The impact of disease activity and tumour necrosis factor-α inhibitor therapy on cytokine levels in juvenile idiopathic arthritis
Summary
The aim of this study was to evaluate prospectively cytokine levels and disease activity in juvenile idiopathic arthritis (JIA) patients treated with and without tumour necrosis factor (TNF)-α inhibitors. TNF-α inhibitor-naive JIA subjects were followed prospectively for 6 months. Cytokine levels of TNF-α, interleukin (IL)−1β, IL-6, IL-8, IL-10 and IL-17 were measured at baseline for JIA subjects and healthy controls (HCs). Cytokine levels were then measured at four time-points after initiation of TNF-α inhibition for anti-TNF-α-treated (anti-TNF) JIA subjects, and at two subsequent time-points for other JIA (non-TNF) subjects. JIA disease activity by Childhood Health Assessment Questionnaire (CHAQ) disability index/pain score and physician joint count/global assessment was recorded. Sixteen anti-TNF, 31 non-TNF and 16 HCs were analysed. Among JIA subjects, those with higher baseline disease activity (subsequent anti-TNFs) had higher baseline TNF-α, IL-6 and IL-8 than those with lower disease activity (non-TNFs) (P < 0·05). TNF-α and IL-10 increased, and IL-6 and IL-8 no longer remained significantly higher after TNF-α inhibitor initiation in anti-TNF subjects. Subgroup analysis of etanercept versus adalimumab-treated subjects showed that TNF-α and IL-17 increased significantly in etanercept but not adalimumab-treated subjects, despite clinical improvement in both groups of subjects. JIA subjects with increased disease activity at baseline had higher serum proinflammatory cytokines. TNF-α inhibition resulted in suppression of IL-6 and IL-8 in parallel with clinical improvement in all anti-TNF-treated subjects, but was also associated with elevated TNF-α and IL-17 in etanercept-treated subjects.
Introduction
Tumour necrosis factor (TNF)-α is an important cytokine implicated in the pathogenesis of juvenile idiopathic arthritis (JIA), and can induce expression of other proinflammatory cytokines such as interleukin (IL)-1β, IL-6 and IL-8, leading to an overall protracted inflammatory response 1-4. In the normal physiological state, proinflammatory cytokines, including TNF-α, are maintained in equilibrium with anti-inflammatory cytokines, such as IL-10. However, inflammatory disease states, such as JIA, may be due to a shift in this balance 2.
Several studies have attempted to characterize patterns of cytokine expression in serum and synovial fluid (SF) of JIA patients, with varied results 5-12. Some studies found increased TNF-α, IL-6 and IL-8 levels in children with various JIA subtypes when compared to other subtypes and/or healthy controls (HCs). Other studies only reported significantly increased levels of serum TNF-α, IL-1β, IL-6 and IL-17 in JIA patients with active disease when compared to those with inactive disease and/or HCs 5-12. However, these studies have several limitations. Most were cross-sectional, included patients already on immunosuppressive therapy and lacked age-matched HCs. Cytokine levels evaluated at one point in time may not be reliable and may be affected by many variables, including age, co-morbidities, infection, medications and circadian rhythms 13. Many of these studies also included systemic-onset JIA (SoJIA) subjects, who probably have a distinct cytokine profile and pathogenesis.
JIA patients with high disease activity are being treated increasingly with TNF-α inhibitors, including etanercept, adalimumab, infliximab and certolizumab pegol. The impact of biologicals on the overall cytokine milieu in JIA patients is not well understood. Additionally, little is known about the alterations of cytokine levels in relation to treatment response. Research suggests that JIA and other chronic inflammatory diseases depend upon a large network of cytokines that are inter-related. Targeting one of the integral cytokines, such as TNF-α, may disrupt this cytokine network and lead to control of disease by down-regulating TNF-α, as well as other inter-related cytokines and bioactivities downstream in this inflammatory pathway 14, 15. However, we still have much to learn about these complicated systems.
The purpose of this study was to characterize cytokine profiles longitudinally, specifically TNF-α, IL-1β, IL-6, IL-8, IL-10 and IL-17, in JIA patients, and to describe associations between cytokine alterations, disease activity and treatment with TNF-α inhibitors. Delineation of cytokine patterns may have clinical implications for JIA patients if certain cytokine values at baseline and/or follow-up help to predict disease activity and therapeutic response. Our previous research suggests that uncontrolled JIA disease activity may be associated with increased risk of infection in JIA patients, and although infection risk must be considered during treatment with TNF-α inhibitors, this therapy may not increase infection rates consistently in JIA patients 16. Future analysis of the relationship between disease activity, cytokine profiles, treatment regimens and infection rates may help to tailor therapeutic plans for JIA patients.
Materials and methods
Study design
This prospective cohort study included TNF-α inhibitor-naive JIA subjects and HCs between the ages of 1 and 21 years from a single tertiary medical centre. This study was approved by the Institutional Review Board at the Hospital for Special Surgery, and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave their informed consent prior to their inclusion in the study. Informed consent was provided by legal guardians for all minors under the age of 18 years. All participants between the ages of 7 and 17 years gave their assent prior to their inclusion in the study.
JIA subjects had a diagnosis of oligoarticular JIA, polyarticular JIA, enthesitis-related arthritis, psoriatic arthritis or undifferentiated juvenile arthritis. Subjects with SoJIA were excluded. HC subjects were recruited from orthopaedic clinics if preoperative blood work was to be drawn, and were excluded if they had any medical condition suspected to alter cytokine levels. HCs were also excluded if they 1 had a physician-diagnosed infection (with or without antibiotic treatment), 2 received immunizations within 1 month prior to enrolment or 3 had a fever within 2 weeks prior to enrolment. Similarly, cytokine levels were not drawn for JIA subjects if they 1 had a physician-diagnosed infection (with or without antibiotic treatment), 2 received immunizations within 1 month prior to a visit or 3 had a fever within 2 weeks prior to a visit. Subjects with primary or acquired immunodeficiencies were excluded from both groups.
Baseline data were collected on all subjects. After enrolment, JIA subjects who were initiated on a TNF-α inhibitor at the discretion of the treating physician were analysed within the anti-TNF group. Dosing of TNF-α inhibitors was based on standard of care for paediatric patients with age- and weight-based dosing. Subjects treated without TNF-α inhibitors were analysed in the non-TNF group. Subjects in either JIA group could be treated with non-steroidal anti-inflammatory drugs (NSAIDs), oral or intra-articular corticosteroids, methotrexate or other disease-modifying anti-rheumatic drugs (DMARDs), as deemed necessary by the treating physician.
HCs completed only a baseline visit in order to obtain cytokine levels to compare with JIA subjects. JIA subjects in the anti-TNF group were evaluated at baseline, 2–4 weeks, 6–8 weeks, 3–4 months and 6 months after initiation of a TNF-α inhibitor. JIA subjects in the non-TNF group were evaluated at baseline, 3–4 months and 6 months after enrolment. Compliance with medications was ensured at each visit by the treating paediatric rheumatologists and on questionnaires which asked subjects if any medication doses were missed. Study visits were conducted at the time of regularly scheduled follow-up visits. The frequency of follow-up visits was determined by the treating physician. Therefore, study visits were more frequent in the anti-TNF group (with subjects requiring more frequent follow-up for more active disease) than the non-TNF group.
Serum blood samples for cytokine analysis were collected at baseline and the specified time-points outlined above for all enrolled subjects. Study blood was drawn only when subjects were having blood drawn for routine monitoring by the treating physician. Because study blood was drawn at the time of scheduled follow-up with the treating physician, time of day for study blood draws could not be standardized for all subjects to account for circadian rhythms of serum cytokines. Serum protein TNF-α, IL-1β, IL-6, IL-8, IL-10 and IL-17 and corresponding whole blood mRNA gene expression were measured. The baseline blood sample was collected prior to start of TNF-α inhibitor treatment in anti-TNF subjects. At each time-point, blood samples were collected by venipuncture into serum separator tubes (5 cc) for the measurement of serum protein cytokine levels and PAXgene blood RNA tubes (2·5 cc) for the measurement of mRNA gene expression cytokine profiles. All samples were transferred on ice, processed immediately and stored at −70°C to be batched later for analysis.
JIA disease activity was recorded at each visit. Patient measures of disease activity included the Childhood Health Assessment Questionnaire (CHAQ) disability index (scores 0–3·0) and pain visual analogue score (scores 0–100) 17. Physician measures of disease activity included total joint count (of 26 possible swollen, tender or limited joints) and physician global assessment (scores 0–10).
Determination of serum cytokine levels
The serum concentrations of TNF-α, IL-1β, IL-6, IL-8, IL-10 and IL-17 were determined in the General Core Laboratory of the Clinical and Translational Science Center (CTSC) in Weill Cornell Medical College using quantitative electrochemiluminescent 4-plex and single-plex assay kits from Meso Scale Discovery (Gaithersburg, MD, USA), following the manufacturer's instructions. The intra- and interassay coefficients of variation for these cytokine assays were less than 14·2% and 10·8%, respectively. The detection limits of TNF-α, IL-1β, IL-6, IL-8, IL-10 and IL-17 were 0·61, 0·4, 0·5, 0·15, 0·8 and 0·17 pg/ml, respectively. Quality control testing was performed to ensure that the presence of etanercept or adalimumab in the serum of subjects treated with these agents did not affect the TNF-α assay results.
Of note, serum IL-1β levels were found to be undetectable with the above assay in the majority of subjects, and were therefore retested using a quantitative sandwich enzyme immunoassay kit from R&D Systems (Minneapolis, MN, USA), following the manufacturer's instructions. The measurement range for this IL-1β assay was 0·13–8·0 pg/ml. Serum IL-1β levels remained undetectable with this second assay in the majority of subjects, and were therefore unable to be analysed in this study.
Determination of cytokine mRNA levels by real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR)
Levels of TNF-α, IL-1β, IL-6, IL-8, IL-10 and IL-17 mRNA were determined in the Molecular Core Laboratory of the CTSC in Weill Cornell Medical College. Briefly, total cellular RNA was extracted from whole blood using the PAXgene Blood RNA kit (Qiagen Inc., Valencia, CA, USA), according to the manufacturer's instructions. The concentration of RNA was determined using the NanoDrop 2000C. RNAs (1 μg) were converted to cDNAs using the high-capacity cDNA reverse transcription kit from Life Technologies (Grand Island, NY, USA), and real-time PCR was performed using TaqMan® reagents in the ABI HT7900 fast real-time PCR system from Applied Biosystems (Foster City, CA, USA), following the manufacturer's instructions. The primer and probe sets of the six target genes (TNF, assay ID# Hs00174128_m1; IL-1B, assay ID# Hs01555410_m1; IL-6, assay ID# Hs00985639_m1; IL-8, assay ID# Hs00174103_m1; IL-10, assay ID# Hs00961622_m1; IL-17A, assay ID# Hs00174383_m1) and the internal control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (assay ID# Hs99999905_m1) and beta actin (ACTB) (assay ID# Hs99999903_m1) were purchased from Life Technologies. A reaction mix was prepared using the TaqMan® Universal PCR Master Mix (Life Technologies). The thermal cycling conditions were: an initial incubation at 50°C for 2 min, then 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min, as described in the manufacturer's protocol. PCR values were analysed in order to determine mRNA transcription levels for each cytokine, and to determine fold changes in transcription at multiple time-points 18.
Statistical analysis
Descriptive analysis of the patient population was evaluated by calculating means and standard deviations for continuous variables and frequencies and percentages for all discrete variables. Univariate comparisons between the study groups were performed using independent-sample t-tests or one-way analysis of variance (anova) and χ2 or Fisher's exact tests. Simple linear regression models were created to evaluate the correlation of disease activity and cytokine levels. Following the initial analysis, a generalized estimating equation (GEE) model was created to assess all observations of cytokine protein levels over time. Between-group factors analysed included exposure to anti-TNF medication and adjustment for patient serologies. All analyses were performed using sas version 9·2 (SAS Institute, Cary, NC, USA). Differences were considered significant at the P < 0·05 level.
Results
Demographics and patient population
Sixty-three subjects were enrolled, with 16 subjects in the anti-TNF group, 31 subjects in the non-TNF group and 16 subjects in the HC group. Demographics and distribution of JIA subtypes were similar overall between groups (Table 1).
| Anti-TNF subjects (n = 16) | Non-TNF subjects (n = 31) | Healthy control subjects (n = 16) | P-value | |
|---|---|---|---|---|
| Age (years), mean (± s.d.) | 13.1 (± 4.6) | 11.2 (± 4.3) | 11.8 (± 5.1) | 0.426 |
| Females, n (%) | 11 (69) | 22 (71) | 11 (69) | 0.982 |
| Ethnicity, n (%) | ||||
| Hispanic | 2 (13) | 5 (16) | 8 (50) | 0.017 |
| Non-Hispanic | 14 (88) | 26 (84) | 8 (50) | |
| Race, n (%) | ||||
| White | 13 (81) | 30* (97) | 13 (81) | 0.146 |
| African American | 2 (13) | 1 (3) | 0 (0) | 0.215 |
| Asian | 0 (0) | 2 (6) | 0 (0) | 0.344 |
| American Indian or Alaska Native | 1 (6) | 0 (0) | 0 (0) | 0.225 |
| Other | 0 (0) | 1 (3) | 3 (19) | 0.057 |
| Comorbidities, n (%) | ||||
| Uveitis | 0 (0) | 1 (3) | > 0.999 | |
| Psoriasis | 2 (13) | 2 (6) | 0.597 | |
| Thyroid disease | 2 (13) | 1 (3) | 0.264 | |
| Coeliac disease | 0 (0) | 2 (6) | 0.541 | |
| Asthma | 4 (25) | 5 (16) | 0.466 | |
| None | 10 (63) | 23 (74) | 0.506 | |
| Medications, n (%) | ||||
| None | 0 (0) | 2 (6) | 0.541 | |
| NSAIDs | 15 (94) | 28 (90) | > 0.999 | |
| Methotrexate | 1 (6) | 0 (0) | 0.340 | |
| Sulphasalazine | 0 (0) | 1 (3) | > 0.999 | |
| Duration of JIA at enrolment (months), mean (± s.d.) | 6.6 (± 8.3) | 26.5 (± 40.4) | 0.012 | |
| JIA subtype, n (%) | ||||
| Oligoarticular | 1 (6) | 5 (16) | 0.584 | |
| RF+ polyarticular | 1 (6) | 3 (10) | ||
| RF– polyarticular | 2 (13) | 1 (3) | ||
| Enthesitis-related | 9 (56) | 15 (48) | ||
| Psoriatic | 2 (13) | 2 (6) | ||
| Undifferentiated | 1 (6) | 5 (16) | ||
| Baseline serologies, n (%) | ||||
| ANA+ | 8 (50) | 15 (48) | 0.554 | |
| RF+ | 1 (6) | 7 (23) | ||
| CCP+ | 1 (6) | 3 (10) | ||
| HLA-B27+ | 5 (31) | 7 (23) | ||
| Baseline disease activity, mean (± s.d.)† | ||||
| CHAQ disability index | 0.8 (± 0.5) | 0.3 (± 0.4) | 0.001 | |
| CHAQ pain visual analogue score | 43.4 (± 29.2) | 27.6 (± 25.6) | 0.062 | |
| Total joint count | 6.5 (± 4.0) | 1.4 (± 1.4) | < 0.001 | |
| Physician global assessment | 5.4 (±2.0) | 1.6 (± 1.5) | < 0.001 | |
| Baseline ESR (mm/h), mean (± s.d.) | 11.4 (± 13.5) | 6.9 (± 7.8) | 0.232 |
- *Two patients reported white and Asian race, one patient reported white and Other race. †Childhood health assessment questionnaire (CHAQ) disability index range 0–3.0; CHAQ pain visual analogue scale range 0–100; total joint count range 0–26; physician global assessment range 0–10. NSAID: non-steroidal anti-inflammatory drugs; JIA = juvenile idiopathic arthritis; RF = rheumatoid factor; ANA = anti-nuclear antibody; CCP = cyclic citrullinated peptide; HLA = human leucocyte antigen; s.d. = standard deviation; ESR = erythrocyte sedimentation rate.
Of the 47 JIA subjects, 45 were included in the final analysis. One anti-TNF subject withdrew after baseline cytokine levels were drawn, and was therefore included only in analysis of baseline cytokine levels; this subject continued anti-TNF therapy but changed follow-up to another facility. One anti-TNF subject self-discontinued TNF-α inhibitor therapy after 1 month, and was analysed only through 3 months of follow-up in the anti-TNF group. Thirteen subjects (81%) in the anti-TNF group and 11 subjects (35%) in the non-TNF group had at least three blood samples analysed.
Among the 15 anti-TNF group subjects analysed after TNF-α inhibitor initiation, six subjects were treated only with etanercept and seven patients were treated only with adalimumab. The six subjects treated only with only etanercept included one oligoarticular, three polyarticular and two enthesitis-related arthritis subjects. The seven subjects treated only with adalimumab all had enthesitis-related arthritis. Due to uncontrolled disease activity, one subject (with undifferentiated arthritis) was treated with etanercept followed by adalimumab and another subject (with enthesitis-related arthritis) was treated with adalimumab followed by certolizumab pegol. Subgroup analysis comparing subjects treated with etanercept versus adalimumab were conducted only on patients treated with a single agent (six etanercept-treated subjects and seven adalimumab-treated subjects). No patients in this study were treated with infliximab. One anti-TNF subject was also treated with methotrexate. No subject in either group was treated with any other immunosuppressive therapy, including oral or intra-articular corticosteroids.
Serum cytokine levels
Baseline cytokine levels were drawn at enrolment for all subjects, and therefore prior to initiation of a TNF-α inhibitor for anti-TNF group subjects (Table 2). At baseline, we found no significant differences in TNF-α, IL-6, IL-8, IL-10 and IL-17 between JIA subjects overall versus HCs. Only serum IL-8 levels were elevated significantly in the anti-TNF subjects compared to HCs. When analysing JIA subjects in subgroups of anti-TNF subjects (those with more active disease at baseline which resulted in subsequent treatment with a TNF-α inhibitor) and non-TNF subjects (those who did not go on to require TNF-α inhibitor treatment), only serum IL-8 levels were elevated significantly in the anti-TNF group compared to HCs (P = 0·01). Among JIA subjects, baseline levels of TNF-α, IL-6 and IL-8 were significantly higher in anti-TNF versus non-TNF subjects (P < 0·05) (Table 2). Subjects were also analysed by JIA subtype at baseline, with polyarticular JIA subjects showing significantly higher levels of IL-6 (6·61 ± 8·20 pg/ml) than JIA subjects with either enthesitis-related arthritis (1·19 ± 1·43 pg/ml) or undifferentiated JIA (0·94 ± 0·62 pg/ml) (P < 0·05 for both).
| Anti-TNF subjects (n = 16) | Non-TNF subjects (n = 31) | Healthy control subjects (n = 16) | P-value* | |
|---|---|---|---|---|
| Baseline serum cytokine levels (pg/ml), mean (± s.d.) | ||||
| TNF-α† | 4.99 (± 3.86) | 3.16 (± 1.30) | 4.13 (± 1.64) | 0.036 |
| IL-6‡ | 3.63 (± 5.59) | 1.07 (± 0.78) | 2.05(± 2.55) | 0.035 |
| IL-8§ | 14.33 (± 6.74) | 9.55 (± 3.67) | 9.44 (± 3.47) | 0.003 |
| IL-10 | 4.62 (± 2.19) | 3.71 (± 2.49) | 4.62 (± 2.55) | 0.339 |
| IL-17 | 0.67 (± 1.04) | 0.55 (± 0.41) | 0.78 (± 0.97) | 0.619 |
- *Three-way P-value. †Anti-tumour necrosis factor (TNF) versus non-TNF, P = 0.035. ‡Anti-TNF versus non-TNF, P = 0.030. §Anti-TNF versus non-TNF, P = 0.004 and anti-TNF versus healthy controls, P = 0.011. JIA = juvenile idiopathic arthritis; pg = picogram; ml = millilitre; s.d. = standard deviation; IL = interleukin.
After the initiation of TNF-α inhibitor treatment, anti-TNF subjects treated with either TNF-α inhibitor had clinical improvement, as evidenced by significantly decreased disease activity based on total joint count and physician global assessment (P < 0.05 for each). Despite this, anti-TNF subjects had significantly higher serum TNF-α and IL-10 levels than non-TNF subjects during 6 months of follow-up (P < 0.05 for each) (Fig. 1). Although IL-6 and IL-8 were higher at baseline in anti-TNF versus non-TNF subjects, these differences did not remain significant once anti-TNF subjects were started on TNF-α inhibitors.
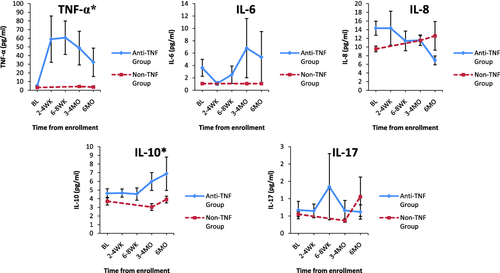
Serum cytokine levels in anti-tumour becrosis factor (TNF) versus non-TNF subjects over time. *Denotes significantly higher serum cytokine levels in anti-TNF versus non-TNF subjects longitudinally over 6 months of follow-up: anti-TNF subjects had significantly higher serum TNF-α and interleukin (IL)−10 levels than non-TNF subjects during 6 months of follow-up (P < 0.05 for each). aError bars are included for both groups. Small variability relative to the scale of the axis makes error bar visualization difficult for the non-TNF group; pg = picogram; ml = millilitre; BL = baseline; WK = weeks; MO = months.
In subgroup analysis of anti-TNF group subjects, TNF-α and IL-17 serum levels were significantly higher during 6 months of follow-up in subjects treated with etanercept (n = 6) versus adalimumab (n = 7) (P < 0.05 for both) (Fig. 2). Other cytokines did not show significant consistent changes between etanercept- and adalimumab-treated subjects during 6 months of follow-up. After TNF-α inhibitor therapy was initiated, mean TNF-α levels were 112.9 pg/ml for subjects on etanercept versus 4·00 pg/ml for subjects on adalimumab (P < 0·001). This occurred despite clinical improvement and a significant decrease in disease activity during 6 months of follow-up for subjects treated with both etanercept and adalimumab (Fig. 3). Higher serum TNF-α levels did not correspond with TNF-α mRNA fold change for either etanercept- or adalimumab-treated subjects (Fig. 4).
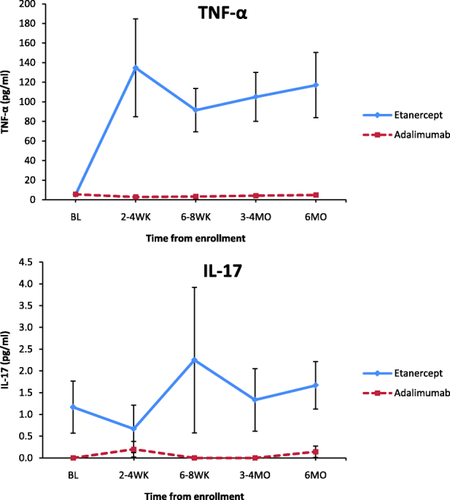
Significant cytokine alterations in etanercept- versus adalimumab-treated subjects over time. Tumour necrosis factor (TNF)-α and interleukin (IL)−17 serum levels were significantly higher during 6 months of follow-up in subjects treated with etanercept (n = 6) versus adalimumab (n = 7) (P < 0.05 for both). aError bars are included for both groups. Small variability relative to the scale of the axis makes error bar visualization difficult for the non-TNF group; pg = picogram; ml = millilitre; BL = baseline; WK = weeks; MO = months.
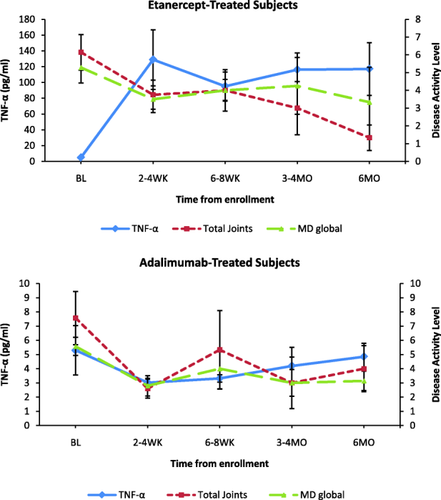
Tumour necrosis factor (TNF)-α levels versus disease activity in etanercept- (n = 6) and adalimumab-treated (n = 7) subjects. Improvement in disease activity measures [total joint count, MD global (physician's global assessment of the overall disease activity)] occurred even in the setting of higher measured serum TNF-α levels in etanercept subjects (P < 0.05). MD global was based on physician overall assessment of disease activity with score of 0–10. Please note varying scales on y-axes; pg = picogram; ml = millilitre; BL = baseline; WK = weeks; MO = months.
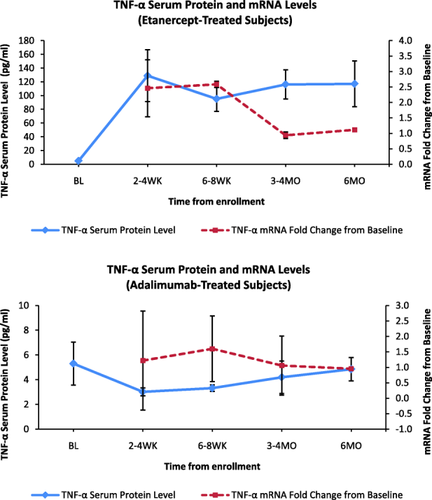
Tumour necrosis factor (TNF)-α serum protein versus mRNA levels in etanercept- and adalimumab-treated subjects. Higher serum TNF-α levels did not correspond with TNF-α mRNA fold change for either etanercept or adalimumab subjects. Please note varying scales on y-axes; pg = picogram; ml = millilitre; BL = baseline; WK = weeks; MO = months.
Serum cytokine levels were assessed for correlation with disease activity (as measured by CHAQ disability index and pain score, total joint count and physician global assessment) for all JIA subjects during the 6 months of follow-up. For all JIA subjects, physician global assessment correlated weakly with TNF-α (r = 0.19) and IL-6 levels (r = 0·32) (P < 0.05 for each). Physician global assessment correlated weakly with IL-6 levels in anti-TNF subjects (r = 0.31, P < 0.05) and in non-TNF subjects (r = 0.40, P < 0.05). Additionally, IL-8 levels correlated weakly with both CHAQ disability index (r = 0.39) and pain score (r = 0·35) (P < 0.05 for each) in non-TNF subjects.
Cytokine mRNA levels
Cytokine mRNA levels were also measured to determine whether they related to serum cytokine levels. mRNA levels were measured by fold change from baseline. No specific patterns of fold changes across time were noted for anti-TNF or non-TNF subjects for any of the five cytokines. Furthermore, fold changes in mRNA expression did not appear to vary reliably with the cytokine levels observed over time, suggesting that the changes in serum cytokines were not due to changes at the level of transcription and, alternatively, that serum cytokines measured may be derived from other cells or tissues, such as the synovium 9.
Discussion
This is the first study to evaluate serum cytokine alterations prospectively in JIA patients treated with and without TNF-α inhibitor therapy. Our aim was to characterize cytokine profiles in JIA patients, to assess cytokine alterations in conjunction with disease activity in JIA and to describe potential alterations in cytokine profiles in JIA patients treated with TNF-α inhibitors. We also investigated whether serum cytokine profiles at the protein level corresponded with serum mRNA cytokine expression at the transcriptional level.
At baseline, we found no significant differences in TNF-α, IL-6, IL-8, IL-10, and IL-17 between JIA subjects overall versus HCs. Only serum IL-8 levels were elevated significantly in the anti-TNF subjects compared to HCs. Comparing JIA subjects to each other in our study, prior to any TNF-α inhibitor exposure, baseline levels of TNF-α, IL-6 and IL-8 were significantly higher in anti-TNF subjects versus non-TNF subjects. The anti-TNF population was started on anti-TNF therapy soon after enrolment due to increased disease activity, suggesting that these three cytokines may contribute to the inflammatory process and more extensive articular disease in JIA subjects. Previous studies found similarly that JIA subjects with polyarticular and oligoarticular JIA may have higher serum levels of TNF-α, IL-6 and IL-8, and others have also described that elevated TNF-α and IL-6 levels (although not IL-8) corresponded with increased disease activity 5, 6, 9.
Within our cohort, IL-6 was the only cytokine that consistently, albeit weakly, corresponded with disease activity level at baseline and throughout follow-up in all JIA subjects. While elevated IL-6 levels have been described in JIA patients with more active disease this is the first study, to our knowledge, showing a decline in IL-6 level with improved disease control 6, 10. Although IL-6 is clearly involved in proinflammatory pathways in JIA, it is unclear why IL-6 was the only cytokine that corresponded with disease activity measures in our JIA cohort. As IL-6 correlated weakly with disease activity, we hypothesize that our subjects treated with etanercept or adalimumab may have altered inflammatory pathways and expression of serum cytokine markers in patterns not observed previously. The weak correlations noted in our study may be explained partially by small numbers and interpatient variability.
TNF-α, IL-6 and IL-8 all contribute to inflammatory pathways that are probably active in JIA patients. TNF-α is derived by macrophages and lymphoid cells that induce expression of other cytokines, including IL-6 and IL-8, thereby initiating an inflammatory response 3, 4. TNF-α and IL-6 have multiple effects on vascular endothelial cells, leucocytes and bone marrow. These effects include induction of leucocyte production by the bone marrow and leucocyte migration to sites of inflammation, as well as acute-phase reactant release by hepatocytes, all contributing to the inflammatory response. IL-6 contributes to the production of acute phase reactants, and assists in both acute and chronic inflammatory cell migration 6, 19. IL-8 also contributes to leucocyte chemotaxis and activation 6.
TNF-α levels did not decrease in JIA subjects treated with either TNF-α inhibitor, despite clinical improvement. While TNF levels were unchanged over time in the adalimumab-treated subjects, etanercept-treated subjects had a paradoxical rise in serum TNF-α levels. Additionally, all anti-TNF-treated subjects had increased IL-10 levels during 6 months of follow-up when compared to non-TNF subjects. IL-10 is produced by T cells, B cells, macrophages and dendritic cells, and helps to control inflammatory responses 20. It is unknown what impact TNF-α inhibitor therapy has on IL-10 expression. One study in adult patients with inflammatory bowel disease (IBD) or psoriasis similarly described increased IL-10 levels after treatment with either etanercept, adalimumab or infliximab, despite clinical improvement 21. We theorize that the higher levels of IL-10 in our anti-TNF subjects may have been an anti-inflammatory response to the higher disease activity in our anti-TNF subjects versus non-TNF subjects. To our knowledge, this has not been reported previously in JIA patients.
The paradoxical increase in levels of serum TNF-α in etanercept-treated subjects merits further discussion. This finding has been described previously, but primarily in adults treated with etanercept. One paediatric study found elevated TNF-α levels in three JIA subjects treated with etanercept when compared to other JIA subjects and HCs, and these subjects remained in remission 22. However, levels were drawn in subjects already on etanercept therapy, and baseline levels of TNF-α were not checked in this study. A single prospective study found that JIA patients who had failed treatment with DMARDS and corticosteroids also had increased TNF-α levels during treatment with etanercept, and this occurred irrespective of clinical course 23. This study did not evaluate other TNF-α inhibitors and did not follow a JIA cohort unexposed to TNF-α inhibitor therapy in parallel. Increased TNF-α levels have been well described in etanercept-treated adults with various diseases, including TNF receptor-associated periodic syndrome (TRAPS), multiple myeloma, metastatic breast cancer, endotoxaemia and orthoclone (OKT)-associated acute clinical syndrome 24-28. In adult ankylosing spondylitis (AS) and rheumatoid arthritis (RA), studies have shown that TNF-α levels increase significantly in etanercept-treated patients, but not in infliximab-treated patients. However, patients treated with either agent showed clinical improvement regardless of measured TNF-α levels 29-31. Our findings in this study were similar when comparing etanercept- and adalimumab-treated subjects, and we had no infliximab-treated subjects for further comparison.
Several theories have been proposed to explain the increased levels of serum TNF-α measured after initiation of etanercept. Etanercept is a TNF-receptor Fc-fusion protein, while adalimumab and infliximab are both monoclonal antibodies that bind TNF-α 32. Similar to naturally occurring soluble TNF-α receptors, etanercept may bind and neutralize TNF-α, but may also stabilize TNF-α and increase its serum half-life 33. Therefore, etanercept-treated patients may have higher measurable levels of TNF-α, but this TNF-α may not be biologically active when it is bound to etanercept 34. Although we found markedly elevated levels of TNF-α in etanercept-treated subjects, these elevated levels of TNF-α did not appear to correspond with increased disease activity, suggesting that the TNF-α measured in these subjects may have been biologically inactive. We hypothesize that adalimumab (and possibly infliximab based on data from other studies) may mask the TNF-α epitopes detected typically by assays used to measure TNF-α, while etanercept may not, leading to differences in measured levels of TNF-α in patients treated with each of these agents 29-31. The fact that other cytokines, including IL-6 and IL-8, remained stable in etanercept-treated subjects also implies that the higher measured levels of TNF-α did not promote an inflammatory cascade as might be expected.
This study is the first to report increased serum IL-17 levels in JIA subjects who improved clinically after treatment with etanercept. IL-17 serum levels were unchanged over time in adalimumab-treated subjects. IL-17 is a highly inflammatory cytokine, produced mainly by CD4+ T helper type 17 (Th17) T cells, which can mediate joint and cartilage destruction 35. Elevated IL-17 levels have been described in the serum and SF of JIA patients, but this has not been found consistently 36-38. No past studies have evaluated IL-17 levels prospectively in JIA patients. One adult study described increased IL-17 levels in patients with psoriasis and IBD treated with etanercept, adalimumab or infliximab 21. This occurred similarly despite clinical improvement in treated subjects. These authors found that while TNF-α inhibitors neutralize the effect of TNF-α in target tissues, TNF-α inhibitors can also enhance peripheral T cell activation.
One limitation of our study was that cytokine levels could not be drawn reliably at the same time of day for each subject (as levels were drawn at subjects’ scheduled visits), which may account for some cytokine variability based on known circadian rhythms of cytokines 13. We attempted, as thoroughly as possible, to address other potential confounders for serum cytokine levels, such as disease activity and consistent sample collection and storage. We also did not collect samples if the patient had recent infection, fever or immunizations, as these factors can all impact cytokine levels. Other limitations included a relatively small sample size, and a lack of subjects treated with infliximab.
JIA is a heterogeneous autoimmune disease in which inflammation is mediated at least partially by cytokines. Paediatric studies have attempted to gain insight into the pathogenesis of JIA by characterizing cytokine levels in the serum and SF of JIA patients. This is the first study ascertaining serum cytokine levels in JIA prospectively, at baseline and during a 6-month period, to explore possible changes in serum cytokine levels with disease activity and TNF-α inhibitor therapy. Proinflammatory cytokines were significantly higher at baseline in JIA subjects with increased disease activity, as expected. In our study, both etanercept and adalimumab were clinically effective in the treatment of JIA. TNF-α inhibition was noted to correspond with suppression of some proinflammatory cytokines (IL-6 and IL-8) in parallel with clinical improvement. Despite decreased disease activity, serum TNF-α levels increased in etanercept- but not adalimumab-treated subjects. Further studies of cytokine patterns in JIA patients are needed in larger paediatric cohorts to reveal critical differences in these biomarkers at baseline and after initiation of therapy for JIA. Delineation of cytokine patterns may help us to understand more clearly the pathogenesis of JIA, and may have clinical implications if cytokine levels are indeed correlated with disease activity and therapeutic response.
Acknowledgements
Study blood work was performed at the Clinical and Translational Science Center (CTSC) at Weill Cornell Medical College. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institute of Health under award number UL1-TR000457. Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Weill Cornell Medical College. REDCap is a secure, web-based application designed to support data capture for research studies. Grant support has been used to fund REDCap and laboratory assays at this institution.
Disclosure
T. L. is a consultant for Abbvie, Amgen, Genentech and Novartis, and is a member of their respective speakers’ bureaus. No other authors have any noted disclosures.




