Probiotics enhances anti-tumor immune response induced by gemcitabine plus cisplatin chemotherapy for urothelial cancer
Abstract
Chemotherapy drugs, such as gemcitabine and cisplatin (GC), are frequently administered to patients with advanced urothelial carcinoma, however the influence of the gut microbiota on their action is unclear. Thus, we investigated the effects of GC on the gut microbiome and determined whether oral supplementation with a probiotics mixture of Lactobacillus casei Shirota and Bifidobacterium breve enhanced the anti-tumor immune response. After subcutaneous inoculation with MBT2 murine bladder cancer cells, syngenic C3H mice were randomly allocated into eight groups. The gut microbiome cluster pattern was altered in both the GC and oral probiotics groups (p = 0.025). Both tumor-bearing conditions (no treatment) and GC chemotherapy influenced Pseudoclostridium, Robinsoniella, Merdimonas, and Phocea in the gut. Furthermore, comparison of the GC-treated and GC + probiotics groups revealed an association of four methyltransferase family enzymes and two short-change fatty acid-related enzymes with oral probiotics use. A significant difference in tumor volume was observed between the GC and GC + probiotics groups at week 2 of treatment. Additionally, decreased recruitment of cancer-associated fibroblasts and regulatory T cells, and activation of CD8+ T cells and dendritic cells were observed in the tumor microenvironment. Our findings reveal the positive effects of a probiotics mixture of Lactobacillus and Bifidobacterium in enhancing anti-tumor effects through the gut–tumor immune response axis. Future clinical trials are needed to evaluate the full benefits of this novel supplement with oral probiotics in patients with advanced urothelial carcinoma.
Abbreviations
-
- 1L
-
- the first-line
-
- aUC
-
- advanced urothelial carcinoma
-
- BY
-
- Bifidobacterium breve strain Yakult
-
- CAF
-
- cancer-associated fibroblast
-
- CFU
-
- colony-forming unit
-
- DC
-
- dendric cell
-
- FCM
-
- flow cytometry
-
- GC
-
- gemcitabine plus cisplatin combination therapy
-
- ICI
-
- immune checkpoint inhibitor
-
- LcS
-
- Lactobacillus casei strain Shirota YIT 9029
-
- PCoA
-
- principal coordinate analysis
-
- SCFA
-
- short-chain fatty acid
1 INTRODUCTION
Advanced, unresectable, and metastatic UC (aUC) is commonly aggressive and refractory to treatment, with a poor prognosis.1-3 Platinum-based drugs, including gemcitabine plus cisplatin (GC) and gemcitabine plus carboplatin, are the first-line chemotherapeutics for aUC.4 First-line chemotherapy is frequently followed by immunotherapy using ICIs such as pembrolizumab and avelumab.5, 6 There is substantial evidence that the first-line chemotherapy responders have an increased response to ICIs compared with the first-line chemotherapy nonresponders.1, 7 Given that ICI responders have significantly improved survival than nonresponders,1, 5 achieving a high response to the first-line chemotherapy is essential to provide favorable outcomes in patients with aUC.
A report from clinical trials IMvigor210 and IMvigor211 demonstrated that concomitant antibiotic use was associated with worse survival in patients with aUC treated with atezolizumab.8 A recent meta-analysis involving 44 cohorts found a negative association of antibiotic use with objective response, progression-free survival, and overall survival in malignancies treated with ICIs.9 Moreover, a systematic evaluation revealed the undervalued effects of the concomitant use of proton pump inhibitors in patients with urothelial cancer (UC) treated with ICIs.10 Accumulating evidence suggests a strong relationship between the gut microbiome and cancer immunotherapy. In contrast, few studies have investigated the relationship between the microbiota and the administration of chemotherapy drugs, such as GC. A preclinical study showed that an SCFA butylate, which is a known postbiotic of intestinal bacteria, can enhance the tumor response to gemcitabine in pancreatic cancer cells.11
Meta-analyses and systematic reviews have revealed that gut dysbiosis is closely related to human health, chemotherapy-related side effects, and surgery-related complications and diseases.12-14 In this study, we investigated the effects of systemic chemotherapy with GC on the gut microbiome. We hypothesized a potential link between chemotherapy-induced gut dysbiosis and decreased anti-tumor immunity, driving us to explore novel interventions for targeted microbiome modulation by supplementing with probiotics, prebiotics, and synbiotics to retain anti-tumor immunity. Herein, we evaluate whether supplementation with a probiotics mixture of LcS and BY could enhance the anti-tumor host immune response in a tumor-bearing syngenic rodent model.
2 MATERIALS AND METHODS
2.1 Drugs and probiotics
Gemcitabine and cisplatin (Tokyo Chemical Industry) were dissolved in sterile water and N,N-dimethylformamide (Nacalai Tesque), respectively. A probiotics mixture consisting of LcS (≥108 CFU/g) and BY (≥108 CFU/g) was purchased from Yakult Honsha Co., Ltd. The probiotics powder was resuspended in sterile water to a final concentration of 0.5 g/ml prior to oral administration.
2.2 Animal experiments
Animal experiments were conducted in compliance with institutional guidelines and regulations after approval from the Committee on Animal Research at Nara Medical University (approval study ID:13068). Specific pathogen-free 5-week-old male C3H mice were purchased from Oriental BioService Inc. Animals were provided ad libitum access to water and chow (Rodent LabDiet Equation 5 L37; Japan SLC, Inc.). The experimental design, treatment, tissue collection, and analytical methods are shown in Figure 1. To prepare tumor-bearing C3H mice, the right flank area of chloral hydrate-anesthetized mice was shaved, followed by subcutaneous inoculation of 2 × 105 MBT2 cells/100 μl in growth factor-reduced Matrigel (BD Biosciences). The six nontumor-bearing mice were allocated randomly into two groups, as shown in Figure 1 (n = 3, each): Group I, nontreated control; and Group II, oral administration of a probiotics mixture (0.1 g twice a day consisting of ≥107 CFU of LcS and ≥107 CFU of BY). When the longest diameter of the tumor reached ≥5 mm, 36 tumor-bearing mice were allocated randomly into six groups (n = 6, each): Group III, nontreated control; Group IV, intraperitoneal injection of gemcitabine (20 mg/kg, three times per week); Group V, intraperitoneal injection of cisplatin (2 mg/kg, three times per week); Group VI, intraperitoneal injection of GC; Group VII, oral administration of a probiotics mixture; and Group VIII, GC plus a probiotics mixture. The mice were euthanized on the day of completion of the 2-week treatment. The samples were collected and preserved for subsequent analysis.
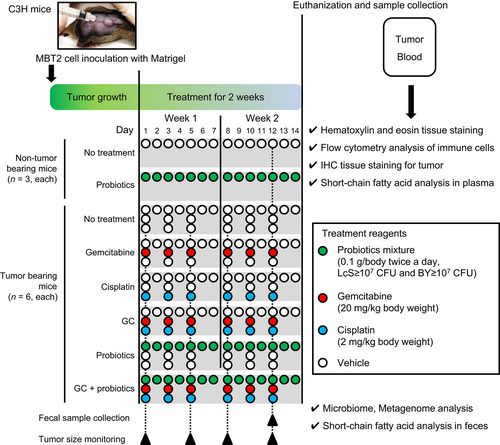
2.3 Bacterial 16S rRNA amplicon sequencing and analysis of fecal samples
A previous study detected Lactobacillus casei in the cecum at 0.5, 3.5, 12, and 24 h after the oral administration of probiotics mixture, with the highest concentration of Lactobacillus casei observed at 3.5 h.15 Based on the data, fecal samples for the analysis of the gut microbiome and SCFAs were collected approximately 4 h after oral administration of the probiotics mixture on day 5 of treatment. 16S rRNA amplicon sequencing was performed at TechnoSuruga Laboratory, Inc. (Shizuoka, Japan). Briefly, bacterial genomic DNA was isolated using a Precellys Evolution homogenizer (Bertin Instruments) and GENE PREP STAR PI-480/NR-201 (Kurabo Industries). The V3–V4 hypervariable regions of 16S rRNA were amplified from the microbial genomic DNA by PCR using universal bacterial primers (341F/R805)16 and the dual-index method.17 All amplicons were sequenced using a MiSeq (Illumina). The read sequences obtained on both sides were joined using fastq-join. The sequences with a quality value of more than 20, with 99% or more bases were extracted using the FASTX-Toolkit following which, the chimeric sequences were removed using QIIME1.8.0 or QIIME2 version 2020.6. Bacterial identification from sequences was performed using the Microbial Identification database NGS-DB-BA 16.0 (TechnoSuruga Laboratory) and Ribosomal Database Project Classifier ver. 2.13. Comparative analyses were performed using the Metagenome@KIN analysis software (World Fusion).
2.4 Predictive metabolic function analysis of fecal samples
PICRUSt ver. 2.3.0-b was used to predict gut microbial metabolic functions based on the 16S rDNA communities. The reference sequence of the Integrated Microbial Genomes database was aligned using HMMER ver. 3.3. The 16S rRNA gene copy numbers were normalized using the caster package ver. 1.6.2 of R ver. 3.6.3. Gene families were predicted based on the database of Clusters of Orthologous Genes and Enzyme Classification numbers. Biological pathways were reconstructed based on the predicted gene families using MinPath ver. 1.4. Gene Ontology analysis was conducted using STAMP (Statistical Analysis of Metagenomic Profiles) software with metagenomic data, allowing comparison of treatment groups.
2.5 Measurement of the SCFAs in fecal and plasma samples
SCFAs are the primary metabolites produced through bacterial fermentation of dietary fiber in the gastrointestinal tract, mainly in the colon.18 The concentrations of nine SCFAs, including succinic acid, lactic acid, formic acid, acetic acid, propionic acid, n-butyric acid, iso-butyric acid, n-valeric acid, and iso-valeric acid, in fresh fecal samples were measured using high-performance liquid chromatography (Shimadzu) at the TechnoSuruga Laboratory. The contents were expressed in mg/g.
Blood was collected by cardiac puncture, and plasma was preserved at −80°C for SCFA analysis. The concentrations of four SCFAs, including acetic acid, propionic acid, butyric acid, and 3-hydroxy butyric acid, in plasma samples, were measured by LCMS-8050 LC–MS/MS (Shimadzu) at Kyushu Pro Search LLP. Concentrations were expressed as μg/ml.
2.6 Immunohistochemical staining (IHC) analysis of resected tumors
At least three vertically cut segments of the fixed tumor were separately embedded in a paraffin section and stained with H&E to evaluate lymphocytic infiltration into the tumor. Immunohistochemistry (IHC) staining using paraffin-embedded, formalin-fixed mouse tumor tissues was performed as previously described.19 Antibodies, staining conditions, and quantification of αSMA (clone 1A4, Sigma-Aldrich) and cleaved caspase-3 (clone 5A1E, Cell Signaling Technology) have been reported in previous studies.19, 20 The number of cleaved caspase-3-positive cells was counted to determine the apoptotic index (% of the total counted cancer cells). The immunoreactivity of αSMA at the tumor border was scored based on the number of αSMA-positive spindle cells (CAFs) as follows: 0, almost no CAFs or a small number of CAFs surrounding less than half of the border; 1, a moderate number of CAFs surrounding less than half of the border; or 2, a large number of CAFs surrounding more than half of the border.20 H&E-stained and IHC-stained sections were examined, and at least five views for each slide were captured at ×200 magnification using a light microscope (EVOS® FL Auto Cell Imaging System, Thermo Fisher Scientific). All sections were reviewed by two experienced investigators (Yuki Oda and Tomomi Fujii) who were blinded to the treatment regimens. The average score was calculated for each treatment group.
2.7 Flow cytometry analysis of resected tumors
Tumors were minced in MACS buffer containing 0.5% BSA and 2 mM EDTA in PBS immediately after resection. Minced tumors were transferred to gentleMACS™ C tubes (Miltenyi Biotec, Germany) and subjected to gentleMACS™ Dissociator (130-093-235; Miltenyi Biotec) to obtain a homogenate. The homogenates were then pushed through Corning™ Cell Strainers (BD Biosciences) to obtain a uniform single-cell suspension. The cells were washed using a washing solution and stained with surface antibodies. The antibodies used in FCM analysis are summarized in Table S1. Intracellular staining of Foxp3, granzyme B, and Ki-67 was performed with monoclonal antibodies against Foxp3 (1:50 dilution), granzyme B (1:50 dilution), Ki67 (1:50 dilution), and Foxp3/Transcription Factor Staining Buffer set (Thermo Fisher Scientific), according to the manufacturer's instructions. After washing, the cells were analyzed using an LSRFortessa or FACSymphony system (BD Biosciences) and FACSDiva (v8.0.1, BD Biosciences), and FlowJo (TreeStar) software. The gating strategy of CD8+ T cells and FoxP3+ regulatory T cells (Tregs) and that of CD45+CD11b+F4/80−CD11c+MHC class II+ antigen-presenting cells (DC) are shown in Figure S1.
2.8 Statistical analysis
PRISM version 9.3.1 (GraphPad Software, Inc.) was used for statistical analysis and for generating graphs. Statistical significance was set at p < 0.05. Most continuous variables are presented as mean ± standard deviation compared using the Kruskal–Wallis test, followed by Dunn's posthoc test. No data set obtained in this study met the prerequisites for parametric tests. Statistical significance was set at p < 0.05.
3 RESULTS
3.1 Analysis of gut microbiota diversity
The tumor and chemotherapy-induced significant changes in the gut microbiota composition (Figure 2). Previous studies have demonstrated that gut dysbiosis was observed even in immunocompetent mouse models of subcutaneous tumors21 and models of liver metastasis in colon cancer cells (CT26).22
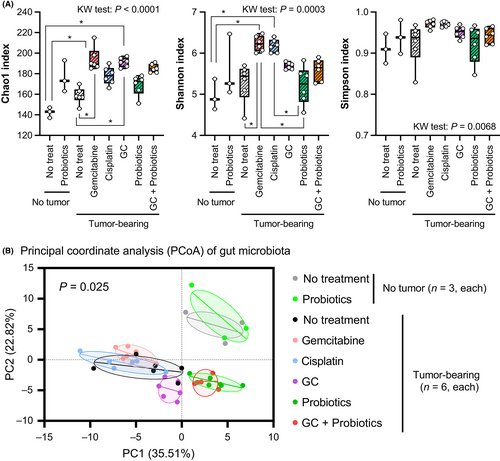
Chao1, Shannon, and Simpson indices were used to evaluate the alpha-diversity of the gut microbiota (Figure 2A). Generally, a higher Chao1 and Shannon index, and a lower Simpson index indicate greater microbial diversity.23 The results of the Chao1 index indicated that tumor-bearing conditions and systemic chemotherapy could promote the richness and diversity of gut microbiota. The Shannon index showed similar results, demonstrating greater gut microbial diversity in tumor-bearing hosts and following systemic chemotherapy, whereas there was no difference in the Simpson index. Principal coordinate analysis was used to evaluate the influence of chemotherapy and probiotics treatment on the composition of the gut microbiota (Figure 2B). No obvious change in the cluster pattern was observed between the nontreated control mice without tumors and the probiotics-treated mice without tumors. The tumor-bearing condition was associated with a cluster pattern distinct from that of the nontumor-bearing condition. In tumor-bearing mice, a combination of GC altered the gut microbiome cluster pattern, whereas monotherapy with gemcitabine or cisplatin did not. Furthermore, probiotics intake in tumor-bearing mice changed the cluster pattern in the PCoA score plots.
3.2 Changes in gut microbiota composition
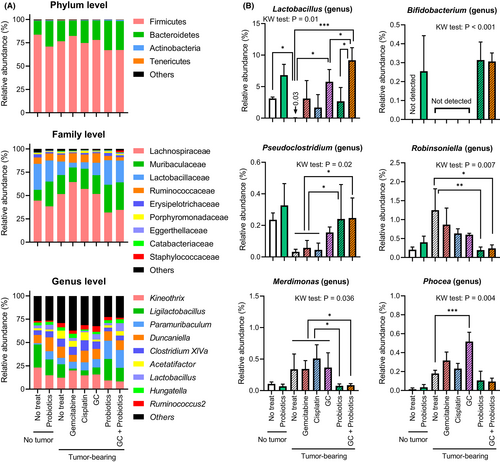
The proportions of bacterial phyla, families, and genera represented in the gut microbiota are expressed as % relative abundance (Figure 3A). There was no significant difference in the Firmicutes-to-Bacteroidetes ratio at the phylum level among the eight treatment groups (p = 0.19, Kruskal–Wallis test, data not shown). However, the dominant bacterial genera differed among the treatment groups. A comparison of six representative bacteria is shown in Figure 3B. As expected, intake of the probiotics mixture was associated with an elevated abundance of Lactobacillus and Bifidobacterium. Bifidobacterium was not detected in mice that did not receive probiotics treatment. Moreover, both tumor-bearing conditions and systemic chemotherapy were shown to directly alter the composition of the gut microbiota, such as Pseudoclostridium, Robinsoniella, Merdimonas, and Phocea. Decreased levels of Pseudoclostridium and increased levels of the other three bacterial species were observed under tumor-bearing conditions, and these effects were attenuated by probiotics intake.
3.3 Predicted metabolic function of the gut microbiota
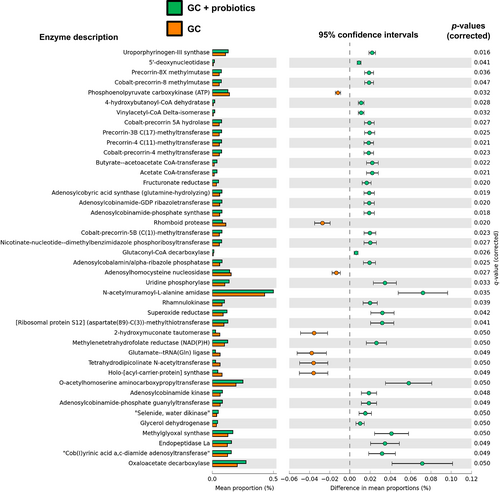
To investigate the metabolic characteristics of gut microbiota under the oral intake of the probiotics mixture, metabolic function analysis using PICRUSt2 was predicted and compared between the GC alone group and the GC plus probiotics group in tumor-bearing mice. Among the 1418 genes assessed, 42 (2.9%) showed p-values ≤0.05, including 35 genes that were upregulated and seven genes which were downregulated, following supplementation with oral probiotics (Figure 4). The results revealed methyltransferase family of enzymes was involved in probiotics mixture intervention, such as precorrin-3B C(17)-methyltransferase, precorrin-4 C(17)-methyltransferase, cobalt-precorrin-4-methyltransferase, and cobalt-precorrin-5B C(1)-methyltransferase. Two enzymes related to SCFAs (EC:2.8.3.8, acetate CoA-transferase, and EC:2.8.3.9, butyrate-acetoacetate CoA-transferase) were upregulated in the GC plus probiotics group.
3.4 The changes in short-chain fatty acids in feces and plasma
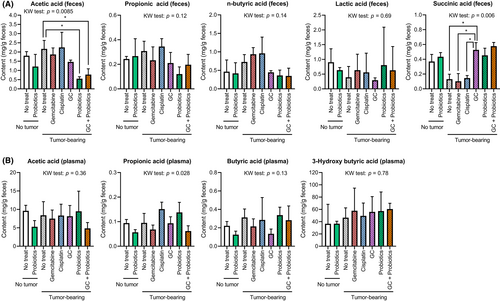
Given that SCFAs are vital mediators of the immune system, the concentrations of nine SCFAs in feces (Figure 5A) and four SCFAs in plasma (Figure 5B) were measured to investigate whether each treatment and condition could affect SCFA composition. Significant differences in fecal concentrations of acetic acid and succinic acid and plasma concentrations of propionic acid were observed between the treatment groups. Specifically, decreased fecal acetic acid levels and increased plasma succinic acid levels were observed in mice treated with probiotics and/or GC chemotherapy. Our findings suggested that chemotherapy can affect the gut microbiota, through the production of SCFAs.
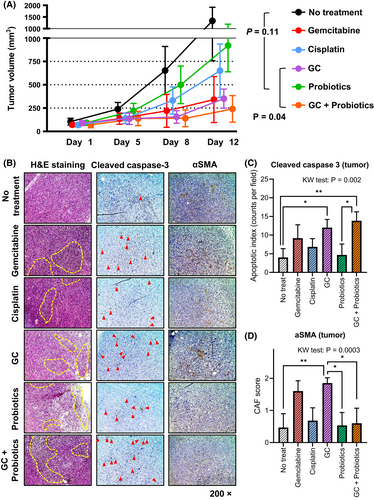
3.5 The anti-tumor effect and modulation of tumor microenvironment by chemotherapy and probiotics
As depicted in Figure 1, tumor volumes were monitored on days 1, 5, 8, and 12 during treatment. There was no difference in the tumor volume on day 5; however, on days 8 and 12, a significant difference was observed among the treatment groups (Figure 5A). Tumor volumes (mm3, mean ± SD) of the nontreated, probiotics alone, GC, and GC plus probiotics group were 1338 ± 580, 927 ± 282, 355 ± 103, and 244 ± 142, respectively. There was no significant difference in tumor volumes between the nontreated group and the probiotics alone group (p = 0.11), whereas there was a significant difference between the GC and GC plus probiotics groups (p = 0.04). Microscopic observation of the H&E-stained images revealed that chemotherapy alone, probiotics alone, and their combination led to the recruitment of tumor-infiltrating immune cells (Figure 5B). Cleaved caspase-3 staining analysis showed a significantly higher number of apoptotic tumor cells in the GC and GC + probiotics groups than in the untreated group. Additionally, αSMA staining analysis demonstrated that GC chemotherapy-induced CAF was attenuated by probiotics.
3.6 FCM analysis of the tumor immune cells
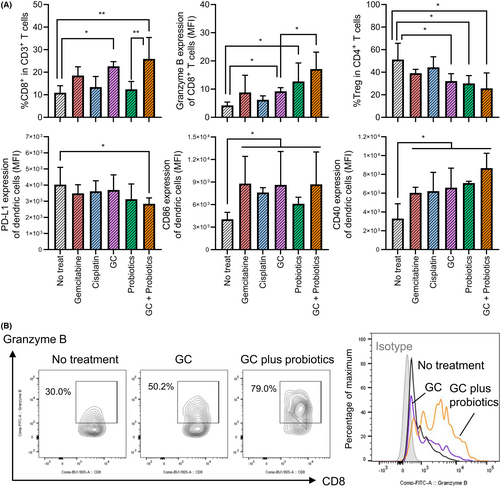
We investigated the immunological landscape of the treated tumors using FCM analysis (Figure 7). GC-treated tumors and GC + probiotics-treated tumors showed significantly higher CD8+ T cell infiltration levels than in the nontreated tumor groups. Notably, when compared with nontreated tumors, granzyme B expression in CD8+ T cells was significantly elevated in probiotics-treated and GC + probiotics-treated tumors, but not in GC-treated tumors (Figure 7B). In addition, when compared with the nontreated tumors, PD-L1 expression in tumor-infiltrating DC was upregulated, whereas the expression of co-stimulatory factors, CD86 and CD40, was downregulated in the tumor-infiltrating DC of the GC + probiotics-treated tumors. Together, GC chemotherapy induced an innate anti-tumor immune response via the activation of antigen-presenting cells and cytotoxic T cells, which was enhanced by supplementation with oral probiotics.
4 DISCUSSION
There is evidence of the association between gut microbiota and cancer initiation, progression, treatment response, and side effects.11-14, 24, 25 The gut microbiota modulates the host immune system, interacts with host metabolism, and produces toxins and metabolites which influence the tumor microenvironment.26 However, few studies have investigated the effects of intravenous administration of chemotherapy drugs on the gut microbiota. In this study, we investigated the change in microbiota following GC chemotherapy and supplementation with a probiotics mixture of LcS and BY in an immunocompetent mouse tumor model. A previous study11 demonstrated that gemcitabine monotherapy elevated the α-diversity of the gut microbiota, which is consistent with our findings (Figure 2A). Additionally, we found that both cisplatin monotherapy and GC combination therapy affected the diversity of the gut microbiota. Interestingly, α-diversity analysis on the PCoA plot demonstrated a clear distinction from nontreated tumors only in the GC combination, but not in the gemcitabine or cisplatin monotherapy; these findings are not consistent with that of a previous report on pancreatic cancer cells.11 This discrepancy could be due to differences in the dose of gemcitabine administered (50 mg/kg gemcitabine intraperitoneally once a week vs. 20 mg/kg gemcitabine intraperitoneally three times a week) and the type of cancer cells (pancreatic cancer vs. UC) being studied.
A comprehensive review of human, animal, and in vitro studies illustrated that probiotics intervention can exert anti-tumor effects through cancer cell apoptosis and immunomodulatory, anti-angiogenesis, and antimetastatic activities.27 The favorable role of probiotics includes attenuation of chemotherapy-induced side effects. Lactobacillus casei strains, such as CRL 431 and Shirota, increase host cellular immunity (such as NK cells, macrophages, and CD8+ T cells) and the production of cytokines (such as TNF-α, IFN-γ, and interleukins) as a result of alterations in the intestinal microflora.28 In particular, a prospective, randomized, controlled trial concluded that oral Lactobacillus casei prevents bladder cancer recurrence after transurethral resection of nonmuscle invasive bladder UC.29 More evidence exists on the therapeutic potential of the Bifidobacterium strain, however that of the Lactobacillus strain has been lesser studied. Li et al. found that oral Bifidobacterium breve inhibited tumor proliferation and induced cancer apoptosis in murine squamous cell carcinoma cells.30 This finding was dependent on the recruitment of tumor-infiltrating lymphocytes, DCs in the tumor microenvironment and intestinal villi, and the DC-related chemokines CCL20 and interleukin 12. Yoon et al. demonstrated that Bifidobacterium breve boosted the anti-tumor effect of systemic cancer treatments of colorectal cancer, including PD-1 inhibitors and oxaliplatin, through augmented lymphocyte-mediated anti-cancer immunity.31 A previous study showed that oral administration of a mixture of Lactobacillus reuteri and Bifidobacterium breve downregulated the expression of pivotal oncogenes HER-2 and PTGS-2 in a mouse model of colorectal cancer.32 This translational and clinical evidence had a great impact, driving us to hypothesize that the probiotics mixture of LcS and BY could enhance anti-tumor immunity in a metastatic UC model.
In our experimental design, probiotics monotherapy did not have a significant anti-tumor effect (Figure 6A, p = 0.11) but augmented the anti-tumor effect of probiotics supplementation (GC vs. GC + probiotics: p = 0.04). Chemotherapy alone or in combination with probiotics had a substantial impact on the immune-modulating effect by recruiting tumor-infiltrating immune cells (Figure 6B). IHC staining of tumors suggested that the augmentation was involved in attenuating the recruitment of CAF to the tumor microenvironment. Previous studies have shown that CAF plays a key role in disease progression and cisplatin resistance in UC, predominantly via a paracrine mechanism.20, 33 Moreover, the percentage of CD8 in CD3+ T cells, granzyme B expression of CD8+ T cells, and CD40 expression of DCs were highest in the GC + probiotics-treated tumors, whereas the percentages of Treg in CD4+ T cells and PD-L1 expression in DCs were the lowest (Figure 7A). Clinical research on patients with rectal cancer undergoing neoadjuvant radio-chemotherapy demonstrated that induction of CD8+/granzyme B+ cytotoxic T cells to the tumor stroma contributed to local tumor control and better clinical outcomes.34 Our results strongly support previous evidence that supplementation with probiotics could activate antigen-presenting cells (DCs) and recruit cytotoxic T cells, resulting in enhanced anti-tumor effects.
Tumor-bearing conditions, systemic chemotherapy, and oral probiotics influence the host gut microbiome composition. The genus Lactobacillus decreased in the nontreated tumor-bearing mice, whereas both chemotherapy and probiotics restored or increased the abundance of Lactobacillus (Figure 3B). No Bifidobacterium strains were present in the gut of SPF mice fed at our animal facility. Four microorganisms in the phylum Firmicutes, including Pseudoclostridium, Robinsoniella, Merdimonas, and Phocea, were significantly different among the six treatment groups. The abundance of Pseudoclostridium was decreased in nontreated tumor-bearing mice, as well as in mice treated with only gemcitabine or cisplatin, but was restored by oral probiotics. Pseudoclostridium species have been reported to ferment sugar to succinic acid,35 which explains the association between the abundance of gut Pseudoclostridium and the high concentration of succinic acid in the fecal samples analyzed in our study (Figure 5A). SCFAs are known to be key signal mediators in the gut–tumor microenvironment axis. A recent publication reported that supplementation with sodium butyrate (butyric acid) can beneficially modulate the gut microbiome and enhance the host immune response to malignant tumors.11, 22 Our results did not show any changes in the fecal or plasma concentrations of butyric acid (Figure 5). A previous review reported that Lactobacillus and Bifidobacterium strains do not produce butyric acid in the human gastrointestinal tract.36 In contrast, the remaining three bacteria showed a tendency to increase in nontreated and treated-tumor-bearing mice that did not receive oral probiotics (Figure 3B). One strain of Robinsoniella was identified in 2009 as a Gram-positive, spore-forming, anaerobic rod that was originally isolated from swine manure and can cause lethal septic shock in patients with cancer.37 To date, there is limited or no evidence regarding the association between cancer progression and treatment with Robinsoniella, Merdimonas, and Phocea species.
As predicted by PICRust2 analysis based on profiles of microbiota composition, a comparison of fecal samples between the GC-treated group and GC + probiotics-treated group revealed the involvement of many methyltransferase family enzymes in the probiotics mixture intervention (Figure 4). Methyltransferases are a large group of enzymes that methylate their substrates in the epigenome, but can be split into several subclasses depending on their structural features. Recent studies have demonstrated that dietary bioactive compounds target the enzymes associated with epigenetic gene regulation, including DNA methyltransferases, histone acetyltransferases, deacetylases, and demethylases.38 Moreover, two SCFA-related enzymes were identified in genes upregulated by supplementary probiotics. Acetate CoA-transferase (EC 2.8.3.8) catalyzes the chemical reaction acyl-CoA + acetate ⇌ a fatty acid anion + acetyl-CoA,39 and butyrate-acetoacetate CoA-transferase (EC 2.8.3.9) catalyzes the chemical reaction butanoyl-CoA + acetoacetate ⇌ butanoate + acetoacetyl-CoA.40 Gut bacteria produced intestinal SCFAs including acetate and butanoate, derivatives of acetic acid and butanoic acid. The intestinal SCFAs can be secreted into blood and became circulating SCFAs, which are more directly linked to the improvement or deterioration of metabolic health and systemic immune function. Significant changes in feces SCFAs and plasma SCFAs were observed in some of analyses. This evidence supports the idea that oral supplementation with probiotics affects pathological and metabolic microbial changes induced by GC chemotherapy in mice with UC.
This study had several limitations. First, the dosage and treatment duration of the chemotherapeutic drug and probiotics mixture was fixed and may have influenced the results obtained. Second, we did not analyze immune cells in the spleen, blood, and mesenteric lymph nodes, which may have provided important data on systemic host immunity. Third, our study did not evaluate the expression of immune checkpoint molecules in tumors. Fourth, we did not assess the potential attenuating effects of probiotics against chemotherapy-induced side effects, such as nausea, pancytopenia, diarrhea, constipation, and renal injury. However, there was no significant difference in body weight between the eight groups during treatment and monitoring (data not shown). Fifth, we could not evaluate the difference in the interaction between gemcitabine + probiotics and that between cisplatin + probiotics because our experimental design did not include those treatment groups. In the current clinical setting, gemcitabine monotherapy and cisplatin monotherapy are not commonly used as a systemic treatment for patients with advanced or metastatic UC. Another future experiment might be needed to unveil the difference in those interactions.
This is the first study to provide evidence of the positive effects of a probiotics mixture of LcS and BY in enhancing the anti-tumor effect of chemotherapeutic agents via the gut–immune response axis. In addition, this study highlighted the effects of the tumor and systemic GC chemotherapy on gut microbiota. Furthermore, shotgun metagenomic analyses of the intestinal mucosa, feces, and plasma-associated microbiome would help to determine potential predictive markers of treatment response. Further clinical trials are needed to evaluate the complete benefits of this novel oral probiotics supplement in conjunction with cancer chemotherapy.
AUTHOR CONTRIBUTIONS
Conceptualization, M.M. and K.F.; data curation, Y.O and S.O.; formal analysis, T.O., K.I., T.F., N.N., T.M., and S.O.; funding acquisition, M.M and K.F.; investigation, T.S., K.O., S.H. and Y.M.; methodology, T.O., K.I., D.G. and K.T.; supervision, N.T. and K.F.; validation, Y.N.; writing – original draft, M.M.; writing – review and editing, K.F.
ACKNOWLEDGMENTS
We would like to thank the TechnoSuruga Laboratory (Shizuoka, Japan) for supporting data analysis. We would like to thank Editage (www.editage.com) for English language editing.
FUNDING INFORMATION
This research was supported in part by the Japanese Urological Association Young Researcher Promotion Grant 2019 (M.M.), JSPS KAKENHI Grant Numbers 16K20159 (M.M.) and 26861290 (K.F.), and Fiscal Years 2015–2016 Nara Medical University Grant-in-Aid for the Collaborative Research Projects (K.F. and M.M.).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A
Animal Studies: The Registration No. 13068.
Open Research
DATA AVAILABILITY
The data generated in this study are available within the article and its supplementary data files.




