HDAC5, negatively regulated by miR-148a-3p, promotes colon cancer cell migration
Chunli OuYang and Guang Shu contributed equally to this work.
Abstract
Histone deacetylases (HDACs) are involved in many processes including tumor cell growth and proliferation and regulation of gene expression. To clarify the role of class IIa HDACs in the metastasis of colon adenocarcinoma, we used the class IIa HDAC inhibitor TMP269 and found that it effectively inhibited the migration ability of colon adenocarcinoma cells. Next, we silenced the member of class IIa HDACs and confirmed that the migratory ability of colon adenocarcinoma cells was significantly inhibited by silencing HDAC5 or HDAC7. HDAC5 plays a variety of roles in human cancers. Here, we examined the role of HDAC5 in colon adenocarcinoma. The results indicated that HDAC5 was highly expressed in tumor tissues and negatively correlated with the expression of miR-148a-3p. Moreover, the expression of HDAC5 was correlated with tumor progression. HDAC5 markedly increased the invasion and migration of cancer cells in vitro, an effect that could be inhibited by overexpression of miR-148a-3p. Following an intraperitoneal injection of colon adenocarcinoma cells in athymic nude mice, HDAC5 promoted tumor implant. Together, these findings showed that HDAC5 overexpression in colon adenocarcinoma is consistent with tumor progression and tumor cell migration and the impact of HDAC5 overexpression is reduced by miR-148a-3p.
Abbreviations
-
- 3’UTR
-
- three prime untranslated region
-
- CDS
-
- coding sequence
-
- CRC
-
- colorectal cancer
-
- EMT
-
- epithelial-mesenchymal transition
-
- HDACs
-
- histone deacetylases
-
- IHC
-
- immunohistochemistry
-
- miRNA
-
- microRNA
-
- NLS
-
- nuclear localization signal
-
- siRNA
-
- short interfering RNA
-
- TCGA
-
- The Cancer Genome Atlas
1 INTRODUCTION
Colon adenocarcinoma is one of the most common gastrointestinal cancers worldwide, and both its incidence rates and mortality rates are continuously increasing.1 Although improvements in the surgical procedure have noticeably increased the 5-year survival rate of patients with colon adenocarcinoma, the mortality rates were still high due to metastasis or recurrence.2-4 Therefore, further explorations of the tumor metastasis mechanism are required to develop a more focused therapeutic strategy for colon adenocarcinoma.
Histone deacetylases are a class of enzymes that deacetylate the lysine side chain of histones and restore a positive charge, which subsequently condense or close local chromatin structures and silence local genetic transcription.5-7 HDAC5 belongs to the class IIa HDAC family, which contains a conserved deacetylation activation domain at its carboxyl terminus and a protein-binding region together with phosphorylation sites at its amino terminus.6, 8 HDAC5 predominantly mediates histone deacetylation, indicating that HDAC5 plays a vital role in growth and development. According to an increasing number of studies, HDAC5 is an oncogene in many malignant tumors, such as breast cancer, neuroblastoma, and lung cancer.9 However, the particular functions of HDAC5 in colon adenocarcinoma remain unclear.
MicroRNAs (miRNAs), a class of single-stranded RNAs with an average length of 19-25 nucleotides, have been a major focus of the field of cancer biology in the past 10 years. They directly bind to the 3’ untranslated region (3’UTR) of targeted messenger RNAs (mRNAs) and limit their stability to regulate gene expression.10 The abnormal expression of miRNAs is one of the signs of tumor progression.11-13 Notably, miRNAs play important roles in regulating many biological processes, and dysfunctional miRNA regulation has been observed in numerous human diseases, including human cancer.10 As shown in our previous study, microRNA-222-3p inhibits epithelial ovarian cancer cell growth and is associated with favorable effects on total survival.14, 15 Moreover, a miR-148a imbalance is associated with breast cancer resistance.16 Although miR-148a has been reported to be associated with a good prognosis for patients with colon adenocarcinoma and miR-148a is associated with tumor metastasis, the specific mechanism of action remains unclear.17, 18
As shown in the present study, HDAC5 could promote the migration of colon adenocarcinoma cells. HDAC5 was expressed at higher levels in colon adenocarcinoma tissues than in adjacent tissues, and the expression of HDAC5 in tumor tissues was related to tumor stage, differentiation, and the overall survival of patients. Meanwhile, mirRNA-148a-3p expression displayed an opposite pattern to HDAC5. miR-148a-3p directly targeted HDAC5 and inhibited the expression of HDAC5. Based on these results, HDAC5 potentially contributes to tumor cells migration, and the miR-148a-3p/HDAC5 axis might be a novel therapeutic target for colon adenocarcinoma.
2 MATERIALS AND METHODS
2.1 Cell lines and patient samples
HCT116 and SW480 cell lines were cultured in RPMI1640 (BI) containing 10% fetal bovine serum (FBS, BI), and HEK-293 cell line was cultured in Dulbecco's Modified Eagle's Medium (DMEM) (BI) containing 10% FBS. All cells were cultured at 37°C in a humidified 5% CO2 incubator.
Nineteen pairs of colorectal tumors and tumor-adjacent tissues were obtained from the Department of Gastrointestinal Surgery of Xiangya Hospital (Central South University, Changsha, China). Paraffin-embedded tissues were obtained from other 94 patients with colon adenocarcinoma paired with 43 tumor-adjacent tissue samples. Systematic clinical and follow-up data were obtained from the Department of Pathology, Xiangya Hospital. The study was approved by the Ethics Review Committee of Xiangya Hospital, Central South University. All procedures involving human participants were performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
2.2 Western blot (WB)
The methods can be found in an earlier study.19 The polyclonal antibody against HDAC5 was obtained from ABclonal (1:1000). GAPDH (Utibody) was used as a control (1:5000, 60,004-1-Ig, proteintech).
2.3 Cell migration assay
The migration ability of the cells was determined by Transwell assays and wound-healing assay, which were carried out as previously described.19
2.4 Cell invasion assay
A total of 200 μl of cells at a density of 2 × 106 cells/ml was placed in the upper chamber coated with 30 μl of Matrigel (Clontech). Cells in five randomly selected fields of view were counted under an inverted microscope.
2.5 MiRNA target prediction
Two miRNA target prediction databases, TargetScan (release 7.1, http://www.targetscan.org/) and microRNA (http://www.microrna.org/) were used to predict miRNAs that might target HDAC5. A colon adenocarcinoma tissue microarray (EMBL-EBI/ArrayExpress/E-EGOE 54088) was analyzed to detect downregulated miRNAs in the colon adenocarcinoma tissues. The miRNAs that were downregulated in all three databases were selected as our potential targets.
2.6 Plasmid construction
A long fragment of the HDAC5 CDS was amplified from cDNAs extracted from HEK-293 cells and inserted into the pcDNA3.1 or pEGFP-N1 vector. The 3’UTR fragment of HDAC5 containing the putative miR-148a-3p binding site was amplified from human genomic DNA. Then, the wild-type (WT) HDAC5 3’UTR was cloned into the psi-CHECK™-2 vector. HDAC5 3’UTR mutant plasmids (MUT) whose target binding sites were mutated were obtained using the Mut Express-II Fast Mutagenesis Kit (Vazyme).
The sequences of the PCR primers are shown in Table S2.
2.7 Animal experiments
Animal care and experiments were performed in strict accordance with the “Guide for the Care and Use of Laboratory Animals” and the “Principles for the Utilization and Care of Vertebrate Animals” and were approved by the Animal Research Committee of The Third Xiangya Hospital Cancer Center. Five-week-old congenitally athymic male nude mice were used in all tumor xenograft experiments. Approximately 5 × 106 cells in 200 μl of PBS was subperitoneally injected into nude mice. Mice were euthanized at the end of the experiment (60 days after cell inoculation).
2.8 Statistical analysis
All assays were performed in triplicate. The statistical significance of differences in data were calculated using a one-way ANOVA or t test with SPSS PASW Statistics 18.0 or GraphPad Prism 7 software. Survival curves were analyzed with the log-rank test using the Kaplan-Meier method. The chi-square test was used to assess the correlation between miR-148a-3p expression and various clinicopathological parameters. The correlation between miR-148a-3p and HDAC5 expression was determined by calculating Pearson's correlation coefficient. All results are presented as average values ± SEM. p < 0.05 indicates a statistically significant difference.
3 RESULTS
3.1 The high expression of HDAC5 is positively correlated with the clinicopathological characteristics of colon adenocarcinoma
To clarify the effect of class IIa HADCs on the migration ability of colorectal cancer (CRC) cells, we first examined the expression of HDAC4, HDAC5, HDAC7, and HDAC9 in CRC cell lines by qRT-PCR and then selected SW480 cells for the next step of investigation (Figure S1A). Next, we detected the changes of migration ability of SW480 cells by using TMP269 (class IIa HADCs inhibitor). The results show that TMP269 can effectively inhibit the migration ability of SW480 cells (Figure 1A). To further determine which member of class IIa HADCs plays a major role in CRC cell migration, we specifically knocked down each member to detect its effect on cell migration ability (Figure S1B). The results showed that knockdown of HDAC5 or HDAC7 effectively inhibited the migratory ability of the cells, while the expression of HDAC4 or HADC9 did not affect the migratory ability of the cells (Figure 1B). The above results indicated that HDAC5 or HDAC7 may be an important molecule in promoting CRC cell migration. Therefore, we first selected HDAC5 as the focus of this study.
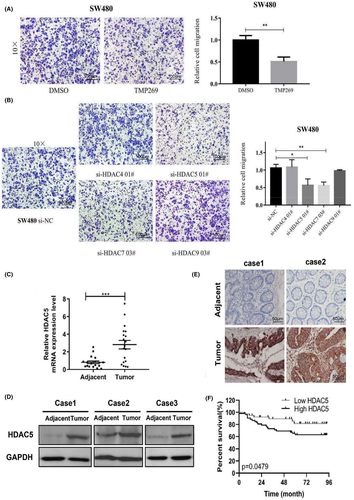
We collected 19 pairs of matched fresh tumor and adjacent tissue samples. The qRT-PCR analysis revealed that HDAC5 was significantly upregulated in tumor tissue samples (Figure 1C). More importantly, the WB results showed increased levels of the HDAC5 protein in tumor tissues (Figure 1D), which was consistent with the mRNA levels.
Then, immunohistochemistry (IHC) analysis was used to further examine HDAC5 expression in 43 adjacent noncancer tissue samples and 94 colon adenocarcinoma tissue samples. As shown in Figure 1E, we observed that the expression of HDAC5 was obviously higher in tumor tissues than in the adjacent tissues.
Finally, we analyzed the potential associations between the expression of HDAC5 and the clinicopathological characteristics of colon adenocarcinoma patients. The results showed that the expression of HDAC5 was positively correlated with histological differentiation, clinical stage, T-classification, N-classification, M-classification, and mortality during follow-up (Table S1). Kaplan-Meier analysis revealed that patients with high HDAC5 expression had worse clinical outcomes than those with low HDAC5 expression. (Figure 1F). Based on the results presented above, HDAC5 was significantly upregulated in colon adenocarcinoma and positively correlated with the clinicopathological features.
3.2 HDAC5 promotes the motility, invasion, and migration of colon adenocarcinoma cells in vitro
We examined the mRNA and protein levels of HDAC5 in colon adenocarcinoma cell lines by qRT-PCR and WB assays, respectively. As shown in Figure 2A-B, HDAC5 was expressed at higher levels in a variety of colon adenocarcinoma cell lines, particularly in SW480 cells, than in NCM460 cells (normal human colonic epithelial cells).
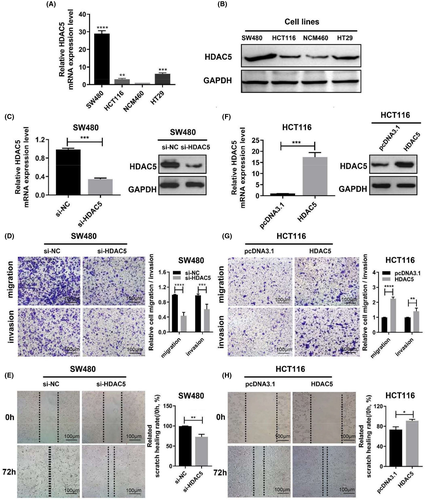
Next, we transfected the siRNAs of HDAC5 into SW480 cells to further explore the potential role of HDAC5 in tumorigenesis. We examined the knockdown efficiency of HDAC5 by WB and qRT-PCR analysis and observed a significant decrease in the levels of HDAC5 protein and mRNA (Figure 2C). Afterward, we assessed the role of HDAC5 in cell migration and invasion. We found that HDAC5 knockdown markedly impeded the migration and invasion of SW480 cells (Figure 2D). Moreover, the wound-healing ability of SW480 cells was significantly inhibited after silencing the expression of HDAC5 (Figure 2E). Thus, we highly suspect that the aberrant expression of HDAC5 is an important cause of colon adenocarcinoma cells migration. Furthermore, we transfected the full-length WT HDAC5 flag-tagged fusion vector into HCT116 cells (Figure 2F). We found that the migration and invasion ability of HCT116 cells was greatly enhanced due to the overexpression of HDAC5 (Figure 2G). At the same time, overexpression of HDAC5 increased the motility of HCT116 cells (Figure 2H). Thus, the above results suggest that the migratory and invasive behaviors of colon adenocarcinoma cells were modulated by HDAC5 in vitro, which suggests that HDAC5 may function as a potential oncogene in colon adenocarcinoma.
3.3 Absence of nuclear localization signal of HDAC5 leads to loss of migration and invasion ability
The localization of a gene in a cell often determines its function, so we next wanted to know the localization of HDAC5. By using immunofluorescence and high-content system, we found that endogenous HDAC5 was expressed in both cytoplasm and nucleus (Figure S2A). Also, we showed that the cellular localization of HDAC5 after exogenous overexpression in SW480 was consistent with the cellular localization of endogenously expressed HDAC5, both of which were whole-cell localized (Figure S2B). We further examined the cellular localization of HDAC5 by nuclear-cytoplasmic separation assay and found that both endogenous HDAC5 and exogenous HDAC5 were expressed in the nucleus and cytoplasm of SW480 cells, and the expression was higher in the cytoplasm (Figure 3A,B).
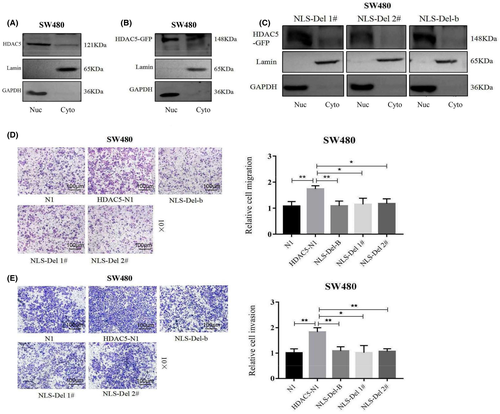
HDAC5 is a protein that can shuttle between the nucleus and the cytoplasm, but it is not clear whether it functions in the nucleus or in the cytoplasm. Therefore, we searched for the nuclear localization signal of HDAC5 through the Motif Scan website (https://myhits.isb-sib.ch/cgi-bin/motif_scan). The results showed that HDAC5 has two nuclear localization signals, amino acids 256-271 and 284-298, respectively (Figure S2C).
To investigate the functions played by HDAC5 in the nucleus, we constructed HDAC5 nuclear localization signal deletion plasmids NLS-Del 1# (NLS-Delete 256-271), NLS-Del 2# (NLS-Delete 284-298), and NLS-Del-B (NLS-Delete 256-271 and 284-298) (Figure S2D). The results of the high-content system showed that green fluorescence was expressed in the cytoplasm after transfection of NLS-Del 1#, NLS-Del 2#, and NLS-Del-B plasmids in SW480, while green fluorescence in the nucleus was almost absent; moreover, this phenomenon was most obvious in NLS-Del-B. This indicates that HDAC5 cannot enter the nucleus after the loss of nuclear localization signal and can only be expressed in the cytoplasm (Figure S2E). To further determine the localization of HDAC5 after deletion of the nuclear localization signal. We performed nuclear-cytoplasmic separation assay in SW480 cells transfected with the nuclear localization signal deletion plasmid (Figure 3C). The results showed that HDAC5 lost its ability to enter the nucleus and was retained in the cytoplasm when the cells were transfected with plasmid mutated in HDAC5 nucleus localization signal. This suggests that the nuclear entry signal peptide is vital for the nuclear transports of HDAC5-GFP, and we also found that transfection of NLS-Del 2# and NLS-Del-B plasmids had the simliar effect on the nuclear transport. This suggests that the nuclear localization signal at positions 284-298 is more important than that at positions 256-271 in helping HDAC5 to enter the nucleus. Afterward, we assessed whether the deletion of HDAC5 nuclear localization signal affects cell migration and invasion ability. We found that the deletion of nuclear localization signals resulted in the loss of HDAC5’s ability to promote migration (Figure 3D and S3A) and invasion (Figure 3E).
3.4 miR-148a-3p directly targets HDAC5
We hypothesized that miRNAs targeting HDAC5 may be downregulated in colon adenocarcinoma tissues, subsequently leading to higher expression of HDAC5 in colon adenocarcinoma metastases. Based on this conjecture, we first downloaded microarray data E-GEOD-54088 (https://www.ebi.ac.uk/arrayexpress/experiments/E-GEOD-54088) from the EBI website for colon adenocarcinoma metastatic foci tissue samples. We analyzed the colon adenocarcinoma tissue microarray to identify miRNAs whose expression was reduced in colon adenocarcinoma liver metastasis samples. We obtained 15 miRNAs, as shown in Figure 4A. The expression of these miRNAs was reduced 20-fold in metastatic tissue samples compared with the primary tumor. These miRNAs may directly or indirectly regulate the expression of HDAC5.
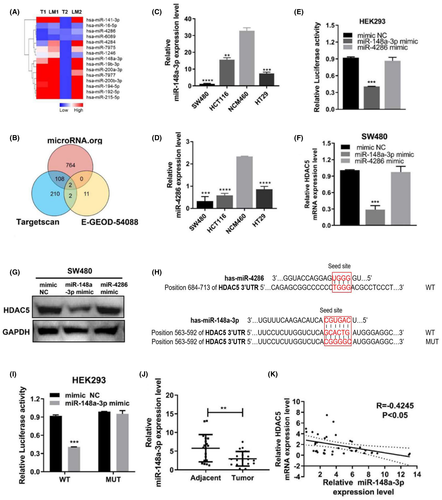
A common mechanism by which miRNAs target gene expression is to bind the 3’UTR. Next, we used two bioinformatics sites, TargetScan (http://www.targetscan.org/vert_71/) and microRNA (http://www.microrna.org/), to predict miRNAs that might directly target HDAC5. TargetScan predicted that 322 miRNAs directly target HDAC5, and microRNA predicted that 874 miRNAs target HDAC5. The two algorithms were superimposed to obtain an intersection of 110 candidate miRNAs that could potentially target HDAC5. Then, we performed a secondary analysis of the expression of 15 of the 110 candidate miRNAs in the colon adenocarcinoma tissue microarray (E-EGOE 54088) and obtained the overlapping miRNAs miR-148a-3p and miR-4286 (Figure 4B). Therefore, we used these two miRNAs as our candidate miRNAs.
We examined the expression levels of miR-148a-3p and miR-4286. In colon adenocarcinoma cell lines, lower expression levels of miR-148a-3p and miR-4286 were observed than in NCM460 cells. Moreover, among those colon adenocarcinoma cell lines, the expression level of miR-148a-3p was relatively low in SW480 cells with relatively high expression of HDAC5, and in contrast, its expression level was relatively high in HCT116 cells with relatively low expression of HDAC5 (Figure 4C,D). Meanwhile, the differences in miR-4286 expression levels were not significant in colon adenocarcinoma cell lines (Figure 4C,D). Furthermore, we cloned the WT 3’UTR of HDAC5 into the dual-luciferase reporter vector CHECK2 for in-depth verification of the prediction. The results showed that only miR-148a-3p, but not miR-4286, markedly reduced luciferase activity (Figure 4E). qRT-PCR and WB assays showed miR-148a-3p significantly reduced the protein and mRNA levels of HDAC5. However, there was little effect of miR-4286 on HDAC5 expression levels (Figure 4F,G).
Further, the luciferase reporter results showed that after mutating the binding site, miR-148a-3p mimics were not able to affect the luciferase activity (Figure 4H,I). This provides further support for our hypothesis that miR-148a-3p directly targets the HDAC5 3’UTR. We also examined the expression of miR-148a-3p in colon adenocarcinoma tissues. qRT-PCR results showed that its expression was downregulated in cancerous tissues compared with normal tissues. More importantly, correlation analysis showed that HDAC5 was inversely correlated with the expression level of miR-148a-3p (Figure 3J,K).
Therefore, based on the evidence from both the bioinformatics and molecular assays, it is reasonable to assume that miR-148a-3p directly targets HDAC5.
3.5 miR-148a-3p inhibits the motility, invasion, and migration of colon adenocarcinoma cells
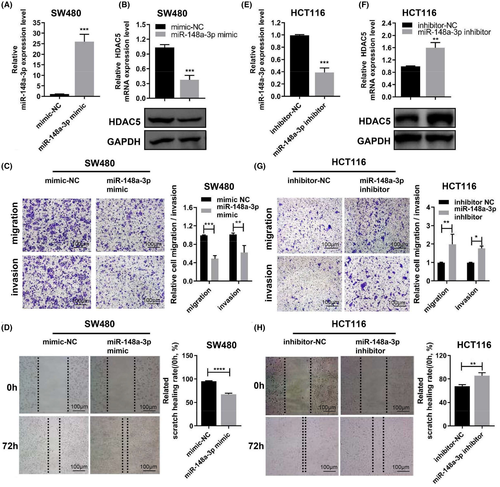
Next, we aimed to further explore the mechanism of miR-148a-3p targeting HDAC5 in colon adenocarcinoma. First, we found that miR-148a-3p mimic could inhibit HDAC5 expression in SW480 by qRT-PCR and WB, while miR-148a-3p inhibitor could promote the expression of HDAC5 (Figure 5A,B,E,F). Based on the finding that miR-148a-3p regulates the expression levels of HDAC5 in colon adenocarcinoma cells, we speculated that miR-148a-3p may inhibit the biological behavior of colon adenocarcinoma cells by regulating HDAC5 expression. Here, the overexpression of miR-148a-3p induced by transfecting miR-148a-3p mimics into SW480 cells significantly increased the invasion and migration of colon adenocarcinoma cells (Figure 5C). Similarly, the transfection of miR-148a-3p mimics into SW480 cells inhibited cell motility (Figure 5D). Conversely, the inhibition of miR-148a-3p obviously inhibited the ability of invasion and migration of colon adenocarcinoma cells (Figure 5G). Moreover, the transfection of the miR-148a-3p inhibitor into HCT116 cells increased cell migration and invasion as well (Figure 5H). These results strongly suggested a tumor suppressor role for miR-148a-3p. Thus, miR-148a-3p, in contrast to HDAC5, inhibits the motility, invasion, and migration of colon adenocarcinoma cells.
3.6 The miR-148a-3p/HDAC5 axis inhibits the motility, invasion, and migration of colon adenocarcinoma cells
We rescued HDAC5 expression by cotransfecting SW480 cells with HDAC5 and miR-148a-3p inhibitors to further examine whether the effects of miR-148-3p on colon adenocarcinoma cells would be altered by HDAC5. The results showed decreased levels of HDAC5 expression in cells transfected with HDAC5 siRNA compared with the control group. However, cotransfection with HDAC5 siRNA and miR-148a-3p inhibitor reversed the levels of the HDAC5 protein to the control level (Figure 6A,B). According to the results of the transwell assays, the migration and invasion ability of the cells transfected with HDAC5 siRNA were decreased compared with the control group. However, the migration and invasion ability of the cells cotransfected with HDAC5 siRNA and miR-148a-3p inhibitor was restored to the control level (Figure 6C,D).
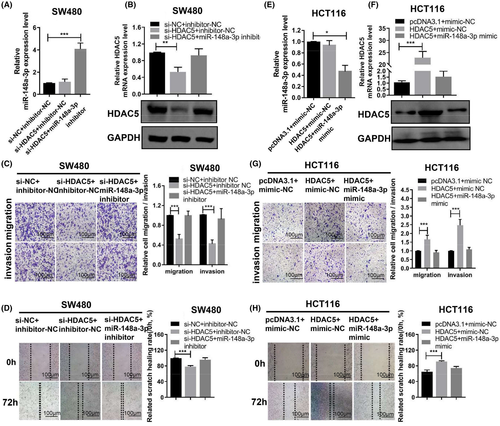
Furthermore, we rescued HDAC5 expression by cotransfected HDAC5 overexpression plasmid (without 3’UTR) and miR-148a-3p mimic in HCT116 cells. qRT-PCR and WB assays showed increased levels of the HDAC5 expression in cells transfected with the HDAC5 overexpression plasmid compared with the control group. However, cotransfection with the HDAC5 overexpression plasmid and miR-148a-3p mimics reversed the level of the HDAC5 protein to the control level (Figure 6E,F). According to the results of the transwell and wound-healing assays, the migration and invasion ability of the cells transfected with the HDAC5 overexpression plasmid was decreased compared with the control group. However, the migration and invasion ability of the cells cotransfected with HDAC5 overexpression plasmid and miR-148a-3p mimics returned to the control level. Silencing mediated by miR-148a-3p mimics eliminated the effects of HDAC5 overexpression on HCT116 cells (Figure 6G,H). Based on these data, miR-148a-3p inhibited the motility, invasion, and migration of colon adenocarcinoma cells by directly targeting HDAC5. Therefore, miR-148a-3p affects colon adenocarcinoma cell migration and scratch wound healing by targeting HDAC5.
3.7 The miR-148a-3p/HDAC5 axis regulates vimentin expression
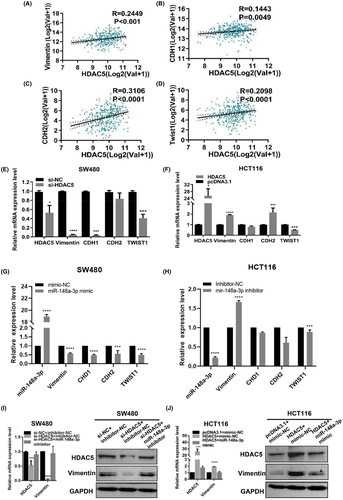
The epithelial-mesenchymal transition (EMT) is inextricably linked to colon adenocarcinoma. Therefore, we aimed to determine the relevance of HDAC5 expression and EMT markers in colon adenocarcinoma tissues in TCGA. As shown in Figure 7A-D, TCGA data validated the positive associations of HDAC5 expression with vimentin, CDH1, CDH2, and TWIST1 expression. The results of qPT-PCR showed that silencing HDAC5 downregulated the mRNA levels of vimentin, CDH1, and TWIST1 in SW480 cells (Figure 7E), and the overexpression of HDAC5 significantly upregulated the mRNA levels of vimentin and CDH2 in HCT116 cells (Figure 7F). We also detected the effect of miR-148a-3p on the expression levels of these four genes in SW480 or HCT116 by qPCR. The result showed that miR-148a-3p inhibited the expression of vimentin (Figure G-H). Furthermore, when we silenced HDAC5, the level of the vimentin protein and mRNA was reduced, but when we used miR-148a-3p inhibitor to restore the expression of HDAC5, the expression of vimentin was restored (Figure 7I). However, upon HDAC5 overexpression, the level of the vimentin protein and mRNA was elevated, consistent with the results of the correlation analysis of TCGA database (Figure 7J). When we cotransfected the miR-148a-3p mimics and HDAC5 overexpression plasmids in HCT116 cells, the expression of vimentin was restored to the original level (Figure 7J). Therefore, according to the result, the miR-148a-3p/HDAC5 axis could regulate vimentin expression.
3.8 Stable overexpression of HDAC5 promotes seeding density of colon adenocarcinoma cells in vivo
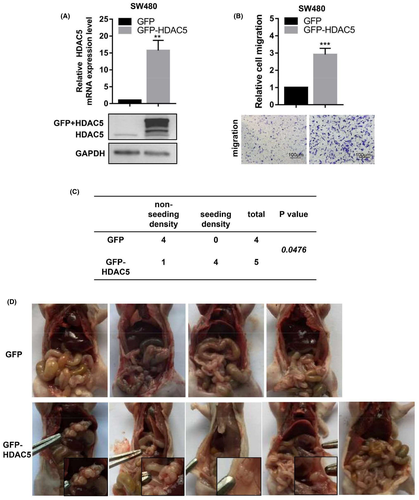
A cell line stably overexpressing HDAC5 was established with a vector containing HDAC5 CDS fused to a GFP reporter gene, as determined based on GFP expression, to further confirm that HDAC5 promoted cell migration. qRT-PCR and WB assays revealed that HDAC5 expression was significantly increased in the GFP-HDAC5 group compared with the GFP control group (Figure 8A). Transwell assays also revealed increased migration of cells in the GFP-HDAC5 group compared with the GFP group (Figure 8B). We injected 1 × 107 cells that stably overexpressed GFP-HDAC5 or GFP into the peritoneal cavity of nude mice. At 8 weeks post injection, HDAC5 overexpression promoted colon adenocarcinoma seeding density compared with the control group (Figure 8C). In the GFP-HDAC5 group, tumor cells implanted to the diaphragm, peritoneum, and intestine, as well as the boundary between nontumor tissues, while the abdominal cavity in the control group showed no tumor implantation (Figure 8D). Based on these results, the stable overexpression of HDAC5 promotes tumor seeding density.
4 DISCUSSION
HDAC5 is a member of the class IIa HDAC family, which was initially shown to deacetylate the Lys 9 and Lys 14 side chain of histones.20 This characteristic has made it an important regulator of cellular activity. Subtle changes in HDAC5 expression dictate critical outcomes in tumor initiation and progression in vivo. In hepatocellular carcinoma, HDAC5 expression is significantly increased compared with the adjacent tissues.20 CD13 promotes the progression of HCC and induces sorafenib resistance by interacting with HDAC5.21 In breast cancer, high expression of HDAC5 affects the proliferation, invasion, and migration of tumor cells.22 In NSCLC and medulloblastoma, high HDAC5 expression affects tumor initiation and progression.23 Similarly, our IHC results revealed higher levels of HDAC5-positive staining in cancer tissues than in the adjacent normal tissues. Additionally, HDAC5 expression was positively correlated with tumor differentiation, TNM staging, and total survival, suggesting that HDAC5 might represent a potential promoter for colon adenocarcinoma progression. According to our data, HDAC5 expression was noticeably increased in patients with distant metastasis compared with patients without metastasis, suggesting that HDAC5 might promote colon adenocarcinoma progression. HDAC5 significantly increased the motility, invasion, and migration of colon adenocarcinoma cells. HDAC5 also promoted tumor seeding density in vivo. Remarkably, recent research on lung cancer is consistent with our results,23 and thus we postulate that HDAC5 plays a similar role in colon adenocarcinoma.
MicroRNAs have also been reported to regulate HDAC5 expression. Importantly, the miR-2861-HDAC5 axis might also contribute to the maintenance of the stemness of lung cancer stem cells.24 The miR-124 and miR-9-HDAC5 axes also inhibit neurite elongation and development.25 The miR-589-3p-HDAC5 axis suppresses NSCLC cell migration, invasion, and tumorigenicity.26 In the present study, miR-148a-3p directly targeted HDAC5 and inhibited its expression. The expression of miR-148a-3p has been reported to correlate with multiple cancers. Unusually high miR-148a-3p expression is observed in prostate cancer tissues compared with the surrounding areas, implying a role in tumor development.27 The expression of miR-148a-3p is downregulated in both patient tissues and epithelial ovarian carcinoma (EOC) cell lines, and it can regulate the invasion and proliferation ability of EOC cells by targeting c-Met.28 As shown in a previous study, miR-148a-3p regulates ERBB3/AKT2/c-myc and ERBB3/AKT2/Snail signaling to inhibit bladder cancer cell proliferation and the EMT.29 In our study, miR-148a-3p functioned as a potential tumor suppressor in colon adenocarcinoma. By inhibiting HDAC5, the overexpression of miR-148a-3p impairs the motility, invasion and migration of colon adenocarcinoma cells. We also observed decreased miR-148a-3p expression in samples from patients with colon adenocarcinoma. Therefore, our results indicated that miR-148a-3p was negatively correlated with HDAC5 and was an inhibitor of HDAC5.
The EMT destroys the intracellular junctions of tumors, weakens the adhesion between cells, and increases migration.30 This process is associated with tumor invasion and distant metastasis.31, 32 Therefore, we used TCGA data to analyze the relationship between the expression of HDAC5 and EMT markers. HDAC5 expression positively correlated with vimentin, CDH1, CDH2, and TWIST1 expression. Although the correlations were very weak, we were still very confused about the positive correlation between CDH1 and HDAC5 expression. A subsequent RT-PCR analysis did not confirm the relationship between the miR-148a-3p/HDAC5 axis and CDH1 expression, and only vimentin expression was positively correlated with HDAC5 expression. Thus, in our study, we can found that the miR-148a-3p/HDAC5 axis regulated cell metastasis in CRC.
In the present study, HDAC5 promoted tumor cell migration and tumor seeding density by functioning as an oncogene in colon adenocarcinoma. HDAC5 was highly expressed in tumor tissues and correlated with tumor progression. The effect of HDAC5 was rescued by the HDAC5 inhibitor miR-148a-3p in colon adenocarcinoma. Moreover, the function of the miR-148a-3p/HDAC5 axis might be correlated with the EMT. Thus, HDAC5 overexpression in colon adenocarcinoma is consistent with tumor progression and tumor cells migration, and the impact of HDAC5 overexpression is opposed by miR-148a-3p. The miR-148a-3p/HDAC5 axis represents a potential novel therapeutic target for colon adenocarcinoma.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (NO. 82173376); the National Key Project R&D Program of Hunan of China, Stem Cell, and Translation Research (NO. 2016YF0102000); the Key Project of Hunan Province 2016 (NO. 2016JC2036); and the Graduate Research Innovation Project in Central South University (NO. 1053320180978).
DISCLOSURE
The authors have no conflict of interest.




