The cancer epigenome: Non-cell autonomous player in tumor immunity
Abstract
Dysregulation of the tumor-intrinsic epigenetic circuit is a key driver event for the development of cancer. Accumulating evidence suggests that epigenetic and/or genetic drivers stimulate intrinsic oncogenic pathways as well as extrinsic factors that modulate the immune system. These modulations indeed shape the tumor microenvironment (TME), allowing pro-oncogenic factors to become oncogenic, thereby contributing to cancer development and progression. Here we review the epigenetic dysregulation arising in cancer cells that disseminates throughout the TME and beyond. Recent CRISPR screening has elucidated key epigenetic drivers that play important roles in the proliferation of cancer cells (intrinsic) and inhibition of antitumor immunity (extrinsic), which lead to the development and progression of cancer. These epigenetic players can serve as promising targets for cancer therapy as a dual (two-in-one)-targeted approach. Considering the interplay between cancer and the immune system as a key determinant of immunotherapy, we discuss a novel lineage-tracing technology that enables longitudinal monitoring of cancer and immune phenotypic heterogeneity and fate paths during cancer development, progression, and therapeutic interventions.
1 INTRODUCTION
Chromatin is an essential medium through which transcription factors, signaling pathways, and other cues alter gene activity and cellular phenotypes. Consistent with this broad functional purview in cellular state and regulation, chromatin aberrations have been associated with a wide range of diseases. The fundamental unit of chromatin architecture is the nucleosome, a histone octamer that is wrapped by 147 base pairs of DNA in the nucleus. The positioning and dynamics of nucleosomes are tightly associated with the chromatin accessibility of DNA-binding or histone-binding protein complexes and indeed chromatin 3D structure. Rewiring the epigenetic landscape is a relatively common event in cancer, owing to alterations in epigenetic regulatory genes. A critical component of chromatin-mediated epigenetic regulation is post-translational modifications of histones, which modulate various extra/intracellular signals with chromatin-dependent functions. During cancer development, mutations in histone H3 occur with high genetic penetrance in rare pediatric gliomas (H3K27M), sarcomas (H3G34W/L), and other cancers (H3K36M), suggesting a direct link between aberrant histone modifications and cancer development. Notably, the oncogenic histone H3 or other histone H1/H2/H4 mutations mostly occur at or near key modifications in the N-terminal histone tails, and the mutated histone proteins often act as dominant-negative histone modifiers.1 These oncogenic histone mutations do not only homogeneously dilute but selectively coordinate certain histone methylations on key tumor suppressor subsets (e.g., CDKN2A) across genomic loci.2-6 As an alternative mechanism for aberrant histone modifications, the molecules that write, read, and erase these oncogenic histone marks are also frequently and dynamically altered in cancer.7 In many cases, epigenetic modifiers' loss- or gain-of-function mutations act as tumor suppressors or oncogenic drivers in tumor development and progression.
Of note, it has recently been shown that epigenetic hyperactivation of oncogenic pathways modulates the immune system besides cancer cell-intrinsic oncogenic reprogramming.8-10 Accompanying extensive modifications, including histone modifications, of the cancer cell epigenome drives rewiring of immune cell chromatin landscapes and reconfiguration of immune suppressive tumor microenvironment (TME),11 thereby leading to deterioration of the antitumor immune response and the potential response to immunotherapy. The epigenetic reprogramming, a hallmark of cancer, enables cancer development and progression via adapting to several environmental conditions, such as immune surveillance and a lack/competition of nutrients in the tumor microenvironment (TME).12 Therefore, cancer development may require cancer cell epigenetic reprogramming that exerts both cell autonomous and non-cell autonomous effects.
In this review, we describe epigenetic reprogramming that modulates the interaction between cancer cells and the immune system in the TME. This interaction shapes tumor developmental trajectories and determines a response to immunotherapy. Thus, cancer cell-intrinsic epigenetic dysregulation impacts the surrounding TME beyond the cancer cells. Furthermore, identifying and leveraging such intrinsic oncogenic epigenetic molecules or machineries may provide a rational combinatorial therapeutic approach that improves the potency of cancer immunotherapy.11, 13, 14 Although histone modifiers, such as histone methyltransferases, are emerging and particular druggable targets among epigenetic modulators,15 the epigenetic dependency and program are diverse across developmental lineages or subtypes of cancer and are therefore context-dependent.16 In consequence, tracing the spatiotemporal evolutionary trajectory of cancer and immune cells is required to figure out key epigenetic molecules during a course of immunotherapy.
2 CANCER-INTRINSIC EPIGENETIC DYSREGULATION AND IMMUNE EVASION
2.1 EZH2 histone methyltransferase
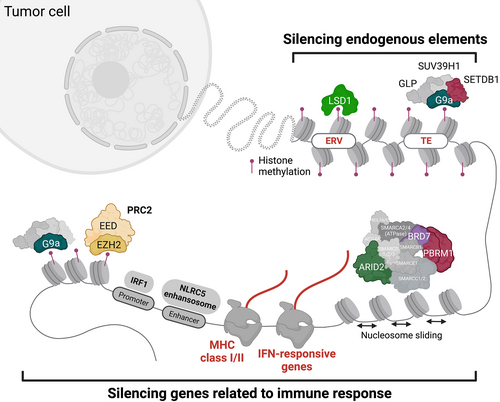
Oncogenic redistribution of histone modifications can also be caused by gain-of-function mutations in histone methyltransferases. Enhancer of zeste homolog 2 (EZH2) is one of the most frequently targeted su(var)3-9, enhancer-of-zeste and trithorax (SET) domain-containing histone methyltransferases by recurrent activating mutations that occur at Y641 or A677, which is the catalytic center of methyltransferase in B-cell lymphoma (follicular lymphoma [FL] and diffused large B-cell lymphoma [DLBCL]), malignant melanoma,17 and Ewing sarcoma.18 EZH2 comprises a writer complex, polycomb repressive complex 2 (PRC2) and mediates H3K27 methylations, such as H3K27 trimethylation (H3K27me3), which is associated with transcriptional repression. These EZH2 mutants have a differential substrate preference, namely, decreased or increased binding affinity to H3K27me0/1 or H3K27me2, respectively.19 In addition to the elevation in H3K27me3 levels by activating EZH2 mutations, certain EZH2 mutations cause a global redistribution of H3K27me3 away from focal peaks (e.g., near the transcription start site) across broad regions, including gene bodies and intergenic regions, resulting in changes in PRC2-dependent gene expression.20 Intriguingly, these oncogenic EZH2 activating mutations are significantly enriched in patients with germinal center B-cell-like DLBCL who lack the expression of major histocompatibility complex (MHC) class I and class II,21 indicating that EZH2 contributes to immune evasion. Moreover, EZH2 hyperactivation leads to inhibition of antigen presentation pathways via H3K27me3-mediated repression of NLRC5, which is a key transactivator of MHC class I and II.22 The mechanism has been reported in DLBCL, mouse embryonic stem cells (mESCs), tissue-specific progenitor cells, and cancer cells that exhibit an embryonic neural crest-like or dedifferentiated phenotype23-25 (Figure 1), therefore immune evasion may be a feature of cells that normally exist in a dedifferentiated state.26 Moreover, cancer cells responding to oncogenic signals that promote dedifferentiation may exploit evolutionarily conserved mechanisms for immune evasion through certain epigenetic mechanisms.
The redistribution of H3K27me3 by EZH2 mutated at Y641 is strictly limited to selected topology-associating domains (TADs) that contain critical tumor suppressor genes such as SESN1, FOXO3, and ARMC2 in the development of B-cell lymphoma.27 Interestingly, in addition to those tumor suppressors, the integrative epigenetic and transcriptomic analysis of this study further suggests that inactive TADs by EZH2 Y641 mutant are significantly enriched for immune-related genes. TADs including the human leukocyte antigen (HLA) gene cluster loci at chromosome 6 show a neutral effect by either EZH2 mutants or EZH2 catalytic inhibitors in FL and DLBCL, suggesting a distinct mechanism by which EZH2 controls immune evasion through the reorganization of chromatin 3D architecture in cancer cells. Although it remains unclear how EZH2 recognizes and reorganizes these advantageous TADs in cancer cells, the inactivation of TADs and silencing of tumor suppressors and immune-related genes are responsible for the acceleration of lymphomagenesis, which can be reversed by EZH2 catalytic inhibitors.27 Recent live-cell single-molecule tracking has illustrated that H3K27M prolongs the residence and search time of EZH2/PRC2 to bind without notable effects on its abundance bound to chromatin.28 This novel technology visualizes the aberrant histone modifications that may rewire the epigenetic and transcriptional landscape by disrupting the ability of epigenetic modifiers to recognize their binding sites, thereby providing mechanistic insights into a redistribution of histone modifiers and oncogenic histone modifications in specific TADs, including the genes encoding immune-related pathways.
2.2 G9a histone methyltransferase
The link between oncogenic epigenetic dysregulation (intrinsic) and the evasion from the immune system (extrinsic) within the TME has been implicated as represented by EZH2. To comprehensively and unbiasedly identify the key epigenetic players in cancer cells that harness the immune system, a systematic approach using clustered regulatory interspaced short palindromic repeat (CRISPR)-Cas9-mediated perturbations successfully identified some candidate molecules that suppress immune-related pathways in murine tumor models in vitro29, 30 and in vivo31, 32 (Figure 1). Based on the result of in vitro CRISPR screening,30 inactivation of genes encoding histone methyltransferases, EZH2/PRC2, was confirmed to enhance the upregulation of the cell surface expression of MHC class I by IFN-γ, as well as NK-cell-mediated killing, in human chronic myelogenous leukemia cell line K562 and other cancer types.23 This finding strongly supports the immune suppressive functions of EZH2 in cancer cells, as described in section 2.1 (EZH2 as an epigenetic driver of tumorigenesis and immune evasion).
More importantly, the genome-wide CRISPR screening identified another novel SET domain-containing histone methyltransferase as a potential epigenetic driver of immune evasion, G9a.30 G9a (EHMT2) is an H3K9 methyltransferase that catalyzes mono- and di-methylation of H3K9 (H3K9me1 and H3K9me2, respectively) in the heterodimeric complex with the G9a-like protein (GLP) (EHMT1).33 H3K9me1 and H3K9me2/3 are known to be associated with transcriptional activation and repression, respectively. Similar to EZH2, G9a is often targeted by gain-of-function genetic alterations, which elevate H3K9me2 levels in the presence of GLP and accelerate tumorigenesis by repressing the WNT/β-catenin antagonist DKK1 in malignant melanoma34 (Figure 2). The latest integrated 3D epigenome analysis suggests compartment-specific epigenetic machinery and distinct function mediated by G9a/GLP-dependent H3K9me2.35 For instance, the G9a/GLP complex mainly deposits H3K9me2 in compartment A (euchromatin) or B (heterochromatin) in mESCs or mouse liver cells, respectively,35, 36 suggesting that G9a/GLP-dependent H3K9me2 contributes to lineage differentiation states through distinct repressive 3D chromatin states. A distinct function of G9a-dependent H3K9me2 has been termed “large organized chromatin K9-modifications” (LOCKs), which are implicated in a differentiation state, and almost all H3K9me3 marks do not overlap LOCKs.37 Wild-type and mutant G9a may protect against a repression histone mark, H3K9me2, and prevent subsequent accumulation of another transcription repressive mark, H3K9me3, in a compartment- or site-dependent fashion, physically competing with histone demethylases or methyltransferases that might change the H3K9me2 mark38, 39 (Figure 2). The proposed “gatekeeper” model of G9a may control the immune response by stimulating melanocyte-lineage developmental pathways (e.g., WNT/β-catenin) that can promote intrinsic and extrinsic tumorigenesis programs.40 In fact, G9a contributes to the development of a noninflamed TME in various cancer types,34 thus suggesting a dual mode of oncogenesis through intrinsic and extrinsic cell pathways simultaneously activated by epigenetic regulators.
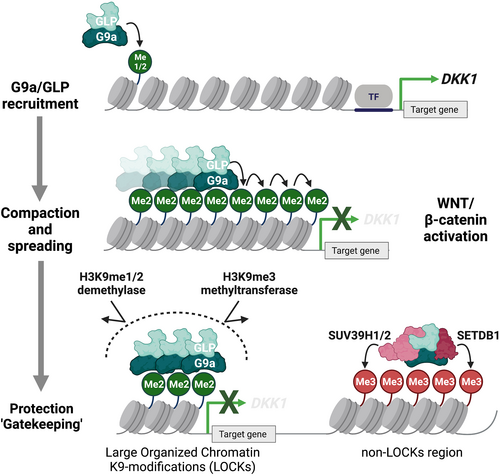
With the recent in vivo CRISPR screens31, 32 specifically targeting epigenetic regulators in multiple murine cancer cell lines, including B16 (melanoma), LLC (lung carcinoma), and lung adenocarcinoma cells derived from the KrasG12D/Trp53−/− lung adenocarcinoma model, it has been shown that cell type-specific epigenetic regulators and a few common genes such as KDM1A and SETDB1 suppress antitumor immune responses. Besides the tumor-intrinsic oncogenic addiction,41, 42 KDM1A and SETDB1 decrease tumor immunogenicity by silencing the expression of immunologically important repetitive genomic elements—endogenous retrovirus elements (ERVs)43 or transposable elements (TEs)—where immune-associated gene clusters are enriched at loci associated with segmental duplicates32 (Figure 1). While a distinct complex of LSD1/KDM1A or SETDB1 with KAP1/TRIM28 corepressor is responsible for the silencing of ERVs as well as TEs in mESCs,44 some specific retroelements are cooperatively regulated by other H3K9 methyltransferases, G9a/GLP and SUV39H1.45-47 Perturbing the ability of this complex to deposit H3K9me2, either by depleting SUV39H1 or by inhibiting the enzymatic activity of G9a/GLP, resulted in the upregulation of TEs or pattern recognition receptors (RIG-I and MDA5) and subsequent mitochondrial antiviral signaling protein activation in acute myeloid leukemia (AML).48 By contrast, G9a and SUV39H1 are not required for KDM5B/SETDB1-mediated repression of endogenous retroelements and subsequent immune evasion in melanoma.49 Therefore, G9a/GLP-dependent H3K9me2 marks may cooperate to silence specific retroelements with other H3K9 methyltransferases such as SETDB1 and SUV39H1 in cancer cells. Taken together, H3K9 methyltransferases and their complexes serve as immunologically and evolutionarily conserved defense systems that allow cancer cells to escape from immune surveillance triggered by endogenous elements, such as ERVs or TEs.
3 THERAPEUTIC BENEFIT OF EPIGENETIC THERAPY AS A DUAL-TARGETED APPROACH
Although recent breakthroughs in cancer treatment have been made by the development of immune checkpoint blockade (ICB) therapy, clinical responses are limited.50 Given the critical involvement of cancer cell epigenetic abnormalities in the immune system in preclinical models, the modulation of epigenetic molecules potentially improves the therapeutic benefits of cancer immunotherapy and increases the proportion of patients with durable clinical responses. Among the oncogenic histone methyltransferases, EZH2 and G9a could be promising targets to induce/activate antitumor immune responses to improve the therapeutic effect of ICB therapy in malignant melanoma, ovarian cancer, and bladder cancer.34, 51-54 In addition, EZH2 inhibition potentially augments the efficacy of adoptive cell therapies targeting surface antigens expressed by cancer cells. Pharmacological inhibition of EZH2 released silencing of ganglioside synthase and induced selective upregulation of GD2 expression on Ewing sarcoma cells, thereby sensitizing the cancer cells to GD2-specific chimeric antigen receptor T cells (CAR-T cells).55 Hence, various EZH2 inhibitors with or without ICB treatment are currently being evaluated in clinical trials, although G9a inhibitors have not yet been tested (Table 1).
| Target | Inhibitor | Combination with ICB | Phase | Trial number | Cancer type | Status |
|---|---|---|---|---|---|---|
| EZH2 | Valemetostat | 2 | NCT04842877 | Relapsed/refractory B-cell lymphoma | Recruiting | |
| Tazemetostat | 2 | NCT03456726 | Relapsed or refractory B-cell non-Hodgkin's lymphoma | Completed | ||
| 2 | NCT02601950 | INI1-negative tumors or relapsed/refractory synovital sarcoma | Active, not recruiting | |||
| 1 | NCT05228158 | Relapsed or refractory follicular lymphoma | Recruiting | |||
| Pembrolizumab | 1/2 | NCT05467748 | Advanced NSCLC | Not yet recruiting | ||
| 1/2 | NCT01897571 | Advanced solid tumors or B-cell lymphoma (DLBCL) | Completed | |||
| 2 | NCT03213665 | Relapsed or refractory advanced solid tumors, non-Hodgkin lymphoma, or histiocytic disorders | Active, not recruiting | |||
| 2 | NCT02860286 | Malignant mesothelioma | Completed | |||
| 1 | NCT02601937 | Pediatric subjects with relapsed or refractory INI1-negative tumors or synovial sarcoma | Completed | |||
| 2 | NCT05467943 | Relapsed/refractory follicular lymphoma | Not yet recruiting | |||
| 2 | NCT04762160 | Relapsed/refractory follicular lymphoma | Recruiting | |||
| 1/2 | NCT04557956 | Metastatic melanoma | Recruiting | |||
| 2 | NCT04590820 | Relapsed/refractory follicular lymphoma | Terminated | |||
| 2 | NCT05023655 | Solid tumors harboring an ARID1A mutation | Recruiting | |||
| 1/2 | NCT05205252 | Relapsed/refractory hematologic malignancies | Recruiting | |||
| 1/2 | NCT04179864 | Castration-resistant prostate cancer | Recruiting | |||
| Pembrolizumab | 1/2 | NCT03854474 | Advanced urothelial carcinoma | Recruiting | ||
| 1 | NCT03028103 | B-cell lymphoma or advanced solid tumor | Completed | |||
| 2 | NCT04917042 | Peripheral nerve sheath tumors | Recruiting | |||
| 2 | NCT03155620 | Relapsed or refractory advanced solid tumors, non-Hodgkin lymphomas or histiocytic disorders | Recruiting | |||
| Unknown | NCT04225429 | Epithelioid sarcoma | No longer available | |||
| 3 | NCT04224493 | Relapsed/refractory follicular lymphoma | Recruiting | |||
| 1 | NCT03217253 | Metastatic or unresectable solid tumors or B-cell lymphomas with liver dysfunction | Withdrawn | |||
| SHR2554 | 1 | NCT03603951 | Relapsed and refractory mature lymphoid neoplasms | Recruiting | ||
| 1/2 | NCT04407741 | Advanced solid tumors and B-cell lymphomas | Recruiting | |||
| 2 | NCT04355858 | Molecularly targeted umbrella study in luminal advanced breast cancer (MULAN) | Recruiting | |||
| PF-06821497 | 1 | NCT03460977 | Relapsed/refractory SCLC, castration-resistant prostate cancer, and follicular lymphoma | Recruiting | ||
| MAK683 | 1/2 | NCT02900651 | Advanced malignancies (DLBCL) | Recruiting | ||
| GSK2816126 | 1 | NCT02082977 | Relapsed/refractory diffuse large B-cell lymphoma, transformed follicular lymphoma, other non-hodgkin's lymphoma, solid tumors and multiple myeloma | Terminated | ||
| CPI-1205 | 1 | NCT02395601 | B-cell lymphomas | Completed | ||
| 1/2 | NCT03480646 | Metastatic castration resistant prostate cancer (mCRPC) | Active, not recruiting | |||
| 1 | NCT03525795 | Advanced solid tumors | Completed | |||
| 1/2 | NCT04104776 | Advanced solid tumors and lymphomas | Recruiting | |||
| LSD1 | Tranylcypromine | 1/2 | NCT02717884 | Non-M3 AML blasts (AML, myelodysplastic syndrome) | Recruiting | |
| 1 | NCT02273102 | AML and MDS (acute myelogenous leukemia, myelodysplastic syndromes, leukemia) | Completed | |||
| 1/2 | NCT02261779 | Relapsed or refractory AML and no intensive treatment is possible | Recruiting | |||
| SP2577 (Seclidemstat) | 1 | NCT03895684 | Advanced solid tumors | Completed | ||
| 1 | NCT03600649 | Relapsed or refractory Ewing or Ewing-related sarcomas | Recruiting | |||
| Pembrolizumab | 1 | NCT04611139 | Gynecologic cancers (SCCOHT, ovarian clear cell tumor, ovarian endometrioid adenocarcinoma, Endometrial Cancer) | Withdrawn | ||
| 1/2 | NCT04734990 | Myelodysplastic syndrome or chronic myelomonocytic leukemia | Recruiting | |||
| ORY-1001 (Ladademstat) | NCT02913443 | SCLC | Completed | |||
| Unknown | AML | Unknown | ||||
| JBI-802 | 1/2 | NCT05268666 | Advanced solid tumors (locally advanced solid tumor, metastatic solid tumor) | Recruiting | ||
| INCB059872 | Pembrolizumab | 1/2 | NCT02959437 | Advanced solid tumors (solid tumors, advanced malignancies, metastatic cancer) | Terminated | |
| 1 | NCT03514407 | Relapsed or refractory Ewing sarcoma | Terminated | |||
| 1/2 | NCT02712905 | Advanced malignancies (solid tumors and hematologic malignancy) | Terminated | |||
| IMG-7289 | 1 | NCT02842827 | Advanced myeloid malignancies (AML, myelodysplastic syndrome) | Completed | ||
| GSK2879552 | 1 | NCT02177812 | AML | Terminated | ||
| 1 | NCT02034123 | Relapsed/refractory SCLC | Terminated | |||
| CC-90011 | Nivolumab | 2 | NCT04350463 | Advanced cancers (SCLC and squamous NSCLC) | Active, not recruiting | |
| 1 | NCT04628988 | Prostate cancer | Recruiting | |||
| 1 | NCT04748848 | Relapsed/refractory AML | Completed | |||
| DOT1L | EPZ-5676 (Pinometostat) | 1 | NCT02141828 | Relapsed/refractory leukemias bearing a rearrangement of the MLL gene | Completed | |
| 1/2 | NCT03701295 | Relapsed, refractory, or newly diagnosed AML with 11q23 rearrangement | Completed | |||
| 1/2 | NCT03724084 | Newly diagnosed AML and MLL gene rearrangement | Active, not recruiting | |||
- Abbreviation: AML, acute myeloid leukemia; DLBCL, diffused large B-cell lymphoma; MLL, mixed lineage leukemia; NSCLC, non-small-cell lung cancer; SCCOHT, small-cell carcinoma of the ovary, hypercalcemic type; SCLC, small-cell lung cancer.
Besides histone modifiers, another genome-wide CRISPR screening has identified the loss of ARID2, PBRM1, or BRD7, which encode distinct components of the SWI/SNF chromatin remodeler complex,56 resulting in an increase in T-cell-mediated killing via an elevation in chromatin accessibility for IFN-γ-responsive genes in the murine melanoma cell line B16-F1029 (Figure 1). The SWI/SNF complex acts as a tumor suppressor and is often targeted by loss-of-function mutations in various types of cancer.56 Thus, it is still challenging to directly target loss-of-function mutants of chromatin modulators. Continuous efforts are required to identify druggable targets in patients harboring a loss-of-function mutation in the SWI/SNF complex.57
The dedifferentiation state of cancer cells can be another factor that promotes immune evasion and acquired resistance to ICB treatment.58-60 For example, studies on malignant melanoma indicate cancer cell dedifferentiation and adoption of a stem- or progenitor-like phenotype that leads to a loss of melanocytic differentiation antigens (gp100 or Melan-A) in response to inflammatory stimuli, such as IFN-γ and TNF-α, thereby escaping from immune recognition by adoptively transferred gp100- or Melan-A-specific T cells in both preclinical animal models and clinical trials.61-63 Two factors contribute to this immune evasion: (1) a loss of differentiation-associated antigens and/or MHC molecules by potent therapeutic pressure and (2) dedifferentiation-associated epigenetic and transcriptional reprogramming, including c-Jun/AP-1-mediated induction of Ccl2 and Ccl5, that results in the recruitment of immunosuppressive myeloid cells (Figure 3).64 Mouse melanoma can resist cancer immunotherapy through inflammation-induced reprogramming into a dedifferentiated state, reminiscent of the drug-tolerant neural-crest stem cell state.65 In addition, the neural crest or NGFRhigh signatures are associated with immune exclusion (poor T-cell infiltration) and resistance to Melan A-specific T cells or anti-PD-1 antibody treatment.66 Notably, pharmacological inhibition of NGFR signaling reverts the resistant phenotype and restores the sensitivity to T-cell-mediated killing. Consistently, high scores of melanocytic plasticity signatures (MPSs), which represent undifferentiated or neural-crest-like states, are linked with resistance to ICB treatment in both preclinical murine models and clinical trials.67 Longitudinal evolutionary tracking of therapeutic resistance to ICB treatment identifies the emergence of a distinct dedifferentiated neural-crest-like (PD-L1high/NGFRhigh) melanoma population that exhibits spatial proximity to CD45+ immune cells,68 suggesting that an interaction of dedifferentiated cancer cells with certain immune cells causes an immunosuppressive TME.
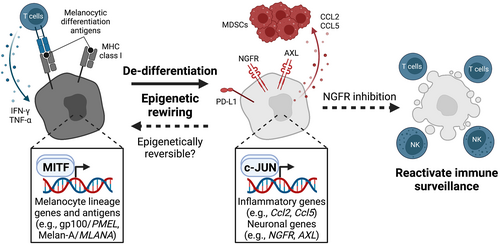
In squamous cell carcinoma, dedifferentiated cancer cells acquire stem-like properties and induce exhaustion of CD8+ T cells by enhancing CTLA-4 expression, leading to escape from T-cell-mediated immune attack.69 Dedifferentiated tumor-initiating leukemia cells can evade immune surveillance via NKG2D dysregulation.70 Single-cell analysis has revealed that lung cancer cells possess heterogeneous differentiation states that correspond to various stages of normal development or regeneration of lung epithelium, which is specified by SOX transcription factors.71 These distinct states characterized by SOX2 and SOX9 are associated with the expression of MHC class I genes and the sensitivity to killing by natural killer (NK) cells. In particular, transition to SOX9high progenitor states leads to MHC class I re-expression and escape from killing by NK cells, consequently expanding quiescent disseminated tumor cells to form macroscopic metastases.
Similarly, CAR-T-cell therapy directed against CD19 in B-cell malignancies, particularly B progenitor acute lymphoblastic leukemia (B-ALL), has shown the emergence of resistance by losing the expression of CD19 antigen via transcriptional silencing or genetic mutation.72 Alternatively, in patients with acute lymphoblastic leukemia (ALL) harboring translocation of the mixed lineage leukemia (MLL, also known as KMT2A) gene, the oncogenic MLL-fusion protein can reinstate stem cell transcription programs in committed progenitors or enable transdifferentiation from a phenotypic B-cell malignancy to a myeloid malignancy that no longer expresses CD19 antigen. Thus, in both solid tumors and hematological malignancies, potent therapeutic pressure can induce cellular plasticity that results in lineage switching, thereby facilitating immune evasion. Understanding the epigenetic events employed in phenotype switching is critical to developing therapeutic strategies that constrain the plasticity of cancer cells and prevent resistance to immunotherapy.
4 FUTURE PERSPECTIVES
The examples presented above demonstrate that intrinsic epigenetic circuits dysregulated in cancer cells affect multiple extrinsic factors that modulate the immune responses in the TME and vice versa; however, the entire picture of such complex interactions between cancer cells and immune cells remains unclear. Recent advances in single-cell technology allow us to precisely trace the evolutionary trajectories in the TME during cancer development, progression, and therapeutic interventions. DNA barcoding is used to monitor clonal dynamics of cancer progression and drug resistance by tracking individual cells (up to 1 million). Cellular barcoding technology can be applied to detect pre-existing resistant clones without any prior knowledge of the underlying molecular resistance mechanism73, 74 and is thus advantageous over other targeted detection methods such as digital PCR. Nevertheless, direct examination of the transcriptional landscape of a single cell carrying a DNA barcode is challenging because cells are destroyed during single-cell RNA-seq analysis. To overcome this issue, the DNA barcode library, called lineage and RNA recovery (LARRY), which can be read by single-cell RNA-seq, was developed for clonal tracing of transcriptomes over time.75 These strategies have been further refined by the Watermelon system, named after its mNeonGreen protein, whose 3′-untranslated region is fused to a semi-random expressed DNA barcode and doxycycline-inducible H2B-mCherry red fluorescent proteins.76 In this system, lineage tracing is achieved by mapping an individual (clone-specific) DNA barcode, whereas proliferation history is monitored by the dilution of H2B-mCherry, followed by doxycycline removal, which allows tracking of the lineage, transcriptional state, and proliferative history of individual cells. These barcoding technologies have enabled us to identify and characterize each cell lineage even in perturbed conditions of differentiation processes. Moreover, although the barcoding system has only been available to study a certain cell type to date (i.e., cancer cells, hematopoietic cells, and human pluripotent stem cells), the methodology is applicable to simultaneously trace multiple cell types when used in combination with different barcode libraries. These new techniques could pave the way for a better understanding of the co-evolution of cancer cells and immune cells in the TME, thereby discovering novel therapeutic strategies to further improve clinical outcomes.
5 CONCLUSIONS
This review summarizes the epigenetic aberrations that drive cancer development and evasion from the immune system: EZH2/PRC2- or G9a-mediated epigenetic rewiring reprograms oncogenic (intrinsic) and immunological (extrinsic) phenotypes in cancers. While various druggable epigenetic abnormalities are being evaluated in pre-clinical models and clinical trials, none of them are successfully translated into the clinic. This failure may partially be attributed to the difficulty of fully capturing the interplay using only snapshot data (even with a single-cell analysis) during cancer development and progression. Much deeper insight into the spatiotemporal dynamics of epigenetic machinery is required to understand functional links between dynamic epigenetic changes in cancer cells and immune responses in the TME. To illustrate common or cancer-type-specific epigenetic events that may predispose to therapeutic failure, DNA barcode technology that integrates lineage tracing and epigenetic and transcriptomic landscapes are required to pursue the fate of the individual clone during the treatment, including cancer immunotherapy. In addition, as cancer cell plasticity and dedifferentiation epigenetically regulate or drive immune evasion,63, 77 in vitro and in vivo CRISPR screens focusing on tissue-specific and differentiation states are required to identify druggable epigenetic molecules. Altogether, novel combinatorial therapeutic strategies need to be developed to modulate immune evasion and tumor evolutionary trajectories.
FUNDING INFORMATION
This work was supported by JSPS KAKENHI Grants-in-Aid for the Scientific Research (A) grant no. 22H00455 (to H.N.), Promotion of Joint International Research [Fostering Joint International Research (B)] grant no. JP20KK0184, Scientific Research (B) grant no. JP20H03512, Challenging Research (Exploratory) grant no. JP20K21542, Challenging Research (Pioneering) grant no. JP22K18381 (to K.H.), and Scientific Research (C) grant no. JP22K07148, Young Scientists grant no. JP20K16301, and Scientific Research on Innovative Areas grant no. JP21H0421 (to S.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Projects for Cancer Research by Therapeutic Evolution [P-CREATE, no. JP20cm0106166h0002 (to K.H.), and no. JP20cm0106703h0001 (to S.K.)] and Projects for Promotion of Cancer Research and Therapeutic Evolution [P-PROMOTE, no. JP22ama221301h0001 (to H.N.), no. JP22cm0106186h0002 (to K.H.), and no. JP22ama221110h0001 (to S.K.)], the Practical Research for Innovative Cancer Control [22ck0106772h0001 (to S.K.) and no. 22ck0106724h0001 (to H.N.)], and the Development of Technology for Patient Stratification Biomarker Discovery grant [no. 19ae0101074s0401 (to H.N.)] from the Japan Agency for Medical Research and Development (AMED).
CONFLICT OF INTEREST
S.K. received funds from the Takeda Science Foundation. Y.M., K.W., and H.N. are co-founders of Sustainable Cell Therapeutics. D.S. received research funding and scholarship from the Hori Sciences and Arts Foundation. H.N. received research funding and honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Chugai Pharmaceutical, and MSD, and from Taiho Pharmaceutical, Daiichi-Sankyo, Kyowa Kirin, Zenyaku Kogyo, Oncolys BioPharma, Debiopharma, Asahi-Kasei, Sysmex, Fujifilm, SRL, Astellas Pharmaceutical, Sumitomo Dainippon Pharma, and BD Japan outside of this study. H.N. is a current Editorial Board Member of Cancer Science. K.H. received funds from the Takeda Science Foundation, the MSD Life Science Foundation, and the Life Science Foundation of Japan.
ETHICS STATEMENTS
Approval of the research protocol by an institutional reviewer board: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.




