Opportunities to Modulate Tumor Ecosystem Toward Successful Glioblastoma Immunotherapy
Funding: This work was supported by Seth M Boyer Foundation. Frankel Innovation Initiative at the University of Michigan. Rogel Cancer Center, University of Michigan. American Brain Tumor Association. Brain Research Foundation. All of Us Research Program.
ABSTRACT
Over the past decade, the failure of multiple clinical trials has confirmed the need for a systematic and comprehensive understanding of glioblastoma (GBM). Current immunotherapies aiming to harness the immune system to achieve anti-tumor effects remain largely ineffective, highlighting the complexities of the GBM microenvironment. However, our recent understanding of immune niches within the central nervous system provides both opportunities and challenges in translating these insights into successful immunotherapy implementation. We discuss these strategies, including targeting multiple antigens within the heterogeneous GBM microenvironment, identifying new druggable targets to abrogate immunosuppression, and understanding niche-specific immune cell functionality to modulate tumor-immune-stroma interactions.
1 Introduction
Tumors of the central nervous system (CNS) represent a diverse entity of malignancies that vary in clinical outcome, therapeutic response, and underlying biology. The World Health Organization (WHO) classification combines phenotypes and genotypes for diagnosis to develop a classification of the tumors with a degree of homogeneity in the survival of each patient pool [1]. Glioblastoma (hereafter GBM), now defined as isocitrate dehydrogenase (IDH)-wild type tumors, is the most aggressive form of primary brain tumor with a disproportionately high mortality [2]. Transcriptome profiling studies have identified transcriptional subtypes in GBM, such as proneural (PN), classical (CL), and mesenchymal (MES), reflecting the developmental constraints and the distinct tumor microenvironment [3]. Subtype transitions in patient tumors suggest that tumor phenotype and therapeutic efficacy may be determined by dynamic tumor-niche interactions [4]. A detailed personalized map of the cellular components and their interactions is required to understand common therapeutic failures and improve GBM treatment outcomes.
Cancer immunotherapy has emerged as a transformative treatment modality, based on the concept that the immune system targets tumor cells by recognizing tumor-specific characteristics. The remarkable success of immune checkpoint blockade therapy (ICBT), which entails the use of monoclonal antibodies to block intercellular signaling (e.g., anti-programmed cell death-1 (PD-1), anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)), has repelled skepticism toward cancer immunotherapy among clinical oncologists. Another breakthrough, chimeric antigen receptor (CAR)-T cell therapy, encourages the development of advanced cell engineering strategies. These advancements have been actively integrated into clinical trials across the nations, as of December 26, 2024 (Tables 1 and 2). However, initial immunotherapy trials for GBM seem abortive in producing consistent signs of clinical benefits observed in other cancers [5]. This contrast prompts us to reconsider the mechanism and the impact of immune-tumor and -niche interactions in a spatiotemporal manner.
| Modality | Immunological target | Trial ID | Trial name | Phase | No. of Patients | Tumor description | Registration Date | Status |
|---|---|---|---|---|---|---|---|---|
| Combination therapy | ||||||||
| Antibody (Pembrolizumab) & tumor treating fields | PD-1 | NCT06556563 | EF-41/KEYNOTE D58: Phase 3 Study of Optune Concomitant With Temozolomide Plus Pembrolizumab in Newly Diagnosed Glioblastoma | Phase III | 741 | Newly Diagnosed Glioblastoma | 2024–12 | Recruiting |
| Immunotoxin (D2C7-IT) & antibody (2141-V11) | CD40, Immunotoxin | NCT06455605 | D2C7-IT +2141-V11 Combination Post-resection in rGBM | Phase I | 46 | Recurrent Glioblastoma | 2024–11 | Not yet recruiting |
| Autologous tumor-infiltrating lymphocytes & antibody (Pembrolizumab) | TILs, PD1 | NCT06640582 | TIL Therapy Combined With Pembrolizumab for Advanced Brain Cancer Including Gliomas and Meningiomas | Phase I/II | 85 | Brain Tumors (Brain Metastases, Gliomas, Glioblastoma, Meningioma) | 2024–10 | Recruiting |
| CMV-specific T cells & pembrolizumab | PD-1, Tumor Virus Antigen | NCT06157541 | T Cells and Pembrolizumab for Recurrent and Newly Diagnosed Glioblastoma | Phase I/II | 58 | Glioblastoma | 2024–02 | Recruiting |
| Ultrasound (Sonocloud-9) & antibody (Balstilimab) | BBB, CTLA-4, PD-1 | NCT05864534 | Phase 2a Immune Modulation With Ultrasound for Newly Diagnosed Glioblastoma | Phase III | 25 | Newly Diagnosed Glioblastoma | 2024–01 | Recruiting |
| Immunotoxin (D2C7-IT) & antibody (2141-V11) | CD40, Immunotoxin | NCT05734560 | D2C7-IT and 2141-V11 in Newly Diagnosed GBM Patients | Phase I/II | 50 | Newly Diagnosed MGMT Unmethylated Glioblastoma | 2023–09 | Recruiting |
| Antibody (Crizanlizumab, Nivolumab) | P-selectin, PD1 | NCT05909618 | Crizanlizumab Alone or in Combination With Nivolumab for Glioblastoma and Melanoma With Brain Metastases | Phase II | 33 | Glioblastoma, Metastatic Melanoma in the Central Nervous System | 2023–07 | Recruiting |
| Immune checkpoint blockade | ||||||||
| Antibody (Ipilimumab, Nivolumab) | CTLA-4, PD1 | NCT06097975 | A Clinical Trial on Combined (Neo-)Adjuvant Intravenous Plus Intracranial Administration of Ipilimumab and Nivolumab in Recurrent Glioblastoma | Phase I | 18 | Recurrent Glioblastoma | 2024–01 | Not yet recruiting |
| Antibody (Ipilimumab, Pembrolizumab) | CTLA-4, PD1 | NCT06047379 | Safety and Efficacy of NEO212 in Patients with Astrocytoma IDH-mutant, Glioblastoma IDH-wildtype or Brain Metastasis | Phase I/II | 134 | Diffuse Astrocytoma, IDH-mutant Gliomas, IDH-wt glioblastoma, Brain Metastases, other solid cancers | 2023–11 | Recruiting |
| Antibody (Pembrolizumab) | PD-1 | NCT05235737 | The Assessment of Immune Response in Newly Diagnosed Glioblastoma Patients Treated With Pembrolizumab | Phase IV | 36 | Newly Diagnosed Glioblastoma | 2022–03 | Recruiting |
| Antibody (Pembrolizumab) | PD-1 | NCT04977375 | Trial of Anti-PD-1 Immunotherapy and Stereotactic Radiation in Patients With Recurrent Glioblastoma | Phase I/II | 10 | Glioblastoma | 2021–12 | Recruiting |
| Antibody (AB154, AB122) | TIGIT, PD-1 | NCT04656535 | AB154 Combined with AB122 for Recurrent Glioblastoma | Early Phase I | 46 | Glioblastoma | 2021–04 | Recruiting |
| Cell therapy | ||||||||
| CAR T cell | EGFRvIII, EphA2/IL-13Ralpha2, synNotch system | NCT06186401 | Anti-EGFRvIII synNotch Receptor Induced Anti-EphA2/IL-13Ralpha2 CAR (E-SYNC) T Cells | Phase I | 20 | EGFR mutated Glioblastoma | 2024–09 | Recruiting |
| Engineered NK cell | PD-L1, IL15 superagonist | NCT06061809 | N-803 and PD-L1 t-haNK Combined With Bevacizumab for Recurrent or Progressive Glioblastoma | Phase II | 20 | Glioblastoma | 2024–08 | Recruiting |
| Neoantigen-specific T cell | Tumor Antigens | NCT05685004 | Study of Neoantigen-specific Adoptive T Cell Therapy for Newly Diagnosed MGMT Negative Glioblastoma Multiforme (GBM) | Phase II/III | 120 | Glioblastoma | 2023–09 | Recruiting |
| Tris-CAR-T cell | CD44, CD133, truncated IL7Ra system | NCT05577091 | Tris-CAR-T Cell Therapy for Recurrent Glioblastoma | Phase I | 10 | Recurrent Glioblastoma | 2023–09 | Recruiting |
| CAR T cell | CD70, IL-8 Receptor | NCT05353530 | Phase I Study of IL-8 Receptor-modified CD70 CAR T Cell Therapy in CD70+ Adult Glioblastoma (IMPACT) | Phase I | 18 | Glioblastoma | 2023–07 | Recruiting |
| CAR T cell | EGFRvIII, EGFR, T-cell–engaging antibody molecules | NCT05660369 | CARv3-TEAM-E T Cells in Glioblastoma | Phase I | 21 | Glioblastoma | 2023–03 | Recruiting |
| Dendritic cell | Tumor Stem Cell Antigens | NCT03548571 | Dendritic Cell Immunotherapy Against Cancer Stem Cells in Glioblastoma Patients Receiving Standard Therapy | Phase II/III | 60 | Glioblastoma | 2018–04 | Recruiting |
| Dendritic cell | Tumor Antigens | NCT04801147 | Immunotherapy With Autologous Tumor Lysate-Loaded Dendritic Cells In Patients With Newly Diagnosed Glioblastoma Multiforme | Phase I/II | 76 | Newly Diagnosed Glioblastoma | 2010–06 | Recruiting |
| Virus therapy | ||||||||
| Oncolytic virus (G207) | Engineered herpes simplex virus (HSV) | NCT04482933 | HSV G207 with a Single Radiation Dose in Children with Recurrent High-Grade Glioma | Phase II | 35 | Malignant Gliomas (including Glioblastoma) | 2024–12 | Recruiting |
| Oncolytic virus (rQNestin34.5v.2) | Engineered herpes simplex virus (HSV) | NCT03152318 | A Study of the Treatment of Recurrent Malignant Glioma With rQNestin34.5v.2 | Phase I | 62 | Malignant Gliomas (including Glioblastoma) | 2017–07 | Recruiting |
| Vaccines | ||||||||
| RNA-Lipid Particle (RNA-LP) Vaccines | pp65 mRNA, tumor RNA | NCT06389591 | RNA-Lipid Particle (RNA-LP) Vaccines for Recurrent Adult Glioblastoma (GBM) | Phase I | 24 | Recurrent Glioblastoma | 2024–10 | Recruiting |
| PEP-CMV vaccine | CMV antigens | NCT06132438 | Immunotherapy Targeting of Cytomegalovirus Antigens in Glioblastoma | Phase I | 26 | Glioblastoma | 2023–11 | Not yet recruiting |
| RNA-lipid Particle (RNA-LP) Vaccines | pp65 mRNA, tumor RNA | NCT04573140 | A Study of RNA-lipid Particle (RNA-LP) Vaccines for Newly Diagnosed Pediatric High-Grade Gliomas (pHGG) and Adult Glioblastoma (GBM) | Phase I/II | 52 | Newly Diagnosed Pediatric High-Grade Gliomas and Adult Glioblastoma | 2021–10 | Recruiting |
| VBI-1901 vaccine | CMV antigens | NCT03382977 | Study to Evaluate Safety, Tolerability, and Optimal Dose of Candidate GBM Vaccine VBI-1901 in Recurrent GBM Subjects | Phase I/II | 98 | Recurrent Glioblastoma | 2017–12 | Recruiting |
| Small molecules/cytokine | ||||||||
| Inhibitor (Indoximod) | Indoleamine 2,3-dioxygenase (IDO) | NCT05106296 | Chemo-immunotherapy Using Ibrutinib Plus Indoximod for Patients With Pediatric Brain Cancer | Phase I | 37 | Ependymoma, Medulloblastoma, Glioblastoma, Primary Brain Tumor | 2022–02 | Recruiting |
- Note: Trials identified from ClinicalTrials.gov (https://clinicaltrials.gov) with filtering: Condition/ disease; glioblastoma, Other term; GBM, Intervention/treatment; immunotherapy, Study Status; Recruiting, Not-yet-recruiting studies, Study type; Interventional. This search, as of December 26th, 2024, led to 36 studies, nine of which were excluded from this table because these studies are observational or not for immunotherapy.
| Modality | Immunological Target | Trial ID | Trial Name | Phase | No. of Patients | Tumor Description | Country | Registration Date | Status |
|---|---|---|---|---|---|---|---|---|---|
| Combination therapy | |||||||||
| Antibody (Nivolumab, Ipilimumab) & DC |
PD-1, CTLA-4, Tumor antigens |
2024–517,842–33-01 |
Phase I clinical trial on intra-tumoral ipilimumab plus intravenous nivolumab following the resection of recurrent glioblastoma |
Phase I/II | 113 | Glioblastoma | Belgium | 2024–11 | Not yet recruiting |
| Antibody (Pembrolizumab) & tumor treating fields | PD-1 | jRCT2011240045 | EF-41/KEYNOTE-D58Trial | Phase III | 741 | Newly Diagnosed Glioblastoma | Japan, USA, United Kingdom, Switzerland, Spain, Poland, Italy, Israel, Germany, France, Czech, Canada | 2024–10 | Recruiting |
| Antibody (Nivolumab) & multi-kinase Inhibitor (Regorafenib) | PD-1 | jRCT2031200408 | A Trial to Learn Whether Regorafenib in Combination With Nivolumab Can Improve Tumor Responses and How Safe it is for Participants With Solid Tumors | Phase II | 200 | Solid Tumors (including Glioblastoma) | Belgium, France, Japan, Korea, Italy, United Kingdom, USA | 2021–03 | Complete |
| Antibody (Ezabenlimab) & bispecific nanobody (BI 836880) | PD-1, VEGF, Ang2 | jRCT2031200207 | A study to test different doses of BI 836880 combined with ezabenlimab in patients with advanced non-small cell lung cancer followed by other types of advanced solid tumors | Phase I | 6 | Advanced Solid Tumors (including Glioblastoma) | Japan, Australia, China, France, Germany, Hong Kong, Korea, Poland, Russian, Spain, Taiwan, Ukraine, United Kingdom, USA | 2020–11 | Complete |
| Peptide vaccine (EO2401) & Antibody (Nivolumab) | Microbiome-derived bacterial antigens, PD-1 | 2018–002279-16 | A Multicenter, Open-Label, First-in-Human, Phase Ib/IIa Trial of EO2401, a Novel Multipeptide Therapeutic Vaccine, with and without PD-1 Check Point Inhibitor, Following Standard Treatment in PatIents with Progressive Glioblastoma (Rosalie study) | Phase Ib/IIa | 100 | Glioblastoma | USA, France, Spain, Germany | 2019–08 | Complete |
| Bacterial vaccine vector (VXM01) & Antibody (Avelumab) | PD-L1, VEGFR2 | 2017–003076-31 | An open-label, Phase I/II multicenter clinical trial of VXM01 in combination with avelumab in patients with progressive glioblastoma following standard treatment with or without second surgery. | Phase I/II | 100 | Glioblastoma | Germany, Netherlands | 2018–04 | Ongoing |
| Immune checkpoint blockade | |||||||||
| Inhibitor (ABBV-181) | PD-1 | jRCT2031230510 | A Phase 1 study of ABBV-706 alone or in combination in adult subjects with advanced solid tumors | Phase I | 350 | Advanced Solid Tumors with Potential SEZ6 Expression (including IDH-wt Glioblastoma) | Japan, USA, Israel, Korea | 2023–12 | Recruiting |
| Antibdoy (Pembrolizumab) | PD-1 | 2020–006143-26/2024–517,535–52-00 | A Single center, Open-Label, Randomized Study to Evaluate the Safety and Efficacy of neoadjuvant and adjuvant Pembrolizumab on top of standard Chemo-Radiotherapy (Stupp protocol) in Treatment of Patients with newly diagnosed Glioblastoma Multiforme | Phase IV | 36 | Newly Diagnosed Glioblastoma | Poland | 2021–02 | Recruiting |
| Antibody (Durvalumab/Medi4736) | PD-L1 | 2016–001614-16/2024–515,000–39-00 |
A phase I/II multicenter trial evaluating the association of hypofractionated stereotactic radiation therapy and the anti-PD-L1 Durvalumab (Medi4736) for patients with recurrent glioblastoma. |
phase I/II | 106 | Recurrent Glioblastoma | France | 2016–10 | Complete |
| Antibody (Nivolumab/BMS-936558) | PD-1 | jRCT2080223128 | A Phase 3 Study of ONO-4538/BMS-936558 (ONO-4538-40/CA209548) | Phase III | 693 | Glioblastoma | Japan, North America, Europe, Oceania, Middle East | 2016–03 | Complete |
| Cell therapy | |||||||||
| Dendritic cells | Tumor antigens | jRCTc031230593 | A safety study of combined immunotherapy for glioblastoma | Phase I | 3 | Glioblastoma | Japan | 2024–10 | Not yet recruiting |
| Dendritic cells (DEN-STEM) | Tumor antigens | 2015–002198-40/2024–518,663–35-00 |
Open label randomized phase II/III trial of dendritic cell immunotherapy against cancer stem cells in glioblastoma patients receiving standard therapy (DEN-STEM) |
Phase II/III | 60 | Glioblastoma multiforme | Norway | 2016–09 | Recruiting |
| Dendritic cells (ICT-107) | Tumor antigens | 2015–002685-23 |
A Phase III randomized double-blind, controlled study of ICT-107 with maintenance temozolomide (TMZ) in newly diagnosed glioblastoma following resection and concomitant TMZ chemoradiotherapy. |
Phase III | 414 |
Newly Diagnosed Glioblastoma |
Austria, Canada, France, Germany, Italy, Netherlands, Spain, Switzerland, UK, USA | 2016–04 | Ongoing |
| Dendritic cells (WT1 mRNA-electroporated) | Tumor antigens | 2014–001098-15/2024–515,291–13-00 |
Adjuvant dendritic-cell immunotherapy plus temozolomide following surgery and chemoradiation in patients with newly diagnosed glioblastoma |
Phase II | 20 |
newly diagnosed glioblastoma |
Belgium | 2015–07 | Ongoing |
| Lympohid cells (ALECSAT) | Lymphoid Effector Cells |
2013–003045-42/ NCT02060955 |
An Open-labeled, Randomized Phase II Multicentre Study to Investigate Efficacy of Autologous Lymphoid Effector Cells Specific Against Tumor-Cells (ALECSAT) in Patients with Glioblastoma Multiforme Measured as Progression Free Survival Compared to Avastin/Irinotecan |
Phase II | 175 | Glioblastoma multiforme | Denmark | 2013–10 | Complete |
| Dendritic cells | Tumor antigens | 2010–018447-34 | Investigation of dendritic cell vaccine immunotherapy in pediatric high grade glioma | Phase I | 20 | Malignant glioma | UK | 2011–08 | Ended |
| Dendritic cells (DCm-HGG-L) | Tumor antigens | 2009–018228-14 | HGG-2010. AS phase IIb prospective placebo-controlled double blind randomized clinical trial for the treatment of patients with newly diagnosed glioblastoma multiforme with tumor vaccination as “add-on therapy” to standard primary treatment | Phase II | 146 | Glioblastoma multiforme | Belgium | 2011–04 | Complete |
| Virus therapy | |||||||||
| Oncolytic virus (G47delta) | Engineered herpes simplex virus (HSV) | UMIN000015995 | A phase 2 clinical trial of a replication-competent, recombinant herpes simplex virus type 1 in patients with glioblastoma | Phase II | 30 | Residual or Recurrent Glioblastoma | Japan | 2014–12 | Complete: follow-up complete |
| Oncolytic virus (G47delta) | Engineered herpes simplex virus (HSV) | UMIN000002661 | A clinical study of a replication-competent, recombinant herpes simplex virus type 1 (G47delta) in patients with progressive glioblastoma | Phase I/II | 21 | Recurrent Glioblastoma | Japan | 2009–10 | Complete: follow-up complete |
| Vaccines | |||||||||
| Tumor vaccine (Cellm-001) | Tumor Antigens | jRCT2031200153 | Randomized phase III clinical trial on the therapeutic effect of Cellm-001 for newly diagnosed glioblastoma | Phase III | 112 | Glioblastoma | Japan | 2020–10 | Recruiting |
| WT1 peptides | Tumor antigens | UMIN000026965 | Phase II clinical trial of WT1 peptide-based vaccine combined with Temozoromide for patients with glioblastoma | Phase II | 35 | Glioblastoma | Japan | 2017–09 | Complete: follow-up complete |
| Wt1 peptide (DSP-7888) | Tumor Antigens | jRCT2080223161 | A phase 1/2 study of DSP-7888 in pediatric patients with relapsed or refractory high grade gliomas | Phase I/II | 30 | Pediatric High Grade Gliomas | Japan | 2016–05 | Complete |
| Autologous tumor vaccine | Tumor antigens | UMIN000010602 | Randomized phase IIb/III trial of autologous tumor vaccine with temozolomide for newly diagnosed glioblastoma | Phase IIb/III | 60 | Newly Diagnosed Glioblastoma | Japan | 2013–04 | Complete: follow-up complete |
| Peptide vaccine (ITK-1) | Antigens | UMIN000006970 | A placebo controlled phase III double blind study of ITK-1-The efficacy and safety evaluation of ITK-1 in Temozolomide refractory Glioblastoma patients with HLA-A24 positive | Phase III | 110 | Glioblastoma | Japan | 2012–01 | Complete: follow-up complete |
| Peptide vaccine (ITK-1) | Antigens | jRCT2080221684 | ITK-1 Phase 3 placebo controlled double-blind comparative study | Phase III | 110 | Glioblastoma | Japan | 2011–12 | Complete |
| Peptide vaccine | VEGFR-1/2 | UMIN000005545 | A phase I/II study for peptide vaccine of vascular endothelial growth factor receptor-1/2 in patients with progressive or recurrent glioblastoma | phase I/II | 20 | Glioblastoma | Japan | 2011–05 | Complete: follow-up complete |
| WT1 peptide | Tumor antigens | UMIN000002419 | Immunotherapy using WT1 peptide with surgical treatment, irradiation therapy, and Temozolomide administration against glioblastoma multiforme: A phase I/II study | phase I/II | 45 | Glioblastoma Multiforme | Japan | 2009–09 | Recruiting |
| WT1 peptide | Tumor antigens | UMIN000002024 | Immunotherapy using WT1 peptide against recurrent glioblastoma multiforme: A phase I/II study | phase I/II | 30 | Glioblastoma Multiforme | Japan | 2009–06 | Complete: follow-up complete |
| Autologous tumor vaccine | Tumor antigens | UMIN000001426 | Phase I/IIa trial of autologous tumor vaccine and temozolomide administration in patients with primary glioblastoma | Phase I/IIa | 25 | Glioblastoma | Japan | 2008–11 | Complete: follow-up complete |
| Peptide vaccination | Tumor antigens | UMIN000001243 | Phase II clinical trial of personalized peptide vaccination for standard therapy failure patients with malignant glioma (glioblastoma) | Phase II | 40 | Malignant Glioma (Glioblastoma) | Japan | 2008–07 | Complete: follow-up complete |
| Autologous tumor vaccination | Tumor Antigens | C000000002 | Phase I/IIa trial of autologous tumor vaccination in patients with primary glioblastoma | Phase I/IIa | 25 | Glioblastoma | Japan | 2005–08 | Complete: follow-up complete |
| Small molecules/cytokine | |||||||||
| IFN beta | Cytokine | UMIN000010472 | Phase II trial of polifeprosan 20 with carmustine implant, Interferon-beta and Temozolomide Combination Chemoradiotherapy for Newly Diagnosed Glioblastomas | Phase II | 60 | Newly Diagnosed Glioblastoma | Japan | 2013–04 | Recruiting |
| IFN beta | Cytokine | UMIN000003466 | A Multicenter Randomized Phase II trial of Interferon-beta and Temozolomide Combination Chemoradiotherapy for Newly Diagnosed Glioblastomas(JCOG0911, INTEGRA study (P-II)) | Phase II | 120 | Glioblastoma | Japan | 2010–04 | Complete: follow-up continuing |
- Note: Trials were identified from 1. rctportal (https://rctportal.niph.go.jp/en) with filtering: Glioblastoma and manual filtration for interventional immunotherapy that led to 22 studies, 2. EU Clinical Trials (https://www.clinicaltrialsregister.eu/ctr-search/search, https://euclinicaltrials.eu/?lang=en) with search filtration of “Glioblastoma AND Immunotherapy” or terms: Immunotherapy and condition: Glioblastoma. The data were retrieved on December 26, 2024.
We herein review the current immunotherapy strategies from two perspectives: (1) modulating immune functions and tumor immunogenicity, and (2) targeting brain immune niches. The CNS was historically considered immune privileged due to the blood–brain barrier (BBB) that prevents peripheral immune cell entry into the CNS. However, growing evidence demonstrates that resident and infiltrating innate and adaptive immune cells contribute to normal brain function and tissue repair, whose dysregulation is implicated in the pathogenesis of autoimmune diseases, neuroinflammation, and tumors. Furthermore, recent studies have identified several specialized immunological niches surrounding the brain parenchyma, mediating dynamic interplays between the peripheral immune system and the CNS [6]. This review summarizes the current knowledge in GBM immunotherapy and discusses the potential of underappreciated immune niches to harness anti-tumor immune responses against GBM.
1.1 Modulating Immune Functions and Tumor Immunogenicity
Successful immunotherapy depends on tumor antigen recognition and immune potency (Figure 1). The former has been exploited by synthetic bioengineering and immune education with tumor-derived antigens. ICB and immunomodulatory ligands rejuvenate and activate immune cells to achieve robust anti-tumor immunity. These strategies show synergy when rationally combined; yet further optimization and therapeutic target identification are required.
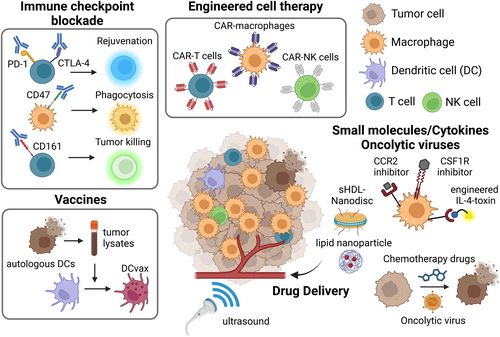
1.2 Immune Checkpoint Blockade
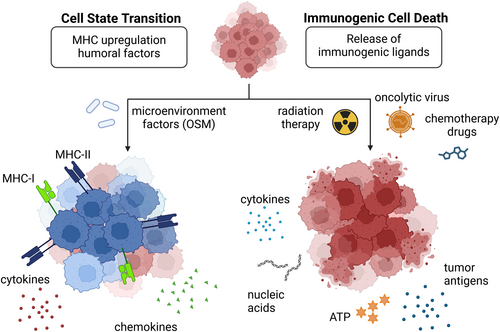
The ICBT primarily targets anti-tumor T cells to restore their functions by blocking T cell inhibitory molecules [7]. Local T cell abundance is the key determinant of ICBT efficacy [7], but transcriptomic studies characterized the immune landscape of GBM as “non-T cell-inflamed” and “macrophage-enriched” [8, 9]. The limited presence and functional exhaustion of T cells—a chronically activated yet hypo-functional state—partly driven by the high abundance of immunosuppressive myeloid cells, may contribute to the poor outcomes observed in clinical trials [10]. The immune-cold microenvironment may be associated with tumor subtypes, as researchers identified higher infiltration of exhausted T cells in MES-enriched tumors [11]. In mouse models of MES-like GBM, tumors exhibited sensitivity to CTLA-4 blockade but not to PD-1 inhibition. This response was associated with enhanced interactions between IFNγ-producing CD4+ T cells and MHC-II+ microglia, ultimately leading to tumor clearance [12]. Single-cell RNA-sequencing (scRNA) of human MES-GBM revealed heterogeneous MHC and inflammatory profiles of tumor cells caused by interactions with Oncostatin M (OSM)-expressing macrophages (Figure 2) [11]. Therefore, approaches for effective ICBT may include the development of a personalized strategy for MES-GBM patients based on specific genetic biomarkers, such as germline POLE mutations and FcγR polymorphism [13]. Other immune checkpoint molecules have been explored, such as CD161, whose blockade induces NK cell-like state activation in T cells [14], and CD47-SIRPα axis to restore myeloid phagocytosis activity [15]. The antibodies targeting these molecules are still in the early stages of therapeutic validation, potentially promising as a combination therapy [16].
1.3 Engineered Cell Therapy
CAR-T cells are made by engineering patients' autologous peripheral T cells to express receptors that recognize tumor antigens [17], but the major limitations include exhaustion and resistance due to antigen heterogeneity that drives immune evasion. Indeed, CAR-T cells targeting single GBM antigens (e.g., EGFRvIII, HER2, IL13Rα) have not reached large-scale trials [18]. Two ongoing clinical trials targeting multiple antigens show promising effects in eradicating GBM (Table 1; NCT05660369, NCT06186401) [19, 20]. Choi et al. designed EGFRvIII-CAR-T cells that secrete a bispecific antibody bridging wild-type EGFR on GBM and CD3 on T cells [19]. Bagley et al. developed bivalent CAR-T cells that recognize both EGFR and IL13Rα [20]. A dual targeting approach is also investigated in CAR-NK cells [21], and GD2-CAR-NK cells [22] or CAR-NKT cells [23] in brain tumors are under investigation. Preclinical studies demonstrated that engineered macrophages expressing CD133-CAR [24] or secreting bispecific T cell engager (BiTE) [25] exhibit great potency in tumor phagocytosis in combination with CD47 blockade [24]. Beyond CAR, engineered macrophages [26], neutrophils [27], and bacteria [28] are utilized as drug-delivery tools when coupled with drug-loaded polymeric backpacks/nanoparticles and multilayered polymeric particles [29]. Advanced synthetic biology will greatly contribute to overcoming tumor heterogeneity and achieving spatiotemporal regulation of engineered cells by controllable molecular switches [30, 31].
1.4 Vaccines
Vaccines educate the host immune system, particularly dendritic cells (DCs), to induce a tumor-specific T-cell response [32]. Multi-peptide vaccines targeting GBM tumor-specific or tumor-associated antigens, such as rindopepimut (EGFRvIII) and SurVax (survivin), are being tested, and several early-phase trials have shown favorable results [32]. A therapeutic vaccine, DCVax-L, which uses autologous tumor lysates to activate DCs for intradermal injection, demonstrated significant improvement in median survival in phase III clinical trials [32]. Human cytomegalovirus (hCMV) pp65 antigen-mRNA loaded DC vaccines also showed promising effects in boosting CD8+ T cell responses in GBM [32]. A recent study achieved the dramatic delivery efficacy of mRNA-containing lipid particle aggregates (RNA-LPA), which rapidly reprogrammed the TME to the “immune-hot” state by activating the RNA sensing pathway in the stroma [33].
1.5 Small Molecules/Drug Delivery System/Oncolytic Virus
Small-molecule drugs have continuously faced challenges in GBM therapy since the approval of temozolomide [2] due to their inability to penetrate the BBB and limited druggable GBM oncogenic drivers. To enhance BBB permeability, nanomaterials for small molecule delivery such as sHDL Nanodisc [34] and nanoparticles encapsulated with the innate immune agonist CpG or nanohydrogel show advantages in activating tumor-associated macrophages and suppressing immune evasion [35]. Non-invasive treatments, Sonodynamic therapy (SDT), Photodynamic therapy (PDT), and magnetic resonance focused ultrasound (MRgFUS), deliver high-intensity ultrasound waves to precisely target tumor tissue with certain sensitizers and physically disrupt the BBB to enhance payload delivery [36, 37]. Traditional chemotherapeutic drugs induce tumor cell death associated with enhanced tumor immunogenicity [38], but the low mutational burden in GBM limits the diversity of molecular target drugs. Our chemical proteomics platform identified previously unrecognized druggable protein targets in 25 cancer types including GBM [39], which may tailor the heterogeneity of GBM cell states reactive to currently available immunotherapies through transcriptional reprogramming. Researchers also focus on CCR2, CSF1R, and CHIL3L1 [40] inhibitors to block immunosuppressive macrophage infiltration and skew phenotypic polarization toward anti-tumoral states. Oncolytic viruses (OVs) have attracted considerable interest for the potency of immunogenic cell death (ICD) induction, even while still facing some obstacles, including host-mediated virus clearance [41]. OVs selectively replicate in tumor cells by their functional deletion of the neurovirulence factors and induce oncolysis [41, 42], leading to extracellular release of damage-associated molecular patterns (DAMPs) such as ATP and HMGB1 (Figure 2), showing synergistic effects with ICBT [43, 44].
1.6 Cytokine/Chemokine Therapy
Cytokine and chemokine therapy aims to remodel the immunosuppressive TME landscape and increase immunogenicity [45]. For pro-inflammatory cytokines such as IL-12, TNFα, and IFNα, various delivery strategies have been developed to control the dose, localization, and toxicity of these soluble factors, including targeted oncolytic viruses [46], lipid nanoparticles [47], and CAR engineering [17]. Administration of the cytokine-conjugated antibody L19-IL-12 and L19-TNFα, which binds to extracellular domain B (ED-B) of fibronectin expressed in tumors, improved overall survival in orthotropic GBM mouse models [48]. A recent clinical trial reported that recombinant IFNα administration enhanced the efficacy of temozolomide and prolonged patient survival. Engineered immunotoxin MDNA55, which consists of circularly permuted IL-4 fused to a truncated Pseudomonas aeruginosa exotoxin A, was shown to induce cell death of IL-4R-high populations, including GBM tumor cells and immunosuppressive macrophages [49]. With an acceptable safety profile and a 50% increase in median overall survival seen in the phase IIb trial, MDNA55 was granted Fast Track and Orphan Drug Status, allowing it to move into a phase III trial. Further development requires rational combination strategies.
2 Targeting Brain Immune Niches
Adaptive and innate immune cells interact with the non-immune cells in the CNS microenvironment—neurons, astrocytes, and oligodendrocytes—to construct distinct immunological niches that tightly regulate brain homeostasis and function [6]. Choroid plexus, meninges, and perivascular niche, as well as skull bone marrow serve as a reservoir necessary for immune homeostasis and allow reparative leukocyte infiltration from the periphery into the brain parenchyma following CNS damage. A groundbreaking discovery of the meningeal lymphatic system [50] has transformed our perception of immune cell trafficking from the CNS to the meningeal borders and the draining cervical lymph nodes. The impact of immunological niche dysregulation on CNS pathologies has been studied mainly in acute CNS injury, neurodegenerative diseases [51], and infectious diseases, including COVID-19 [52]. Although studies characterizing GBM immune niches are still immature, the lessons learned from other brain diseases may provide unique GBM therapeutic interventions targeting immune niches (Figure 3).
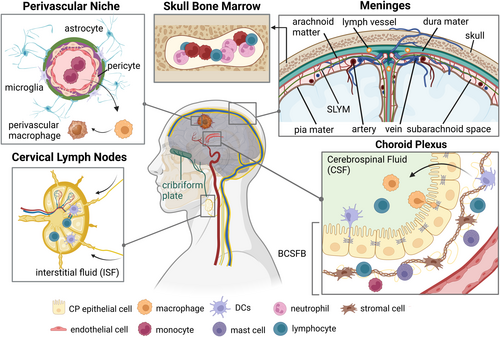
2.1 Choroid Plexus (CP)
The CP consists of specialized epithelial cells surrounded by vascularized stroma, located in the brain ventricles, and functions to produce cerebrospinal fluid (CSF). CSF protects the brain and spinal cord from mechanical trauma, provides nutrients to the CNS, and regulates metabolite homeostasis. CSF follows a multi-route circulation [53] that includes the cribriform plate, lymphatic vessels, olfactory nerves, as well as meningeal and perivascular routes, ultimately reaching the cervical lymph nodes. The Blood-Cerebrospinal Fluid Barrier (BCSFB) is a physiological gateway for immune cells into the CNS. Multiple CP resident immune cells, mainly DCs, macrophages (known as Kolmer's epiplexus cells), and effector memory CD4+T cells, orchestrate to regulate immune homeostasis [54]. Ligand-receptor interactions, such as CCR6-CCL20 [55] or IFNγ-IFNGR [56] between immune cells and CP epithelium induce pathogenic T cell infiltration, and IL-1β [57] and type I IFN [58]. Targeting CP epithelium [59] and CSF [60] might improve drug sensitivity and recruit a specific immune cell population into the GBM microenvironment.
2.2 Meninges
The meninges is membranous connective tissue consisting of four layers: the pia mater, the subarachnoid lymphatic-like membrane (SLYM) [61], the arachnoid mater, and the dura mater (from inner to outer). Each layer has distinct roles in immune homeostasis and inflammatory responses [6], showing unique enrichment of immune cell subsets [62]. Although controversial, anatomical analyses revealed the morphological and functional differences between dorsal meningeal lymphatic vessels (MLVs) and basal MLVs on drainage of CSF-derived macromolecules, immune trafficking [63, 64], and glioma antigen delivery [63, 65]. VEGF-C regulates lymphatic angiogenesis and vascular remodeling [63], and several studies demonstrated that the ectopic expression of VEGF-C enhanced CD8+ T cell migration into glioma tissue through lymphatic drainage [66] and increased ICBT and radiation therapy efficacy [63, 67]. Collectively, utilizing MLV for targeted delivery of immune-stimulating agents and vascular reprogramming might enhance the therapeutic effects of biologics and non-invasive treatment.
2.3 Perivascular Niche (PVN)
The PVN stroma cells function as the critical regulators of glioma stemness, invasion, and therapeutic resistance [68]. The PV endothelium maintains glioma stem-like cell (GSC) properties and induces immunosuppressive macrophages [69]. High abundance of these GSC-derived pericytes is associated with better chemotherapy efficacy [70, 71]. Oligodendrocyte progenitor cell-like GBM tumor cells actively invade into peritumoral brain parenchyma along with PVN through the Olig2-Wnt7 pathway [72], contributing to therapeutic failure. Interestingly, neuron-to-glioma synapses drive glioma progression and neuronal excitability [73, 74] and astrocytes show unique accumulation in the peritumor region away from myeloid cells [75], exacerbating tumorigenesis by mitochondria transfer [76]. PV macrophages show heterogeneity, including yolk sac-derived resident Lyve1+F4/80+CD206+CXCR3+ macrophages [6] and several monocyte-derived macrophages (MDMs) that increase along GBM progression. CD169+ MDMs promote phagocytosis and T cell recruiting [77], whereas GLUT1+ MDMs produce anti-inflammatory IL-10 and suppress T cell activation [78]. Mechanisms of this heterogeneous macrophage differentiation and clinical relevance with spatial analysis [79] need further investigation. Reprogramming macrophages into immune-activating phenotype and discovering new immune checkpoint blockade targeting tumor-stroma-immune interactions might provide therapeutic benefits.
2.4 Cervical Lymph Nodes (CLNs)
The CLNs continuously receive brain-derived molecules and antigens from the CNS via CSF and interstitial fluid (ISF). The drainage of meningeal antigen-presenting cells to the CLNs induces activation and circulation of T cells and B cells to the CP and the meninges [6]. Lymph node resection has historically been considered a diagnostic and therapeutic strategy to assess and avoid solid tumor metastases, respectively [80]. However, emerging evidence suggests that intact CLNs are critical for the successful immunotherapy of solid cancers [81]. Although rare, there are several clinical cases reporting GBM extraneural metastases to CLNs [82]. Considering the drainage by meningeal lymphatics, these metastases might be the result of dysfunctional meninges, potentially dorsal MLVs [63]. Further basic and clinical investigations are required to reveal how these metastases occur under certain conditions and why they are relatively rare.
2.5 Skull Bone Marrow (BM)
Skull BM displays a myeloid-rich signature, mainly monocytes and neutrophils [83, 84]. The ScRNA-seq approach identified “hybrid” neutrophils (TANs) harboring antigen presentation capacity, which arise from skull BM and accumulate in GBM [85]. These TANs induce MHC-II-dependent T cell activation and suppress glioma growth in syngeneic GBM models. Interestingly, the accumulation of GD2-CAR-T cells in skull BM of pediatric glioma patients was observed [86]. Another study reported the enrichment of effector CD8+ memory T cells in skull BM proximal to tumors but not in distal BM [87]. These findings suggest the potential of engineering skull BM resident myeloid cells to redirect anti-tumor T cells, with advanced imaging techniques [88].
3 Concluding Remarks
The following challenges need to be considered for successful GBM immunotherapies: (1) targeting multiple GBM antigens and tumor heterogeneity, (2) identifying new druggable targets that contribute to the development of an immunosuppressive microenvironment, (3) understanding niche-specific immune cell functions and developing strategies to modulate tumor-immune-stroma interactions, (4) advanced disease models recapitulating human GBM biology to enable rationale combinations to address the challenges mentioned above. In addition, detailed spatial analysis of inter- and intra-tumoral heterogeneity with specific treatment history will improve our understanding of GBM biology and expedite the creation of potent strategies.
Author Contributions
Mariko Takahashi: conceptualization, supervision, visualization, writing – original draft. Darina Mukhamejanova: visualization, writing – original draft. Himani Jasewicz: visualization, writing – original draft. Nandini Acharya: visualization, writing – original draft. James J. Moon: conceptualization, visualization, writing – original draft. Toshiro Hara: writing – original draft, writing – review and editing.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
J.J.M. declares financial interests as board membership, a paid consultant, research funding, and/or equity holder in EVOQ Therapeutics and Saros Therapeutics. Other authors declare no conflicts of interest.




