Elotuzumab-mediated ADCC with Th1-like Vγ9Vδ2 T cells to disrupt myeloma–osteoclast interaction
Yusuke Inoue and Hirofumi Tenshin contributed equally to this work.
Abstract
Multiple myeloma (MM) cells and osteoclasts (OCs) activate with each other to cause drug resistance. Human Th1-like Vγ9Vδ2 (γδ) T cells, important effectors against tumors, can be expanded and activated ex vivo by the aminobisphosphonate zoledronic acid in combination with IL-2. We previously reported that the expanded γδ T cells effectively targeted and killed OCs as well as MM cells. Because the expanded γδ T cells expressed CD16 on their surface, we investigated the utilization of the expanded γδ T cells for antibody-dependent cellular cytotoxicity (ADCC). Although the expanded γδ T cells alone induced cell death in MM cell lines, the addition of the anti-SLAMF7 monoclonal antibody elotuzumab (ELO) further enhanced their cytotoxic activity only against SLAMF7-expressing MM cell lines and primary MM cells. Intriguingly, ELO was also able to enhance γδ T cell-induced cell death against OCs cultured alone, and against both MM cells and OCs in their coculture settings. SLAMF7 was found to be highly expressed in OCs differentiated in vitro from monocytes by receptor activator of nuclear factor-κ B ligand and M-CSF, although monocytes only marginally expressed SLAMF7. These results demonstrate that SLAMF7 is highly expressed in both MM cells and OCs, and that the ex vivo-expanded γδ T cells can exert ELO-mediated ADCC against SLAMF7-expressing MM cells and OCs besides their direct cytotoxic activity. Further study is warranted for the innovative utilization of γδ T cells.
1 INTRODUCTION
The implementation of new agents, including therapeutic monoclonal antibodies (mAbs), has improved clinical outcomes of patients with multiple myeloma (MM). However, most patients eventually relapse.1 MM cells and osteoclasts (OCs) activate with each other and form a vicious cycle between tumor expansion and bone destruction to cause drug resistance.2 Human Vγ9Vδ2 (γδ) T cells can be expanded and activated ex vivo by the aminobisphosphonate zoledronic acid (ZA) in combination with IL-2.3 We demonstrated that the expanded γδ T cells effectively target and impair not only MM cells but also OCs to disrupt the MM cell–OC vicious cycle.4 Because of their unique antitumor properties, clinical application of γδ T cells has been drawing considerable attention in cancer immunotherapy.5, 6 However, MM-associated bone marrow microenvironment has been reported to blunt the antitumor activity of γδ T cells.6-8 Therefore, we need to develop maneuvers to empower their anti-MM potential. The expanded γδ T cells are phenotypically and functionally Th1-like with IFN-γ production7 and also express surface molecules similar to those on natural killer (NK) cells, including CD16 (FcγRIIIa).5 Elotuzumab (ELO), a therapeutic mAb against signaling lymphocytic activation molecule family 7 (SLAMF7), can preferentially exert antibody-dependent cellular cytotoxicity (ADCC) with NK cells.9 In the present study, we investigated the utilization of the ex vivo-expanded Th1-like γδ T cells for induction of ADCC in combination with currently available therapeutic mAbs, namely anti-CD38 mAbs and ELO, to target MM cell–OC interaction.
2 MATERIALS AND METHODS
2.1 Human γδ T cell expansion
Th1-like γδ T cells were expanded from peripheral blood mononuclear cells (PBMCs) as previously reported.4, 7 In brief, PBMCs were cultured for 1 week with ZA at 1 μM in combination with IL-2 at 100 IU/mL. γδ T cell expansion was analyzed with the Gallios flow cytometer (Beckman Coulter).
2.2 Human OC differentiation
Monocytes were isolated from PBMCs using the Pan Monocyte Isolation Kit (Miltenyi Biotec) and cultured at 1 × 106 cells/mL in 24-well culture plates for 14 days in the presence of M-CSF (10 ng/mL) and receptor activator of nuclear factor-κB ligand (RANKL) (50 ng/mL) as previously described.10 Culture media were changed every 2 days.
2.3 Cytotoxicity assay by flow cytometry
Target MM cells were stained by PKH26 fluorescent dye and cultured alone or with effector cells; then, dead cells were stained with 7 amino-actinomycin D (7-AAD). The cells were analyzed by flow cytometry, and the distributions of 7-AAD-stained cells were calculated within PKH26-labeled MM cells.
Methods are further described as supporting information including Supplementary Materials and Methods—Data S1 and Table S1.
3 RESULTS AND DISCUSSION
3.1 γδ T cells exert ADCC with ELO
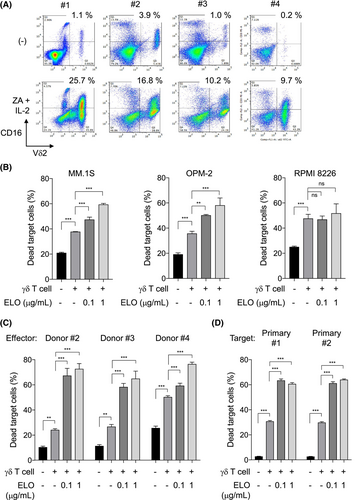
Zoledronic acid and IL-2 expanded Th1-like γδ T cells from PBMCs, which expressed intracellular IFN-γ (Figure S1) and increased CD16 on their surface (Figure 1A). We next examined cytotoxic activity by Th1-like γδ T cells in combination with clinically available therapeutic mAbs. SLAMF7 was expressed on MM.1S and OPM-2 cells but not on RPMI 8226 cells, while CD38 was expressed on all these MM cell lines (Figure S2A). ELO, the anti-CD38 mAbs daratumumab (DARA), or isatuximab (ISA) alone marginally induced cell death in these MM cell lines (Figure S2B). The expanded Th1-like γδ T cells induced cytotoxic activity for these MM cell lines; interestingly, the addition of ELO further enhanced the cytotoxic activity of the Th1-like γδ T cells against SLAMF7-expressing MM.1S and OPM-2 cells but not against the SLAMF7-lacking RPMI 8226 cells (Figure 1B). Furthermore, addition of anti-CD16-blocking Ab mitigated the ELO-mediated cytotoxicity (Figure S3A), indicating ELO-mediated ADCC with the γδ T cells. Although the expanded γδ T cells expressed SLAMF7 on their surface, ELO pretreatment for the γδ T cells only marginally affected their cytotoxic activity against MM cells (Figure S3B, C). ELO-mediated ADCC was further confirmed using γδ T cells expanded from different healthy donors (Figure 1C) and primary CD138+ MM cells isolated from patients with MM (Figure 1D). However, neither DARA nor ISA enhanced the cytotoxic activity of Th1-like γδ T cells in our experimental conditions (Figure S4), indicating good compatibility of ELO with Th1-like γδ T cells. These results suggest that Th1-like γδ T cells can be utilized as an effector for ELO-mediated ADCC.
3.2 Osteoclasts highly express SLAMF7 and are susceptible to ELO's ADCC
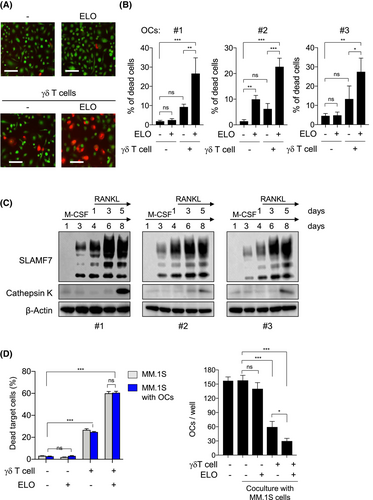
Intriguingly, the addition of ELO also enhanced the cytotoxic activity of Th1-like γδ T cells against OCs (Figure 2A,B), although ELO alone did not induce any cytotoxic activity against OCs (Figure 2B). Blockade of CD16 significantly reduced the ELO-mediated OC death with the γδ T cells (Figure S5A), indicating ELO-mediated ADCC against OCs with the γδ T cells. CD38 was reported to be expressed in monocytes but downregulated in osteoclastic lineage cells differentiated from monocytes.11 In sharp contrast to CD38, SLAMF7 expression was upregulated during osteoclastic differentiation from monocytes, although monocytes only marginally expressed SLAMF7 (Figure 2C and Figure S5B), and confirmed in mature multi-nucleated OCs (Figure S5C). SLAMF7 was robustly upregulated in pre-osteoclasts before expressing cathepsin K, a marker of mature OCs, as well as cathepsin K-expressing mature OCs (Figure 2C). The SLAMF7 expression was induced in parallel with NFATc1 upon RANKL treatment (Figure S5D,E), suggesting RANKL-mediated SLAMF7 induction in osteoclastic lineage cells. Furthermore, ELO was able to enhance γδ T cell-induced cell death against both MM cells and OCs even in their coculture settings (Figure 2D). These results collectively demonstrate that SLAMF7 is highly expressed in both MM cells and OCs, and that OCs as well as MM cells are susceptible to ELO-mediated ADCC with Th1-like γδ T cells. ELO and Th1-like γδ T cells in combination are suggested to target and disrupt the MM cell–OC vicious cycle.
SLAMF7, a therapeutic target of MM cells, is also robustly upregulated in osteoclastic lineage cells. Th1-like γδ T cells expanded by ZA and IL-2 can exert cytotoxic activity against OCs as well as MM cells, which is substantially enhanced through ADCC with ELO. The unique function of Th1-like γδ T cells warrants their further utilization for innovative immunotherapies.
AUTHOR CONTRIBUTIONS
Yusuke Inoue: Conceptualization; data curation; investigation; visualization; writing – original draft; writing – review and editing. Hirofumi Tenshin: Conceptualization; data curation; formal analysis; investigation; validation; visualization. Jumpei Teramachi: Funding acquisition; resources; supervision. Ryohei Sumitani: Resources. Asuka Oda: Data curation; investigation; resources; validation. Yusaku Maeda: Resources. Masahiro Oura: Resources. Kimiko Sogabe: Resources. Tomoko Maruhashi: Resources. Mamiko Takahashi: Resources. Shiro Fujii: Resources. Shingen Nakamura: Resources. Hirokazu Miki: Resources. Tomoyo Hara: Resources. Itsuro Endo: Resources. Kumiko Kagawa: Resources. Shuji Ozaki: Resources. Masahiro Hiasa: Funding acquisition; resources; supervision. Takeshi Harada: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; visualization; writing – original draft; writing – review and editing. Masahiro Abe: Conceptualization; data curation; funding acquisition; project administration; resources; supervision; visualization; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
We thank the Support Center for Advanced Medical Sciences in Tokushima University Graduate School of Biomedical Sciences.
FUNDING INFORMATION
This work was supported in part by JSPS KAKENHI Grant Numbers JP22K08455 to T.H.; JP17H05104, JP19K22719, and JP23H03101 to M.H.; and 21H03111 and 22 K19626 to J.T.; a research grant from Bristol-Myers Squibb to T.H. and M.A.; the Research Clusters program of Tokushima University (2202003) to T.H.; and the Japanese Society of Hematology Research Grants (23195) to T.H.
CONFLICT OF INTEREST STATEMENT
T.H. received research funding and honoraria from Bristol-Myers. M.A. received research funding from Bristol-Myers Squibb and honoraria from Bristol-Myers Squibb, Janssen Pharma K.K., and Sanofi K.K. The other authors declare no competing financial interests.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: 3842 in Tokushima University Hospital, 16–8 in Tokushima Prefectural Central Hospital.
INFORMED CONSENT
Primary samples were obtained from all the patients with written informed consent according to the Declaration of Helsinki.




