Therapeutic implication of genetic variants of IL13 and STAT4 in airway remodelling with bronchial asthma
Summary
Background
Several gene variants identified in bronchial asthmatic patients are associated with a decrease in pulmonary function. The effects of this intervention on pulmonary function have not been fully researched.
Objective
We determined the effects of high-dose inhaled corticosteroids (ICSs) on decreased pulmonary function in asthmatic Japanese patients with variants of IL13 and STAT4 during long-term treatments with low to mild doses of ICS.
Methods
In this study, 411 patients with bronchial asthma who were receiving ICSs and living in Japan were recruited, were genotyped, and underwent pulmonary function tests and fibreoptic examinations. The effects of 2 years of high-dose ICSs administered to asthmatic patients who were homozygous for IL13 AA of rs20541 or STAT4 TT of rs925847 and who progressed to airway remodelling were investigated.
Results
High-dose ICS treatment increased the pulmonary function of patients homozygous for IL13 AA of rs20541 but not of patients homozygous for STAT4 TT of rs925847. The increased concentrations of the mediators IL23, IL11, GMCSF, hyaluronic acid, IL24, and CCL8 in bronchial lavage fluid (BLF) were diminished after high-dose ICS treatment in patients homozygous for IL13 AA of rs20541.
Conclusion and Clinical Relevance
IL13 AA of rs20541 and STAT4 TT of rs925847 are potential genomic biomarkers for predicting lower pulmonary function. The administration of high-dose ICSs to asthmatic patients with genetic variants of IL13 AA may inhibit the advancement of airway remodelling. The genetic variants of STAT4 TT did not respond to high-dose ICSs. Therefore, using medications other than ICSs must be considered even during the initial treatment of bronchial asthma. These genetic variants may aid in the realization of personalized and phenotype-specific therapies for bronchial asthma.
Introduction
Bronchial asthma is a disorder of the conducting airways that leads to variable airflow obstructions in association with airway hyperresponsiveness. Bronchial asthma exhibits a wide clinical spectrum that ranges from mild disease to a severe and intractable state. The severe form of bronchial asthma is characterized by a significant loss of lung function and by a frequent dependence on high-dose inhaled corticosteroids (ICSs). The observed airway obstruction in bronchial asthma is thought to be secondary to airway remodelling 1. The histopathological features of this process include epithelial changes, subepithelial basement membrane thickening, inflammatory cell infiltration, bronchial smooth muscle hypertrophy, and mucus hypersecretion 2, 3. Interleukin 13 (IL13) promotes IgE isotype switching by B cells and is involved in the recruitment of inflammatory cells, including T cells and eosinophils. IL13 is a major effector of Th2 inflammation and tissue remodelling 4. Many recent studies confirmed that single nucleotide polymorphisms (SNPs) in the IL13 promoter and coding regions influence baseline lung function and airway remodelling in asthmatics 5-9. SNPs in the IL13 and IL4Rα genes were associated with lung function in a study of 264 African Americans with asthma and healthy control subjects 5. We demonstrated an association between a variant of the IL13 gene (rs20541 G/A: major allele is G), in which an arginine residue at amino acid 110 is substituted with glutamine, and pulmonary function and hyperresponsiveness in Japanese people 7, 8. We identified increases in IL13, IL23, IL11, GMCSF, hyaluronic acid and CCL8 in bronchial lavage fluid (BLF) from IL13 homozygotes. Airway remodelling is also induced by asthma exacerbation, which may be initiated by a respiratory tract virus infection. We may assume that weaker Th1 responses to viral infections are associated with asthma exacerbation and suggest that viral infections exacerbate pre-existing Th2-dominated lung diseases 10, 11. We associated this possibility with data that showing that decreased IFN-γ production in response to viral infection of monocytes at birth predicts the susceptibility to respiratory tract illness during the first year of life. A study of acute asthma exacerbation in children demonstrated that the expression of genes involved in Th1 pathways decreases during exacerbation, which leads to deficits in baseline lung function 12. Genetic variants in Th1 genes (STAT4, IL12A, IL12RB1, and IRF2) affect lung function as shown by a meta-analysis of genome-wide association studies in 4 European American asthmatic populations 13. Among these genes, STAT4 is essential to IL12 transcription and function and thus plays an important role in IL12-mediated IFN-γ production and the differentiation of Th1 cells 14, 15. Associations have been found between reduced IFN-γ neonate production and increased frequency and severity of childhood wheezing and respiratory diseases 16, 17 and between reduced IFN-γ neonate production and increased cold symptoms after viral infection 18. Moreover, associations have been shown between STAT4 genotype and viral illness, asthma exacerbations, and childhood wheezing 19-22. These associations may be due to defects in IFN-γ response profiles or to a skewing of T-helper cell differentiation toward a Th2 phenotype, implicating STAT4 in Th2 inflammation and airway remodelling 23-25. However, even in airways where ICSs diminished Th2 cells, inflammation proceeded to induce airway remodelling in the patients who had no symptoms but had genetic variants. The most likely explanation is that sufficient doses of ICSs to control asthma symptoms were not adequate to improve airway remodelling in subjects with genetic variants in IL13 and STAT4 who used ICSs regularly. Some evidence for dose-related differences in the effects of corticosteroids on the bronchial mucosa basement membrane thickness also exists.
We hypothesized that (1) IL13 and STAT4 SNPs are associated with low lung function in patients and that (2) phenotyping patients based on IL13 and STAT4 SNPs would identify populations that are associated with a differential rate of FEV1 decrease despite the use of high doses of ICSs. To eliminate the possibility of patients with asthma-COPD overlap syndrome registering for the study, we used many screening questionnaires and a more in-depth clinical history for patient selection. This method enabled more strict determination of bronchial asthma than our previous studies 7 and excluded asthmatic patients with a history of smoking tobacco more than 10-pack-years and with exposure to environmental hazards. We selected post-bronchodilator percent predicted FEV1 and bronchial biopsy specimens to evaluate airway structural changes. No placebo group was included in this study because randomizing patients with moderate asthma to receive a placebo for several years was not considered ethical.
Methods
Study subjects
All study subjects were recruited from the Iwate Medical University Hospital. Patients aged ≥ 18 years were eligible if they had a diagnosis of asthma as defined by the American Thoracic Society criteria for ≥ 5 years and were using ICS at a stable low-mid dose for ≥ 1 year before screening. Well-controlled asthmatic patients who had no other medical disorders, who had smoked less than 10-pack-years and who had not been exposed to environmental hazards were considered for the study. This study was approved by the Iwate Medical University Hospital Ethics Committee (H20-119) and registered with Clinical Trials (JMA-IIA00045 IL13STAT4-ICS). Prospective patients were notified of our desire to include them in our study, and they were asked whether they would be willing to participate. Upon acceptance, the subjects provided written informed consent according to the ethical protocols of our institution. Subjects were assessed for age, gender, asthma duration, smoking history, spirometry, and eosinophil counts. DNA was isolated from lymphocytes using standard procedures. Subjects were genotyped for rs20541 and rs925847 using a 7500 Fast Real-Time PCR System (Life Technologies Japan, Tokyo, Japan) to evaluate the relationship between genotype and airway remodelling. Spirometry was performed, and airway methacholine responsiveness was measured using an Astograph (Jupiter 21, CHEST, Tokyo, Japan) according to the method described by Takishima et al. 26.
Fibreoptic bronchoscopy and specimen handling
BLF collection and bronchial biopsies were performed by inserting a flexible fibreoptic bronchoscope (BF-Q290; Olympus Optical Co Ltd, Tokyo, Japan) under local anaesthesia as described in detail previously 27. BLF collection was always performed before the biopsy to prevent blood contamination. BLF collection was performed in one of the subsegmental bronchi of the left lingular division via the injection of two 20-mL aliquots of sterile saline pre-warmed to 36.5 °C and gentle aspiration back into polypropylene tubes that were kept on ice. We obtained 20–25 mL of BLF from each subject. Mucus was removed from the fluid by filtration through gauze immediately after lavage, and the fluid was centrifuged at 200 × g for 10 min at 4 °C. The supernatant was decanted and stored at −80 °C. The cell pellet was washed in phosphate-buffered saline (PBS), and a total cell count was performed using a disposable cell counting chamber (Cellometer, Nexcelom Bioscience LLC, Lawrence, MA, USA). Then, the cells were diluted with PBS to a concentration of 1 × 106/mL. Cytocentrifuge slides were prepared and stained with May-Grünwald-Giemsa, and a differential cell count was performed on 300 cells. BLF cells were used for immunocytochemistry, and the 10 mL of BLF supernatant was concentrated to 1.0 mL and subsequently stored at −80 °C until use. Five biopsies were taken using forceps (Olympus) in the segmental bronchi of the right lobe and in the bifurcations of the right bronchi. Biopsied specimens were fixed in 4% paraformaldehyde, immersed in sucrose, and embedded in OCT compound. Five-micrometre tissue sections were affixed to microscope slides and prepared for histology. Then, haematoxylin and eosin (HE) staining was performed. The thickness of the subepithelial layer of the HE-stained biopsies was measured. The thickening of the subepithelial layer in individual biopsy specimens was analysed using an Olympus DP70 system (Olympus) and computer image analysis (Image-Pro Plus 4.1; Media Cybernetics, Rockville, MD, USA), and the results were compared among the three genotypes and before and after treatment in patients homozygous for IL13 and STAT4 SNPs. A second round of endobronchial procedures was performed after 1 year of treatment on the opposite side of the lung, and a third round of procedures was performed after 2 years of treatment on the left lower lobe of the lung.
Cytokine concentrations of BLF supernatants
We demonstrated that various cytokine concentrations in BLF supernatants were increased in patients homozygous for IL13 AA (rs20541) compared to wild-type and heterozygotes from a previous study using microarray analysis 7. We also analysed and compared IL23 (LifeSpan BioSciences, Inc., Seattle, WA, USA), IL11 (R&D Systems, Inc., Minneapolis, MN, USA), GMCSF (R&D Systems, Inc.), hyaluronic acid (Echelon Biosciences Inc., Salt Lake City, UT, USA), IL24 (R&D Systems, Inc.), and CCL8 (LifeSpan BioSciences, Inc.) expression. The concentrations of the Th1-type cytokine IFNγ (R&D Systems, Inc.) and Th2-type cytokines IL4 (R&D Systems, Inc.) and IL5 (R&D Systems, Inc.) in BLF were also analysed. SRL Laboratories (Tokyo, Japan) assessed IL13 levels in BLF. The values of IL13 AA and IL13 GG obtained by the kit were adjusted based on known proteins as a standard because of the different sensitivities of AA and GG to this assay.
Treatment comparison study design
Eligible subjects were monitored over a 1-month run-in period. Symptoms and lung function stability were examined. Subjects were eligible for the study if they complied with recording information in a home diary and if their asthma was under control. After the clinical and pulmonary function test data of patients homozygous for the SNP rs20541 or rs925847 were assessed, patients homozygous AA for the SNP rs20541 or homozygous TT for the SNP rs925847 were randomly assigned to either 2 years of add-on high-dose ICS treatment or to the continuance of previous treatment using a 1 : 1 ratio to ensure that the groups contained equal numbers. The subjects were interviewed once every 2 months for 2 years. The subjects recorded the following data twice daily in a home diary: PEFR and symptoms scores, as well as the number of puffs of rescue medication needed. No changes were made to treatments or follow-up procedures during the study, and the prescribed doses of ICSs remained constant until mild exacerbation occurred. In the event of exacerbation, subjects were instructed to contact the investigators immediately and to come to the hospital within 24 h. The investigators assessed each event using pre-established exacerbation criteria, and the dose of ICS was increased threefold for a minimum of 4 days in cases of mild exacerbation or until the PEFR returned to normal values.
A pulmonary function test and endobronchial assessment were performed once annually for 2 years. Subjects had to fulfil the following criteria: no evidence of respiratory tract infection and no asthma exacerbation for ≥ 14 days.
Statistical analysis
Statistical analyses were performed using JMP-program version 11 (SAS Institute Inc., Tokyo, Japan). All data are expressed as the mean ± standard error of the mean. Comparisons of the patients' characteristics, concentrations of cytokines of bronchial lavage fluid, and the thickening of the subepithelial layer between the three genotypes were performed using one-way anova. Comparisons of the pulmonary function, mediator concentrations in BLF, and subepithelial layer thickness between two different treatment groups, including the baseline as well as the first- and second-year measurements, were performed using t-tests. Post hoc multiple comparisons of the differences among all of the groups were performed using the Tukey–Kramer test. P values < 0.05 were considered significant. Methods using the Bonferroni correction were performed to avoid multiplicity of primary end-point analyses.
Results
Subject demographics and enrolment
Of 411 asthmatic patients screened, 387 rs20541 patients and 377 rs925847 patients were successfully genotyped (≥ 92%). We identified 154, 172, and 61 subjects that were GG wild-type, GA heterozygotes, and AA homozygotes, respectively, for IL13 (rs20541) during the run-in period. No significant differences in age at study enrolment, gender, smoking history, eosinophil count status, or serum IgE concentration among the three groups (Table 1). However, the %FEV1 exhibited significant differences among the three groups. AA homozygotes of rs20541 had significantly lower %FEV1 compared to other genotype groups (P < 0.05) (Table 1). In total, 104 subjects were TT homozygotes of the STAT4 SNP (rs925847). Similarly, the %FEV1 was significantly lower in TT homozygotes of the STAT4 SNP between the three genotypes (P < 0.05) (Table 1).
| rs20541 | rs925847 | |||||
|---|---|---|---|---|---|---|
| GG (n = 154) | GA (n = 172) | AA (n = 61) | CC (n = 92) | CT (n = 181) | TT (n = 104) | |
| Age at enrollment (year) | 56.5 ± 1.3 | 58.2 ± 1.2 | 60.2 ± 2.2 | 57.6 ± 1.7 | 57.9 ± 1.2 | 57.4 ± 1.5 |
| Gender, M/F | 70/84 | 64/108 | 30/31 | 45/47 | 67/114 | 50/54 |
| Smoking history (never smoker)% | 57.6 | 57.7 | 55.9 | 54.4 | 60.7 | 51.0 |
| Blood eosinophils/μL | 315 ± 29.3 | 298 ± 16.9 | 347 ± 38.1 | 303 ± 26.2 | 337 ± 26.8 | 289 ± 20.9 |
| Serum total IgE IU/μL | 474 ± 89.8 | 435 ± 120.7 | 514 ± 121.7 | 452 ± 68.4 | 416 ± 79.1 | 607 ± 204 |
| FEV1, %predicted (%) | 89.5 ± 1.8 | 87.1 ± 1.6 | 80.5 ± 2.8‡ | 90.8 ± 2.3 | 86.7 ± 1.5 | 82.8 ± 2.2* |
- Data are expressed as the mean ± standard error of the mean. FEV1, forced expiratory flow volume in on second.
- Within-genotype comparison; ‡P < 0.05 compared with GG, *P < 0.05 compared with CC.
Detection of clinically relevant IL23, IL11, GMCSF, hyaluronic acid, IL24, and CCL8 levels and subepithelial layer basement thickness
We performed a bronchoscopy and collected BLF from three groups to confirm whether AA and TT homozygotes of rs20541 and rs925847, respectively, expressed any increased protein levels that were involved with Th1- and Th2-type cytokines and airway remodelling. Seventeen patients who were genotyped as GG, 16 patients as GA, and 25 patients as AA were accepted for the BLF and bronchial biopsy studies. Twenty of the 104 patients who were genotyped as TT homozygotes of rs925847 agreed to undergo fibreoptic bronchoscopy. Twenty-three patients who were genotyped as CC and 18 patients as CT were accepted for the bronchoscopic examination, which was performed following recommended safety procedures and which was well-tolerated in all subjects. Patients who were genotyped as double homozygotes of AA and TT were not enrolled. IL13, IL23, IL11, GMCSF, hyaluronic acid, IL24, and CCL8 levels were all significantly increased in patients who were homozygous for AA compared to wild-type (Table 2), but no significant difference was found for IL4, IL5, and IFNγ among the three groups. None of these changes differed significantly among the CC, CT, and TT groups in rs925847. The increased concentrations of IL13, IL23, IL11, GMCSF, hyaluronic acid, and CCL8 in AA of IL13 were also examined in our previous data. Although subjects of asthma without COPD were used in this study, similar tendencies have been shown.
| rs20541 | rs925847 | |||||
|---|---|---|---|---|---|---|
| GG (n = 17) | GA (n = 16) | AA (n = 25) | CC (n = 23) | CT (n = 18) | TT (n = 20) | |
| IL4 (pg/mL) | 7.9 ± 1.5 | 9.6 ± 1.7 | 6.1 ± 1.1 | 7.6 ± 1.2 | 8.9 ± 2.1 | 6.6 ± 1.0 |
| IL5 (pg/mL) | 1.5 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.1 | 1.5 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.2 |
| IL13 (pg/mL) | 9.2 ± 1.1 | 11.3 ± 2.2 | 18.7 ± 2.4† | 15.5 ± 2.7 | 16.1 ± 3.6 | 10.3 ± 1.4 |
| IFNγ (pg/mL) | 10.1 ± 1.4 | 10.9 ± 1.4 | 11.4 ± 2.1 | 10.9 ± 1.9 | 11.0 ± 2.5 | 10.8 ± 1.3 |
| IL23 (pg/mL) | 11.5 ± 1.7 | 12.3 ± 2.4 | 22.3 ± 3.0† | 15.6 ± 2.7 | 20.3 ± 3.7 | 14.4 ± 2.5 |
| IL11 (pg/mL) | 8.3 ± 1.1 | 12.7 ± 2.4 | 20.7 ± 2.6† | 17.0 ± 2.8 | 16.6 ± 3.0 | 11.0 ± 1.7 |
| GMCSF (pg/mL) | 23.9 ± 2.0 | 19.7 ± 2.5 | 36.1 ± 4.1† | 33.9 ± 4.0 | 24.1 ± 3.9 | 24.1 ± 2.9 |
| Hyaluronic acid (ng/mL) | 74.6 ± 16.1 | 81.3 ± 11.0 | 175.6 ± 24.1† | 137.8 ± 25.5 | 109.9 ± 28.3 | 107.0 ± 14.0 |
| IL24 (pg/mL) | 9.2 ± 1.5 | 11.0 ± 1.5 | 15.1 ± 1.5† | 13.3 ± 1.6 | 11.4 ± 1.7 | 11.7 ± 1.6 |
| CCL8 (pg/mL) | 12.4 ± 2.6 | 20.2 ± 3.8 | 24.6 ± 3.0† | 20.3 ± 2.7 | 19.3 ± 4.5 | 19.6 ± 3.5 |
| Subepithelial layer thickness (μm) | 8.2 ± 0.7 | 7.7 ± 0.7 | 10.0 ± 0.5† | 8.4 ± 0.6 | 7.7 ± 0.7 | 10.4 ± 0.6* |
- Data are expressed as the mean ± standard error of the mean. BLF, bronchial lavage fluid.
- Within-genotype comparison; †P < 0.05 compared with GG, *P < 0.05 compared with CC.
Thickness of the subepithelial layer basement
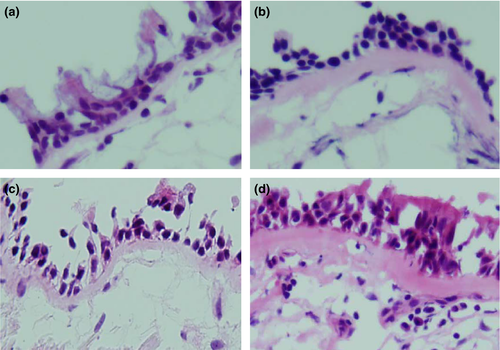
Subsequently, we investigated the differences in the thickness of the subepithelial layer of the bronchial mucosa. The subepithelial layer was significantly thicker in the biopsies taken from the asthmatic subjects who were homozygous compared to wild-type (Fig. 1 and Table 2). Therefore, airway remodelling in homozygotes for AA of rs20541 and TT of rs925847 exhibited increased progression compared to wild-type subjects.
Completed patients
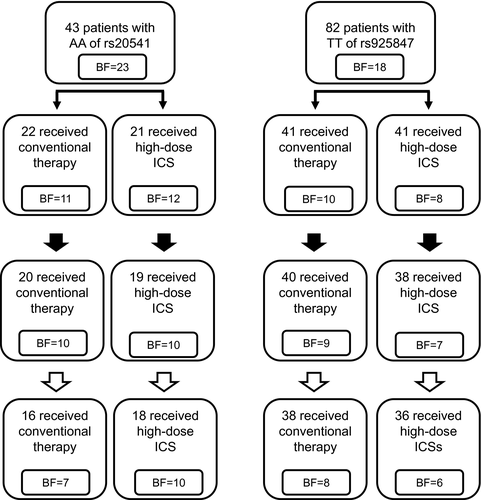
Patients who were genotyped as double homozygotes of AA/TT and were not willing to participate were not enrolled in the 2-year treatment protocol. The enrolled subjects of IL13 AA were divided randomly into two groups; the conventional treatment group (n = 22) and the high-dose ICS treatment group (n = 21). No significant differences were observed between the two groups for the baseline clinical data (data not shown), and the first-round bronchoscopic examination of 23 patients was divided into two groups: 11 patients in the conventional treatment group and 12 patients in the high-dose ICS group (Fig. 2). Paired clinical data were available for 17 homozygotes for AA of rs20541 who completed for 2 years of treatment, of which seven patients were randomized to conventional therapy, and 10 patients were randomized to high-dose ICS treatment. In total, 82 patients who were homozygous for TT of rs925847 were randomized to conventional therapy (n = 41) and to high-dose ICS treatment (n = 41). Seventy-four subjects completed the 2-year protocol in full. Fourteen subjects underwent a third bronchoscopic examination.
Effects of high-dose ICS treatment on %FEVl and airway remodelling
Pulmonary function
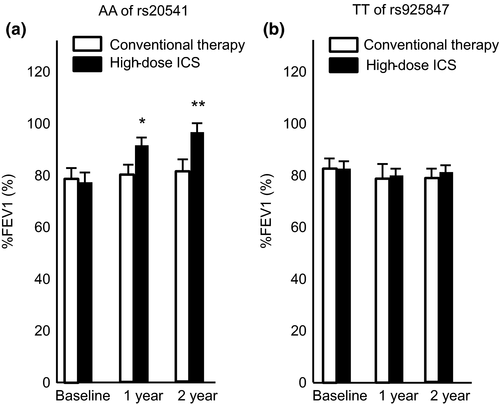
The group analysis of homozygotes for AA of rs20541 revealed a numerically greater increase from the baseline %FEV1 with high-dose ICS treatment in these patients, whereas no increase in %FEV1 was demonstrated when homozygotes for TT of rs925847 were treated with high-dose ICS or conventional treatment (Fig. 3).
Concentrations of mediators in BLF
We performed bronchoscopies and collected BLF and biopsy sample to determine whether individuals homozygous for AA of rs20541 or for TT of rs925847 expressed decreased protein levels in the airways after treatment. One patient in the conventional treatment group and two patients in the high-dose ICS treatment group, all of whom were homozygous for AA of rs20541, had unscheduled hospital visits due to upper respiratory tract infections. Similarly, one patient in the conventional treatment and one patient in the high-dose ICS treatment group, both of whom were homozygous for TT of rs925847, experienced upper respiratory tract infections and could not undergo endobronchial examination after 1 year of treatment. Seven asthmatic subjects using conventional therapy and ten subjects using high-dose ICS treatment, all of whom were homozygous for AA of rs20541, completed a third bronchial examination. Eight asthmatic subjects using conventional therapy and six subjects using high-dose ICSs, all of whom were homozygous for TT of rs925847, were accepted and completed a third bronchial examination (Fig. 2). Bronchoscopic examination with BLF collection was performed with care and was well-tolerated in all subjects after treatment. IL13, IL23, IL11, GMCSF, hyaluronic acid, IL24, and CCL8 levels decreased significantly after treatment with high-dose ICSs compared with conventional therapy in subjects homozygous for AA of rs20541 (Table 3), whereas no significant difference was found in subjects homozygous for TT of rs925847 (Table 4).
| AA of rs20541 of IL13 | |||
|---|---|---|---|
| Baseline (n = 23) | 1 year (n = 20) | 2 year (n = 17) | |
| IL4 (pg/mL) | |||
| Conventional | 5.1 ± 0.9 | 5.6 ± 0.9 | 5.7 ± 0.5 |
| High-dose ICS | 7.5 ± 2.1 | 6.8 ± 1.2 | 6.2 ± 1.1 |
| IL5 (pg/mL)\ | |||
| Conventional | 1.3 ± 0.2 | 1.2 ± 0.1 | 1.4 ± 0.2 |
| High-dose ICS | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.3 ± 0.2 |
| IL13 (pg/mL) | |||
| Conventional | 19.9 ± 2.9 | 23.3 ± 3.1 | 25.2 ± 4.3 |
| High-dose ICS | 19.6 ± 4.0 | 13.6 ± 2.7 | 11.2 ± 0.7‡ |
| IFNγ (pg/mL) | |||
| Conventional | 12.5 ± 3.2 | 9.6 ± 2.0 | 11.7 ± 2.8 |
| High-dose ICS | 11.5 ± 3.4 | 10.0 ± 2.4 | 9.8 ± 2.1 |
| IL23 (pg/mL) | |||
| Conventional | 17.1 ± 4.1 | 21.9 ± 5.2 | 22.9 ± 5.0 |
| High-dose ICS | 25.4 ± 4.5 | 15.4 ± 3.3 | 12.2 ± 1.4‡ |
| IL11 (pg/mL) | |||
| Conventional | 20.7 ± 3.8 | 19.8 ± 3.7 | 25.4 ± 5.1 |
| High-dose ICS | 24.1 ± 4.6 | 16.2 ± 3.6 | 14.0 ± 2.7‡ |
| GMCSF (pg/mL) | |||
| Conventional | 32.9 ± 7.1 | 28.3 ± 6.1 | 27.8 ± 5.7 |
| High-dose ICS | 37.6 ± 5.7 | 18.9 ± 1.9 | 14.0 ± 2.0‡ |
| Hyaluronic acid (ng/mL) | |||
| Conventional | 137.0 ± 24.8 | 167.4 ± 31.9 | 162.9 ± 43.7 |
| High-dose ICS | 210.9 ± 43.6 | 102.7 ± 13.3 | 84.5 ± 14.0‡ |
| IL24 (pg/mL) | |||
| Conventional | 15.8 ± 2.6 | 18.6 ± 3.8 | 20.3 ± 5.7 |
| High-dose ICS | 14.4 ± 2.0 | 10.1 ± 1.1† | 9.2 ± 1.1‡ |
| CCL8 (pg/mL) | |||
| Conventional | 25.8 ± 5.1 | 28.9 ± 4.9 | 28.4 ± 4.3 |
| High-dose ICS | 24.6 ± 3.8 | 17.7 ± 2.6† | 18.9 ± 3.0‡ |
| Subepithelial layer thickness (μm) | |||
| Conventional | 9.3 ± 0.8 | 10.3 ± 0.8 | 10.5 ± 0.8 |
| high-dose ICS | 9.8 ± 0.7 | 8.7 ± 0.7 | 7.9 ± 0.5‡ |
- Data are expressed as the mean ± standard error of the mean. BLF, bronchial lavage fluid. ICS, inhaled corticosteroid. Between the treatment comparison; †P < 0.05 compared with conventional treatment for 1 year; ‡P < 0.05 compared with conventional treatment for 2 year.
| TT of rs925847 of STAT4 | |||
|---|---|---|---|
| Baseline (n = 18) | 1 year (n = 16) | 2 year (n = 14) | |
| IL4 (pg/mL) | |||
| Conventional | 6.4 ± 1.2 | 4.3 ± 0.7 | 6.5 ± 0.9 |
| High-dose ICS | 7.7 ± 2.1 | 7.2 ± 2.5 | 8.4 ± 1.9 |
| IL5 (pg/mL) | |||
| Conventional | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.6 ± 0.1 |
| High-dose ICS | 1.8 ± 0.4 | 1.5 ± 0.2 | 1.3 ± 0.3 |
| IL13 (pg/mL) | |||
| Conventional | 11.2 ± 2.2 | 11.8 ± 3.2 | 12.4 ± 2.5 |
| High-dose ICS | 10.2 ± 2.1 | 10.1 ± 2.2 | 11.4 ± 2.7 |
| IFNγ (pg/mL) | |||
| Conventional | 9.7 ± 1.5 | 14.6 ± 2.1 | 12.7 ± 3.3 |
| High-dose ICS | 7.2 ± 1.7 | 10.1 ± 3.8 | 12.5 ± 2.7 |
| IL23 (pg/mL) | |||
| Conventional | 13.1 ± 3.2 | 14.0 ± 4.5 | 10.7 ± 3.9 |
| High-dose ICS | 11.3 ± 2.2 | 12.5 ± 2.1 | 13.0 ± 3.9 |
| IL11 (pg/mL) | |||
| Conventional | 7.7 ± 1.3 | 8.9 ± 1.1 | 9.3 ± 1.9 |
| High-dose ICS | 9.9 ± 2.1 | 11.2 ± 2.8 | 9.9 ± 2.7 |
| GMCSF (pg/mL) | |||
| Conventional | 21.4 ± 3.8 | 19.0 ± 3.1 | 19.0 ± 1.9 |
| High-dose ICS | 22.2 ± 3.6 | 17.0 ± 3.6 | 16.3 ± 3.7 |
| Hyaluronic acid (ng/mL) | |||
| Conventional | 68.2 ± 11.7 | 75.8 ± 18.3 | 67.4 ± 17.5 |
| High-dose ICS | 50.0 ± 13.2 | 58.7 ± 13.5 | 57.9 ± 13.5 |
| IL24 (pg/mL) | |||
| Conventional | 13.1 ± 2.1 | 13.8 ± 2.9 | 15.1 ± 2.6 |
| High-dose ICS | 8.8 ± 2.5 | 12.5 ± 3.2 | 10.3 ± 4.5 |
| CCL8 (pg/mL) | |||
| Conventional | 15.5 ± 3.8 | 15.9 ± 2.9 | 13.7 ± 2.7 |
| High-dose ICS | 14.3 ± 4.1 | 14.9 ± 3.7 | 13.5 ± 3.8 |
| Subepithelial layer thickness (μm) | |||
| Conventional | 10.4 ± 0.6 | 9.8 ± 1.0 | 9.4 ± 1.1 |
| High-dose ICS | 9.3 ± 0.8 | 8.8 ± 0.8 | 9.5 ± 1.2 |
- Data are expressed as the mean ± standard error of the mean. BLF, bronchial lavage fluid; ICS, inhaled corticosteroid.
Thickness of the subepithelial layer
Significant differences between the subepithelial layer thickness of biopsy specimens taken from asthmatic patients homozygous for AA of rs20541 receiving continuous conventional therapy or high-dose ICS treatment were observed (Table 3), whereas no significant differences in the thickness of the subepithelial layer were observed between treatments in patients homozygous for TT of rs925847 (Table 4).
Discussion
In this study, we investigated genetic variants related to lung function in Japanese asthmatic patients. Several new findings emanated from this study. First, we demonstrated that asthmatic patients with genetic variants of STAT4 (rs925847) exhibited significantly reduced %FEV1 and increased bronchial basement membrane thickness. Second, high-dose ICSs did not result in improvements from baseline in lung function and basement membrane thickness in patients homozygous for TT of rs925847, although patients homozygous for AA of rs20541, which conferred asthmatic airway remodelling, displayed improvements in lung function after high-dose ICS treatment. Third, IL13, IL23, IL11, GMCSF, hyaluronic acid, IL24, and CCL8 levels in BLF from IL13 homozygotes of AA of rs20541 decreased after high-dose ICS treatment, but the levels in BLF from STAT4 homozygotes of TT of rs925847 did not decrease.
Bronchial asthma is one of the most common chronic diseases that affect 300 million individuals of any age worldwide. The response to treatment is also genetically complex and is characterized by high intraindividual repeatability 28 and high interindividual variability 29, with up to 40% of asthmatic patients exhibiting no response to therapy. Studies in families with conditions other than bronchial asthma demonstrated familial segregation and heritability of responses to glucocorticoid medications 30, 31. Previously, we demonstrated an important role for IL13 (rs20541) in airway remodelling using pulmonary function tests and bronchial specimens obtained from asthmatic patients 7. However, because a positive association between IL13 (rs20541) and COPD and impaired lung function was also found 9, we did not use patients with concurrent asthma and COPD in this study, resulting in differences in the background and characteristics of the patients in the present study compared with those used in previous studies. Therapeutic utility of high-dose ICSs aimed at regulating IL13 and Th2-mediated milieu was examined only in asthmatic patients in this study. Corticosteroid treatment generally reduced basement membrane thickness in asthmatic patients in both our study and other studies 32-34. Dose-related differences in the effects of corticosteroids on bronchial mucosal basement membrane thickness are also apparent in some studies 35, 36. Meanwhile, Boulet and Baraket groups did not demonstrate a significant reduction in basement membrane thickness after fluticasone propionate treatment 37, 38. These findings indicate that the diversity of inflammation may reflect a wide repertoire of inflammatory mediators. Whether genetic variants of STAT4 (rs925847) weaken Th1 responses is not known. Kim et al. demonstrated that STAT4-mediated signalling plays a key role in the development of airway hyperresponsiveness and non-eosinophilic inflammation via the actions of Th1 chemokines and macrophages in a Th2 asthma mouse model 39. One explanation for the lack of a steroid effect on lung function in genetic variants of STAT4 (rs925847) subjects may be that this SNP has augmented non-eosinophilic inflammation and macrophage activities. In the present study, our examinations demonstrated no significant differences between the rs20541 (IL13) and rs925847 (STAT4) groups in the levels of PEFR and FeNO, in the levels of the Th1 and Th2 cytokines and in the infiltration of inflammatory cells, including eosinophils, neutrophils, and macrophages in the bronchial wall and lavage fluid (data not shown). Further examinations should be conducted to determine whether TT of rs925847 skewed the balance between M1 and M2 macrophage phenotypes and the ability to produce Th1- and Th2-type cytokines from stimulated BAL cells ex vivo. The AA of rs20541-induced mediators, such as IL13, IL23, IL11, GMCSF, hyaluronic acid, and CCL8, are involved in the process of airway remodelling 7. IL24 was originally termed melanoma differentiation-associated gene 7 and was identified by subtraction hybridization of cDNA libraries from human melanoma cells 40. The tumour suppressor role of IL24 is well-characterized, but its immunomodulatory function and its role in allergic inflammatory diseases remain under scrutiny. Bochkov et al. demonstrated that GMCSF and IL24 gene expression was induced in rhinovirus-infected primary bronchial epithelial cells 41. The exact roles of IL24 in epithelial damage and sustainable airway hyperresponsiveness of asthmatic subjects await further experiments. We demonstrated that corticosteroids are less effective in airway remodelling for the endotype STAT4 TT of rs925847, may be because of the absence of a Th2 process. Non-Th2 asthma likely represents a large proportion of all asthma. However, little is understood regarding this asthma subgroup, the phenotypes underlying it, and the molecular elements that control it compared with Th2 asthma. Non-Th2 asthma may affect more than 50% of corticosteroid-naïve individuals, who exhibit less airway obstruction and hyperreactivity than people with Th2-high asthma do, even though they meet the criteria for asthma.
In conclusion, we demonstrated an important role for IL13 AA of rs20541 and STAT4 TT of rs925847 in airway remodelling with bronchial asthma. Further investigations of high- dose ICS utility aimed at regulating these molecules may provide better control of airway remodelling in asthmatic patients homozygous for IL13 AA of rs20541 but not with patients homozygous for STAT4 TT of rs925847. The mechanism of this different effect may be mediated via the Th1 or Th2 pathways. Thus, IL13 AA of rs20541 and STAT4 TT of rs925847 are potential genomic biomarkers that predict lower pulmonary function. Administration of high-dose ICSs to asthmatic patients with genetic variants of IL13 AA may avoid the advancing of airway remodelling, whereas the genetic variants of STAT4 TT do not respond to high-dose ICSs. Therefore, the use of medications other than ICSs must be considered even in the initial treatment steps of bronchial asthma. These genetic variants could aid in the realization of personalized and phenotype-specific therapies for bronchial asthma.
Acknowledgements
The authors would like to acknowledge Y. Shibata, Y. Tamayama, M. Shibanai, M. Niisato, Prof. Emeritus T. Fujioka (Iwate Medical University School of Medicine), and T. Nishizawa (Shinshu University) for their help in performing the study.
Conflict of interests
The authors declare no conflicts of interest.




