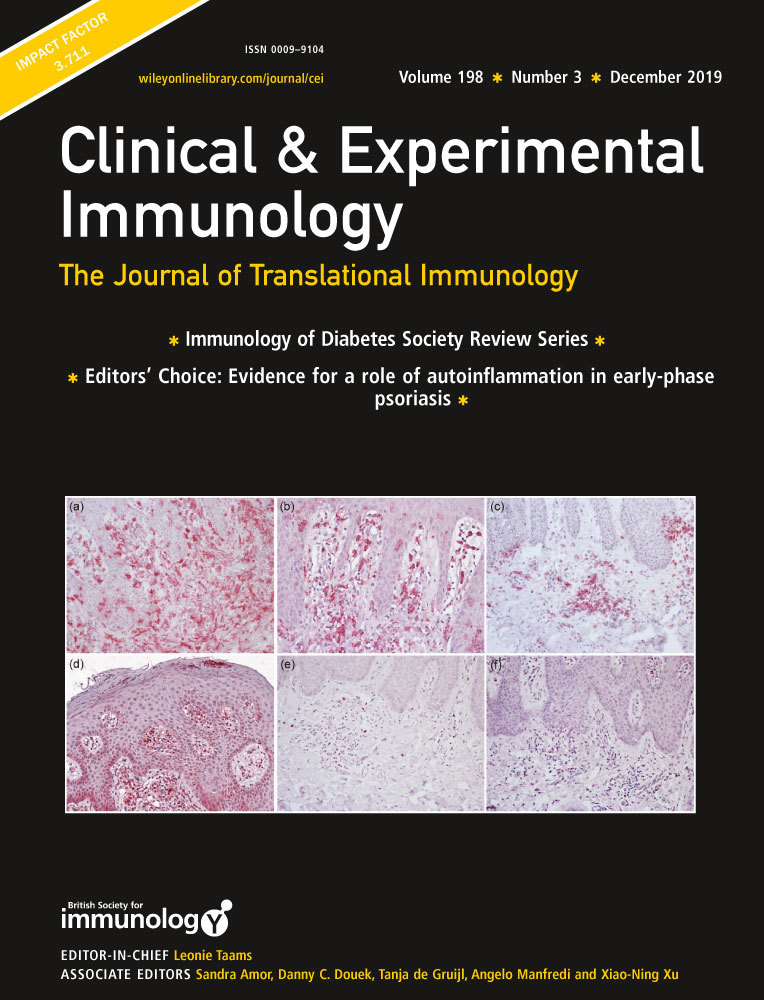
Antibody combinations were subsequently used to select at-risk relatives for clinical trials 58, 65, and it is now well established that the diabetes risk associated with the presence of multiple islet autoantibodies (two or more of IAA, GADA, IA-2A and ZnT8A) is markedly greater than the risk in people with a single autoantibody 66-69. A landmark study, that involved combined analysis of more than 13 000 individuals from three birth cohorts, demonstrated that almost all children with genetic susceptibility to type 1 diabetes who developed multiple islet autoantibodies progressed to diabetes (Fig. 1b) 70. This has paved the way for population-based screening 71.
Natural history of islet autoantibodies
Prospective birth-cohort studies have made invaluable contributions to our knowledge of the appearance and progression of islet autoantibodies in childhood, including the German BABYDIAB Study 72, the Finnish DIPP Project 73, the DAISY from Colorado 74 and the TEDDY Study 75, which have now been running for up to three decades. These studies have shown that in genetically predisposed children, autoantibody seroconversion occurs relatively frequently between the ages of 6 months and 3 years, with the incidence of autoantibodies peaking at an age of 1 year (Fig. 1c) 76-78. The typical natural history of type 1 diabetes in children is the appearance of the first high-affinity autoantibody 79, which is usually IAA in the youngest children, followed by the appearance of other islet autoantibodies 80, usually within 3 years 81, and eventually the development of diabetes. Two islet-autoimmunity endotypes are distinguished 82. One is characterized by the first appearance of IAA in children carrying HLA-DR4, and occurs in the first years of life. The second is characterized by the first appearance of GADA in children carrying HLA-DR3, and is the endotype most frequently observed in children who seroconvert after age 2 years. Based on the different associations of the two endotypes and environmental factors, it has been suggested that the endotypes have different etiologies 82. However, age is an important confounder and it is possible that these differences are merely age-related. In contrast to IAA and GADA, IA-2A usually occurs together with autoantibodies against other β cell antigens and is therefore a very specific and highly predictive immune marker for progression to clinical type 1 diabetes 83, 84, particularly if its reactivity spreads to epitopes on the homologous protein IA-2β 84-86. ZnT8A also usually appears later in the development of the disease 87.
Practical perspectives
Antibody titre, affinity and specificity
There are differences in the target autoantigens and epitopes, the titres, affinities and subclasses of islet autoantibodies. These characteristics are associated with the subject’s age and HLA genotype, and in some cases can help to distinguish diabetes-associated islet autoantibodies from non-disease-associated autoantibody signals 88.
The intensity and maturity of the antibody response are reflected in the antibody titre, affinity, immunoglobulin (Ig)G subclass, and target epitopes on single or multiple islet autoantigens. Islet autoantibodies with high titres usually involve multiple IgG subclasses and are directed against multiple epitopes on the target antigen. Similar to ICA 30, high titres of IAA 64, 84 or IA-2A 84 are associated with faster progression to clinical type 1 diabetes. Moreover, IAA or IA-2A responses that include IgG2, IgG3 and/or IgG4 as well as IgG1 are associated with an increased risk, even if the antibody titres are not high. By combining these antibody characteristics, the 5-year diabetes risk in islet-autoantibody-positive relatives can be stratified from less than 10% to more than 90% 84.
The affinity (binding strength) of the autoantibody to the target antigen is closely related to the intensity of the antibody response. Accordingly, high-affinity islet autoantibodies are associated with progression to clinical type 1 diabetes, even if the antibody titre is relatively low, whereas low-affinity antibodies are unrelated to the development of diabetes, even if the capacity and titre of the antibody are high 79, 89-92. Consistent with their high disease specificity, IA-2A are characterized by high affinity 92. In contrast, both IAA and GADA can range in affinity by more than 1000-fold 79, 89-91. The highest affinities are > 1011 l/mol. For IAA and GADA the low- and high-affinity autoantibodies appear to bind to different epitopes 79, 90, 91. For example, high-affinity IAA require the preservation of amino acids 8–13 in the insulin A chain to bind to human insulin, and also bind proinsulin. In contrast, the majority of low-affinity IAA are dependent on the COOH-terminal residues of the insulin B chain and usually do not bind proinsulin 79. Low-affinity antibodies are seen more frequently in individuals who do not have a strong genetic susceptibility to type 1 diabetes and in children who remain positive for only IAA or GADA 79, 80, 93. The affinities and epitope specificities of IAA and GADA can be used to stratify the progression to type 1 diabetes 79, 90, 94, 95, and those for GADA can predict insulin therapy in individuals with adult-onset diabetes 96, 97. The spread of IA-2A reactivity against epitopes on the homologous IA-2β protein is associated with the rapid development of diabetes 84-86.
Therefore, it is useful to identify and/or exclude low-affinity signals in risk screening for clinical trials, particularly in individuals with only IAA or GADA, who may be at an early stage of the disease process and may progress to producing multiple islet autoantibodies 79, 80, 90. The identification of markers associated with the risk of progression from single to multiple islet autoantibodies has been investigated in studies within the TrialNet Consortium 68, 98-103. Genetic risk may also be used to select single islet autoantibody-positive children who are most likely to progress to producing multiple islet autoantibodies 68, 102, 103. A low GAD autoantibody titre is associated with a low risk of progression to multiple islet autoantibodies 98.
Why do multiple antibodies or multiple tests work?
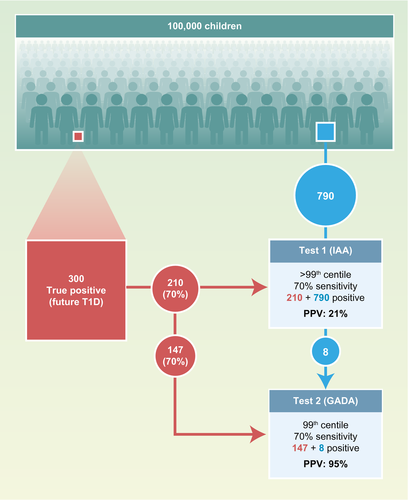
Multiple antibodies and multiple tests have the mathematical advantage of increasing the a priori probability of a true result in the samples selected for the second measurement, as may be expected from Bayes’ theorem 104. This can be illustrated theoretically in the example shown in Fig. 2. The example assumes that 0·3% of preschool children are true positives and will develop type 1 diabetes. In a population of 100 000, this corresponds to 300 children. A single islet autoantibody measurement (e.g. IAA) with a sensitivity of 70% will identify 1000 children when its threshold is set to the 99th percentile of the population. These children will have a 21% risk (positive predictive value) of developing type 1 diabetes. A second test with similar characteristics will identify a similar number of children with a similar risk. However, there will be a marked enrichment of future cases of type 1 diabetes in the children who have both autoantibodies: there would be 155 children with both IAA and GADA, 147 of whom would develop diabetes (95% risk; 49% sensitivity); and of 1690 with only IAA or GADA, 126 would develop diabetes (7·5% risk; 42% sensitivity). Adding more antibodies, such as IA-2A, would identify multiple islet autoantibodies in another 70% (88) of children with a single IAA or GADA who will develop diabetes, thereby increasing the sensitivity (78%) with only a slight reduction in the risk. Adding another antibody (e.g. ZnT8A) will provide a limited improvement in test performance. In reality, IAA and GADA, etc. are not completely independent and there are age relationships, so it is not quite this simple. Nevertheless, for many children who develop the disease, the high risk associated with multiple antibodies has more to do with Bayes’ theorem and perhaps less to do with a multiple-hit disease process that progresses from single to multiple antibodies. A similar principle applies when a second test is performed (e.g. an electrochemiluminescence assay 105, 106, luciferase immunoprecipitation system assay 107, 108 or IAA affinity assay 109, 110) using samples that were previously identified as positive on a radiobinding assay. A very similar outcome would also be expected if the order of the tests were reversed. The thresholds for the different antibodies can also be adjusted to obtain the best possible combination of sensitivity and risk 111.
Modelling islet autoantibody profiles
Prospective studies have shown that the natural progression to type 1 diabetes is not uniform in children and adolescents. Based on the individuals’ different genetic backgrounds and environments, islet autoimmunity may develop at different ages, show different longitudinal autoantibody profiles and progress to clinical diabetes at various rates. Today, we are unable to predict the individual’s progression exactly or to link etiological factors to the dynamics of islet autoantibody patterns over time. However, recent studies have started to develop mathematical algorithms to model complex longitudinal autoantibody profiles and stratify progression rates 112, 113. Children who develop multiple islet autoantibodies can be clustered according to their longitudinal profiles, and it has been shown that the likelihood of progressing from seroconversion to clinical diabetes within 5 years ranges in these clusters from below 10% to above 80%. Those children who seroconverted in the first years of life and expressed stable IAA and IA-2A responses had the highest risk of diabetes. Interestingly, this risk was unaffected by the child’s GADA status 113. A cluster analysis also revealed that losing IAA reactivity was associated with delayed progression to type 1 diabetes in children who were positive for multiple islet autoantibodies 112. Mathematical approaches applied to data from prospective cohorts have strong potential utility as novel tools for the stratification of islet-autoantibody-positive individuals and offer new opportunities to clarify the disease mechanisms.
Islet autoantibodies used to select for trials and as study outcomes
Clinical trials to investigate the treatment of type 1 diabetes commonly use the participant’s autoantibody positivity as an inclusion criterion (Fig. 1c) 58, 65, 114. In trials that recruit individuals with clinical diabetes, islet autoantibodies are used to distinguish type 1 diabetes from other types. In addition to the recruitment of trial participants, islet autoantibodies have been used as outcome markers in several studies (Fig. 1c). These include natural history studies such as TEDDY 115 and primary prevention studies such as BABYDIET 116, TRIGR 117 and POInT 118. The availability of high-quality, high-throughput and harmonized autoantibody tests 119 mean that stable longitudinal measurements of the outcomes are possible. As discussed in above, the definition of outcome can be improved by including confirmation with a second laboratory test or the use of multiple assays.
The age at screening is also important. For first-degree relatives of patients with type 1 diabetes, the risk of developing islet autoantibodies decreases exponentially with age, with a half-life of 3–4 years 120. This has practical implications. First, if we consider that the peak incidence of islet autoantibody seroconversion occurs in the first 3 years of life, screening is likely to be most effective in preschool years. Secondly, relatives who remain negative through to their teenage years will have an eightfold lower risk of developing islet autoantibodies than they had when they were born.
Extension to the population at large
The development of multiple islet autoantibodies has long been recognized as a critical step in the pathogenesis and diagnosis of type 1 diabetes 59, 60, culminating in the finding that almost all children who develop multiple islet autoantibodies will develop clinical symptomatic diabetes, regardless of whether they have an a priori family history of the disease 70. This has led to a new staging strategy for type 1 diabetes, in which the presence of multiple islet autoantibodies is now used as a criterion for the diagnosis of presymptomatic early-stage type 1 diabetes 121. An early diagnosis of type 1 diabetes can prevent the severe metabolic decompensation that is frequently observed at the onset of clinical diabetes 122-124. Screening for islet autoantibodies can be performed with capillary blood samples or dried blood spots 125-128. The Fr1da Study started in 2015 as a model project investigating public health screening for early-stage type 1 diabetes (confirmed with positivity for multiple islet autoantibodies) in Bavaria, Germany 71. It assesses: (1) whether the early diagnosis of type 1 diabetes in the context of regular medical check-ups in childhood is feasible and efficient; (2) whether ketoacidosis and the hospitalization of children can be prevented by screening; and (3) whether psychological distress can be reduced with the early diagnosis of diabetes, education and care. Similar studies have already commenced in Lower Saxony, Germany, with additional screening for low-density lipoprotein–hypercholesterolemia (Fr1dolin Study) 129 and in Colorado, with additional screening for celiac disease (ASK Study) 130.
The future
We envisage two areas of activity in the next few years (Fig. 1d). From a practical perspective, a technology is required that facilitates the widespread use of islet autoantibody testing for the diagnosis of presymptomatic type 1 diabetes in the public health context 131. From the research perspective, activities to identify modified protein targets, both to generate better assays and to identify pathogenetic disease mechanisms, are highly likely.
Technological advances should drive down costs and allow simple high-throughput screening, which will favor its widespread application. Cost is a clear factor, because population-based screening requires that tens or hundreds of thousands of children be tested, more than 99% of whom will be negative. This will require a sensitive first-line test that covers the majority of the major islet autoantibodies (IAA, GADA, IA-2A, ZnT8A), with follow-up tests for those who are positive to confirm and stratify their risk 71, 127, 132, 133. Point-of-care testing may be one approach to achieving this. This technology should be coupled to careful application of Bayes’ modeling, including additional risk factors such as genetics 134, family history and age. This sort of modeling will be possible once much larger numbers of children have been tested and followed, emphasizing the need to introduce broad testing programs in many regions and countries.
Modified islet antigens have been reported in the literature 135, 136, but we conclude that a number of these are unlikely to be validated because of weaknesses in the assays. Increased antibody binding to a modified form of the tetraspanin 7 protein was observed in some patients 137, but it is difficult to determine whether this is a favored in-vivo target or an artificial in-vitro modification. Smart systems that reliably identify antibodies that bind to proteins from unperturbed and perturbed islets should be possible, and will probably reveal a range of variations in the autoantibody–autoantigen targets that we know today.