Treating Hematological Malignancies With OR-2100, an Orally Bioavailable Prodrug of Decitabine
Funding: This work was supported by Shinnihon Foundation of Advanced Medical Treatment Research, OHARA Pharmaceutical Co. Ltd. Nippon Shinyaku, Yasuda Memorial Medical Foundation, JSPS KAKENHI, 17H06956, 20K07593, and 23K06744.
ABSTRACT
DNA methylation is an enzyme-driven epigenetic modification that must be precisely regulated to maintain cellular homeostasis. Aberrant methylation status, especially hypermethylation of the promoter sites of tumor-suppressor genes, is observed in human malignancies and is a proven target for cancer therapy. The first-generation DNA demethylating agents, azacitidine and decitabine, are widely used for treating several hematological malignancies. In addition, orally bioavailable prodrugs of azacitidine and decitabine have recently been approved by the FDA. We have developed a silylated derivative of decitabine, OR-2100, which is resistant to degradation by cytidine deaminase and orally bioavailable. It has efficacy against several human hematological malignancies in xenograft mouse models with less hematotoxicity than decitabine. Since DNA demethylating agents are combined with molecularly targeted drugs in clinical use and trials, we think that the less hematotoxic profile of OR-2100 makes it suitable for use as a combination therapy. In this article, we review the therapeutic approach in hematological malignancies with the DNA demethylating agent OR-2100.
1 Introduction
Epigenetic modifications, such as methylation of DNA and histone proteins, are involved in biological processes including gene expression and chromosome stability [1]. DNA methylation is catalyzed by DNA methyltransferases; DNMT1 is a maintenance transferase that adds a methyl group to hemi-methylated DNA and DNMT3A/B are a de novo methyltransferases that add a methyl group to unmethylated DNA [2]. Since DNA methylation is involved in gene expression and chromatin organization, aberrant DNA methylation disrupts cellular homeostasis, potentially leading to human diseases including cancer [3]. Genetic mutations and aberrant expression levels of methylation-related genes, including DNMT3A, DNMT1 and TET2 have been found in human hematological malignancies [4-6]. Therefore, aberrant DNA methylation status is thought to act as a driver of carcinogenesis and is attracting attention as a therapeutic target.
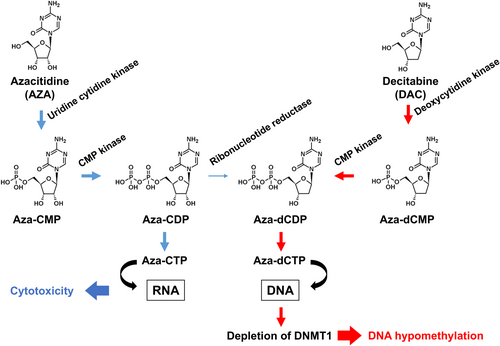
The DNA demethylating drugs azacitidine and decitabine, which were originally synthesized as pyrimidine antimetabolites [7, 8], induce the differentiation of leukemic cells through DNA hypomethylation [9]. Both drugs are metabolized into the deoxycytidine triphosphate analog AZA-dCTP through the pyrimidine metabolism pathway. AZA-dCTP is incorporated into the growing daughter strand of DNA, leading to depletion of DNMT1, and DNA hypomethylation in cells that are dividing [10] (Figure 1). Both drugs are degraded rapidly by cytidine deaminase (CDA) [11] and administered by injection. The second-generation demethylating agents CC-486 [12] and ASTX727 [13], which are orally bioavailable formulations of azacitidine and decitabine, respectively, were approved by the FDA in 2020. To overcome CDA-mediated degradation, we developed OR-2100, an orally bioavailable prodrug of decitabine, and confirmed its efficacy in mouse models of several hematological malignancies (Table 1). OR-2100 is currently in a phase I clinical trial in Japan (Japan Registry Clinical Trials, jRCT2071220035).
| Disease | Treatment | Molecular mechanisms (key molecules) | References |
|---|---|---|---|
| Colon cancer | Monotherapy | [14] | |
| MDS/AML | Monotherapy | Induction of differentiation-related genes | [15] |
| ATL | Monotherapy | Induction of negative regulators of TCR signaling | [16] |
| Lymphocytic leukemia | Monotherapy | Down-regulation of MYC expression. | [17] |
| MDS/AML | Combination therapy (venetoclax) | Induction of ROS, down-regulation of VAMP7 | [18] |
| CML | Combination therapy (ABL TKI) | Induction of PTPN6 | [19] |
| ALCL | Combination therapy (ALK TKI) | Down-regulation of Wnt/β-catenin pathway | [20] |
| ATL | Combination therapy (EZH inhibitor) | Induction of DUSP5 | [21] |
DNA demethylating agents induce unique reprogramming of gene expression and monotherapy shows clinical benefit in patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) [22]. Combining DNA demethylating agents with other anti-cancer drugs has recently been tested. For example, the combination of decitabine or azacitidine with the BCL-2 inhibitor venetoclax showed clinical efficacy, tolerable safety, and favorable overall response rates in AML patients, and this success has changed the standard of care for frail patients with AML [23]. Here, we present the therapeutic approach in hematological malignancies with the DNA demethylating agent OR-2100.
2 Development of OR-2100
2.1 Development of OR-2100 as an Orally Bioavailable Prodrug of Decitabine
The first-generation DNA demethylating agents azacitidine and decitabine are routinely used in clinical settings for treating high-risk MDS [24]. However, these drugs need to be administered via subcutaneous or intravenous injection, meaning that patients require daily hospital visits for ~7 days. In addition, both drugs sometimes induce hematotoxicity. The deoxyribonucleoside decitabine induces DNA hypomethylation at lower concentrations than the ribonucleoside azacitidine, which is predominantly incorporated into RNA [25]. Decitabine prodrugs resistant to CDA-mediated degradation have been developed; ASTX727 is a fixed-dose combination of decitabine and the CDA inhibitor cedazuridine [13] and SGI-110 is a dinucleotide of decitabine and deoxyguanosine that releases decitabine [26, 27]. Both drugs are resistant to CDA-mediated degradation, and ASTX727 can be administered orally but SGI-110 cannot. Therefore, we sought to develop a novel, single-molecule prodrug of decitabine that is orally bioavailable and has reduced hematotoxicity to make it suitable for long-term administration.
To develop a novel decitabine prodrug, several 5′-O-trialkylsilylated decitabines were designed [14, 28]. The DNA demethylating activity of these compounds was screened in a luciferase activity assay; in the absence of demethylating agents, luciferase expression is silenced by promoter hypermethylation [16]. The DNA demethylating activity was further validated by measuring the promoter methylation level of the LINE-1 region [28], representative of global genomic DNA methylation [15]. Considering the logP value, which is widely used to evaluate the lipophilicity of molecules (0–3 indicating good potential for absorption by oral administration) [29], stability to CDA, and half-lives for decitabine release in vitro, OR-2100 was selected for further analysis; The logP value of OR-2100 and decitabine is 2.14 and −0.32, respectively, and decitabine was completely degraded by CDA within 0.5 h, while OR-2100 retained 84% after treatment with CDA for 2 h [14]. Since we wanted to confirm the improved bioavailability of OR-2100 compared to decitabine, which is administered by injection in the clinic, both were administered duodenally and compared directly; a pharmacokinetic study of OR-2100 administered duodenally to macaques showed that OR-2100 had a 20-fold greater rate of migration into the blood than decitabine [30]. Furthermore, duodenal administration of OR-2100 induced hypomethylation in the proximal promoter region of the γ-globin gene in peripheral blood mononuclear cells [28].
Since OR-2100 releases decitabine, the efficacy was expected to be derived from the released decitabine. Indeed, OR-2100 and decitabine demethylated similar genomic regions (determined by genome-wide DNA methylation analysis using Infinium Human MethylationEPIC) and induced similar gene expression changes (determined by genome-wide gene expression analysis using an expression microarray) [14]. In mouse models, OR-2100 exhibited anti-tumor activities with fewer adverse effects such as hematotoxicity and impaired liver function than decitabine [14, 28]. The broad concentration–time profile of decitabine reportedly makes it suitable for non-cytotoxic DNMT1 depletion, with lower-grade hematotoxicity [31]. Thus, OR-2100 demonstrated the potential for safe oral administration through a different pharmacokinetic profile than decitabine due to its prodrug nature.
2.2 OR-2100 Monotherapy in MDS and AML
MDS and AML originate from the impaired differentiation of hematopoietic stem cells and progenitor cells [32]. Aberrant DNA hypermethylation that silences tumor-suppressor genes in these cells is thought to be involved in the leukemogenesis of MDS and AML [33, 34], motivating researchers to test the efficacy of DNA demethylating agents against both malignancies.
Recently, oral azacitidine (formally known as CC-486) was developed. The AZA-MDS-003 study (ClinicalTrials.gov, NCT01566695), a phase III randomized, placebo-controlled trial of CC-486, revealed that people treated with CC-486 had a significantly higher rate of red blood cell transfusion independence than placebo-treated patients, although more deaths occurred in the CC-486 arm due to infections caused by profound neutropenia [35]. Orally available CC-486, which has an estimated bioavailability of 6.3%–20%, is a convenient drug to replace injectable azacitidine [12]. To make hypomethylating agents stable in vivo, ASTX727 was developed as a fixed-dose formulation of decitabine and the CDA inhibitor cedazuridine [13]. Results from ASCERTAIN (ClinicalTrials.gov, NCT03306264), a randomized, crossover, pharmacokinetic phase III study were disclosed in 2024 [36]; the primary endpoint of total exposure of ASTX727 versus intravenous decitabine was 98.93% (90% CI 92.66–105.60), indicating that the drugs had equivalent bioavailability and pharmacodynamics.
Treatment of MDS and AML cell lines with OR-2100 suppressed cell growth associated with DNA hypomethylation and also induced cell differentiation via up-regulation of differentiation-related genes such as CEBPE and GATA-1. The efficacy of OR-2100 was also observed in a xenograft mouse model in which HL-60 cells were inoculated intravenously, and in this model OR-2100 had fewer adverse effects than decitabine [28]. Based on these results, a single-arm, multicenter phase I clinical trial of OR-2100 is ongoing. The trial includes Japanese patients with MDS or chronic myelomonocytic leukemia characterized as intermediate-risk, high-risk, or very high-risk by the Revised International Prognostic Scoring System and disease that is relapsed, refractory, or intolerant to standard therapy (Japan Registry Clinical Trials, jRCT2071220035).
2.3 OR21 Monotherapy in ATL
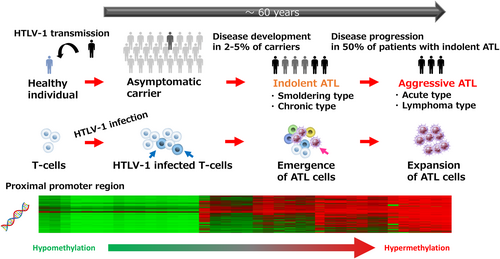
Adult T-cell leukemia/lymphoma (ATL) is an aggressive T-cell malignancy caused by infection with human T-cell leukemia virus type I (HTLV-1). Most HTLV-1-infected individuals remain asymptomatic throughout their lives, but 2%–5% develop ATL with a long latency period [37, 38]. ATL is classified into four clinical subtypes based on clinical features: smoldering, chronic, acute, and lymphoma. Although smoldering and chronic ATL are relatively indolent, about half of cases progress to aggressive ATL subtypes (acute and lymphoma) that require urgent treatment. Despite an increased number of treatment options, the prognosis of aggressive ATL remains poor, with a median survival time of ~1 year, and the survival rate for indolent ATL has not improved in 30 years [39, 40].
HTLV-1-infected T cells accumulate genetic and epigenetic abnormalities during leukemogenesis that result in their capacity for clonal cell expansion. Accumulation of tri-methylated histone H3 at lysine 27 (H3K27me3) is observed in HTLV-1-infected cells and ATL cells and contributes to the multi-step carcinogenesis that leads to ATL [41, 42]. Like DNA methylation, H3K27me3 is well known as a mark of repression of gene expression [43]. Since HTLV-1-infected cells and ATL cells aberrantly overexpress enhancer of zeste homolog 2 (EZH2) [44], which catalyzes the tri-methylation reaction of H3K27, EZH2 is considered a target for ATL therapy. Indeed, an EZH1/2 dual inhibitor (DS-3201, valemetostat) was recently approved in Japan for treating refractory/relapsed ATL [45]. It is important to note that another epigenetic-targeting agent, HBI-8000 (tucidinostat) [46] is also approved in Japan for refractory/relapsed ATL. The successes of epigenetic-targeting drugs in ATL therapy encouraged us to study the involvement of DNA methylation in ATL leukemogenesis.
We identified differentially methylated positions specific to HTLV-infected T cells [30]. Methylation at differentially methylated positions accumulates in HTLV-1-infected cells and ATL cells during leukemogenesis and reflects the development and progression of ATL (Figure 2). Several genes that negatively regulate T-cell receptor signaling, including THEMIS, LAIR1, and RNF130, were down-regulated through promoter hypermethylation in ATL cells [16]. Since active (gain-of-function) mutations in the T-cell receptor–NF-κB pathway are frequently found in, and thought to drive ATL leukemogenesis [17], we tested whether regional DNA hypermethylation contributes to the growth of HTLV-1–infected cells, and whether DNA demethylation efficiently suppresses tumor cell growth.
As expected, OR-2100 inhibited the growth of an HTLV-1-infected cell line inoculated subcutaneously in immunodeficient mice. Notably, the hematotoxicity of OR-2100 in our model was lower than that of decitabine in different xenograft mouse models in separate studies. We also tested the longer-term treatment in an ATL patient-derived xenograft mouse model; treatment with OR-2100 was safely sustained for 100 days, suppressed body weight loss, and prolonged the survival of the mice [30]. Thus, we believe that aberrant regional DNA hypermethylation contributes functionally to ATL leukemogenesis and is a promising therapeutic target in ATL.
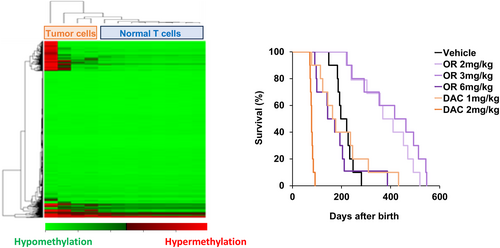
Based on our results in models of ATL, we hypothesized that ATL leukemogenesis could be prevented by continuously suppressing DNA methylation abnormalities. However, this strategy has not been confirmed due to the lack of a suitable animal model of spontaneous leukemogenesis caused by chronic HTLV-1 viral infection. AKR mice spontaneously develop lymphocytic leukemia through retrovirus infection; they produce mink cell focus virus (MCFV) by recombination of a germline proviral AKV retrovirus and a murine leukemia virus. The MCFV is replicated and incorporated into the genome by T-cell lymphocytes in the thymus, resulting in the development of lymphocytic leukemia [47]. Therefore, we used AKR mice to evaluate the efficacy of OR-2100 for cancer chemoprevention. Genome-wide DNA methylation profiling revealed regional DNA hypermethylation in leukemic cells from AKR mice, similar to that seen in ATL cells. AKR mice died of lymphocytic leukemia by Day 200–300, whereas OR-2100 was safely administered for more than 500 days and significantly prolonged survival with less hematological toxicity than decitabine (Figure 3) [48].
3 Combination Therapy With DNA Demethylating Agents and Other Drugs
3.1 OR-2100 Plus Venetoclax for AML
Since the median age at diagnosis of AML is 68 years [49], not many patients are eligible for conventional intensive chemotherapy and hematopoietic stem cell transplantation. Less intensive chemotherapy regimens such as DNA demethylating agents or low-dose cytarabine have been used in these frail patients [50]. The combination of venetoclax, a selective BCL-2 inhibitor, and azacitidine showed high response rates and durable remissions with a good safety profile in patients with AML who are ineligible for intensive chemotherapy in the VIALLE-A clinical trial (ClinicalTrials.gov, NCT02993523) [49]. Following this success, clinical trials are ongoing to evaluate the safety, efficacy, and pharmacokinetics of oral DNA demethylating agents (CC-486 and ASTX727) combined with venetoclax (Table 2).
| DNA demethylating agent | Combination | Disease | References |
|---|---|---|---|
| CC-486 | Venetoclax | Previously untreated high-risk MDS | NCT05782127 |
| CC-486 | Venetoclax | AML | NCT05287568 |
| CC-486 | Gilteritinib | Relapsed/refractory FLT3-mutated AML | NCT06022003 |
| ASTX727 | Venetoclax | Treatment-naïve high-risk MDS or CMML | NCT04655755 |
| ASTX727 | Venetoclax | CMML and other MDS/MPN | NCT05600894 |
| ASTX727 | Eltanexor (KPT-8602) | High-risk MDS | NCT05918055 |
| ASTX727 | Venetoclax, gilteritinib | Relapsed/refractory FLT3-mutated AML or high-risk MDS | NCT05010122 |
| ASTX727 | Venetoclax | Relapsed, refractory, or newly diagnosed AML | NCT04746235 |
| ASTX727 | Thioguanine | Relapsed/refractory AML | NCT06351306 |
| ASTX727 | Entrectinib | Relapsed/refractory TP53 mutated AML | NCT05396859 |
| ASTX727 | Venetoclax | Relapsed AML after donor stem cell transplant | NCT05799079 |
| ASTX727 | Venetoclax, ivosidenib or enasidenib | Relapsed/refractory AML | NCT04774393 |
| ASTX727 | SNDX-5613, venetoclax | AML | NCT05360160 |
We tested the combination of OR-2100 with venetoclax in vitro and, as expected, found synergistic anti-AML activity. In addition, the combination suppressed tumor cell growth in a mouse xenograft model generated using an AML cell line and prolonged survival more than either monotherapy without toxicity [18]. We also found that the synergistic efficacy was a result of ROS induction and down-regulated expression of VAMP7, a protein that regulates autophagy to maintain mitochondrial homeostasis [18]. Since OR-2100 demonstrated comparable efficacy to decitabine with less hematotoxicity, we think it is worth testing whether the combination of OR-2100 and venetoclax has less hematotoxicity than the combination of venetoclax with either decitabine, azacitidine or ASTX727.
3.2 OR-2100 Plus ABL TKIs for CML
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell disorder induced by t(9;22) (q34;q11) translocation. Tyrosine kinase inhibitors (TKIs) targeting ABL have revolutionized the treatment of CML. TKIs dramatically improve the survival of patients with chronic phase CML, resulting in a life expectancy that is similar to that of the general population [51]. Although the current therapeutic strategy for patients with chronic phase CML aims to safely discontinue TKIs [52], approximately half of patients who initially achieve a deep molecular response fail to maintain that response after TKIs are discontinued. The cause of this poor therapeutic response is likely the presence of CML leukemic stem cells (LSCs), which persist in a quiescent state in the bone marrow microenvironment and can survive independently of BCR–ABL1 [53]. Since DNMT1 is essential for the maintenance of normal hematopoietic stem cells [54] and epigenetic reprogramming is also required for the persistence of CML LSCs, DNA demethylating agents have the potential to eradicate CML LSCs in patients with CML.
We confirmed that OR-2100 exhibits anti-tumor effects as monotherapy in lab-based studies, showing that it increased TKI-induced apoptosis of CML cells and inducted expression of tumor-suppressor genes including PTPN6 (which encodes SHP-1) in vitro and in vivo [55]. In addition, the combination of OR-2100 and a TKI targeted LSCs, as demonstrated by a reduced level of secondary transplanted bone marrow cells from primary murine CML models in the presence of the combination [55].
3.3 OR-2100 Plus ALK-TKI for ALK+ALCL
Anaplastic lymphoma kinase (ALK) was first identified as a fusion gene in anaplastic large cell lymphoma (ALCL) and subsequently in non-small cell lung carcinoma (NSCLC) [19, 56]. Because fusion gene products cause constitutive tyrosine kinase activity and drive carcinogenesis, molecular targeting agents against ALK have been developed [57]. ALCL is a type of non-Hodgkin's lymphoma with four subtypes including ALK-positive ALCL (ALK+ALCL), ALK-negative ALCL, primary cutaneous ALCL, and breast-implant-associated ALCL [58]. ALK+ALCL is the most common subtype of ALCL, accounting for ~90% of pediatric and ~50% of young adult cases [59]. The first-generation ALK-targeting TKI, crizotinib, was approved by the FDA for young adult or pediatric patients with relapsed or refractory ALK+ALCL in 2021. The second-generation ALK-TKI, alectinib, also showed favorable clinical activity in patients with ALK+ ALCL who failed to respond to standard chemotherapy [60] and was approved in 2020 in Japan for treating relapsed or refractory ALK+ALCL.
Aberrant DNA methylation occurs in ALK+ALCL cells [61], and treatment with DNA demethylating agents, including OR-2100, suppresses tumor cell growth and re-activates silenced tumor-suppressor genes [62], suggesting that DNA hypomethylating therapy is a compelling approach for treating ALK+ALCL. We also tested the combination of ALK-TKIs with OR-2100 in ALK+ALCL cells. Since a phase III head-to-head study of alectinib and crizotinib in Japanese patients with ALK-inhibitor-naïve ALK+NSCLC showed that alectinib had more favorable progression-free survival and adverse event profiles than crizotinib [63], we focused on the combination of alectinib and OR-2100. As expected, the combination had synergistic efficacy, possibly through actions on the Wnt/β-catenin pathway [62], which is aberrantly activated in a subset of ALCL [20, 64].
3.4 OR-2100 Plus EZH Inhibitors for ATL
Recently, two agents targeting epigenetic regulators have been approved in Japan for relapsed or refractory ATL; the histone deacetylase inhibitor tucidinostat and the EZH1/2 dual inhibitor valemetostat. Since H3K27me3 is aberrantly accumulated during ATL leukemogenesis [41], we tested the combination of EZH inhibitors with OR-2100 in HTLV-1-infected cells or an ATL-cell xenograft mouse model. Our findings showed that the combination had greater efficacy than either monotherapy [21]. In addition, transcriptome analysis revealed that the combination induced a strong reprogramming of gene expression through hypomethylation of DNA and hypomethylation of histone H3 at lysine 27. We also identified that expression of the tumor-suppressor gene DUSP5, which encodes an ERK-specific phosphatase, was significantly up-regulated by the combination. DUSP5 gene expression was down-regulated through DNA and histone hypermethylation in HTLV-1-infected cells and ATL cells during ATL leukemogenesis, and reconstitution of DUSP5 expression suppressed the growth of ATL cells. Thus, the induction of DUSP5 expression is one of the molecular mechanisms underlying the efficacy of the OR-2100 and EZH inhibitor combination in ATL.
Recently, acquired mutations in EZH2 have been identified in approximately half of ATL patients whose disease progresses on valemetostat treatment [45]. Furthermore, a portion of patients without EZH2 mutations had a TET2 mutation or increased DNMT3A expression, and these changes caused de novo DNA methylation and better susceptibility to DNA demethylating agents [45]. Since epigenetic abnormalities have been observed in several human malignancies, we reason that dual targeting of aberrant DNA methylation and histone methylation is a rational therapeutic approach in such malignancies, including ATL.
4 Conclusion
It is 60 years since the first DNA demethylating agent was originally synthesized as an antimetabolite, and 20 years since it was approved for treating MDS. DNA demethylating agents induce the reprogramming of gene expression, leading to unique anti-tumor efficacy. Orally administrable DNA demethylating agents are approved by FDA and the combination of DNA demethylating agents with other molecular targeting agents is being investigated in clinical trials and preclinical studies. As OR-2100 has shown efficacy against several hematological malignancies in our mouse models associated with less hematotoxicity than decitabine, these preclinical data on OR-2100 encourage us to plan clinical trials that expand the use of OR-2100 beyond the MDS and chronic myelomonocytic leukemia indications currently being investigated in the ongoing phase I clinical trial in Japanese patients (Japan Registry Clinical Trials, jRCT2071220035) to the combination therapy with molecular targeting agents or long-term chemopreventive therapy.
Author Contributions
Tatsuro Watanabe: conceptualization, data curation, formal analysis, funding acquisition, investigation, project administration, writing – original draft. Keisuke Kidoguchi: data curation, investigation, visualization, writing – original draft. Shinya Kimura: conceptualization, funding acquisition, project administration, supervision, writing – original draft, writing – review and editing.
Acknowledgments
The authors are grateful to all of the members of our laboratory (Dr. Yuta Yamamoto, Dr. Yuki Kurahashi, Dr. Hiroshi Ureshino, Dr. Kazuharu Kamachi, Dr. Nao Yoshida-Sakai, Dr. Kazunori Kawasoe, Mr. Keisuke Kidoguchi, Ms. Yuki Fukuda-Kurahashi, Ms. Ayaka Tomoda and Ms. Maiko Yamada) for performing numerous experiments and technical assistance in studies cited in this paper. We would also like to express our sincere thanks to Dr. Seiji Ohara, Dr. Kazuki Miyazaki, Dr. Yoshitaka Nakata, Dr. Magoichi Sako and Dr. Yutaka Hayakawa at OHARA Pharmaceutical Co. Ltd. for the synthesis and formulation of compounds and financial support for studies that are cited in this review.
Conflicts of Interest
S.K. has received honoraria from Bristol-Myers Squibb, Novartis, Pfizer, and Otsuka Pharmaceuticals, and research funding from Bristol-Myers Squibb, Novartis, Pfizer, and Otsuka Pharmaceuticals, and OHARA Pharmaceutical. The other authors declare they have no potential conflicts of interest. S.K. is an editorial board member of Cancer Science.




