Emergence of TNIK inhibitors in cancer therapeutics
Funding Information
Japan Agency for Medical Research and Development, (Grant / Award Number: ‘16im0210804h0001’) National Cancer Center Research and Development Fund, (Grant / Award Number: ‘26-A-13 and 26-A-5’)
Abstract
The outcome of patients with metastatic colorectal cancer remains unsatisfactory. To improve patient prognosis, it will be necessary to identify new drug targets based on molecules that are essential for colorectal carcinogenesis, and to develop therapeutics that target such molecules. The great majority of colorectal cancers (>90%) have mutations in at least one Wnt signaling pathway gene. Aberrant activation of Wnt signaling is a major force driving colorectal carcinogenesis. Several therapeutics targeting Wnt pathway molecules, including porcupine, frizzled receptors and tankyrases, have been developed, but none of them have yet been incorporated into clinical practice. Wnt signaling is most frequently activated by loss of function of the adenomatous polyposis coli (APC) tumor suppressor gene. Restoration of APC gene function does not seem to be a realistic therapeutic approach, and, therefore, only Wnt signaling molecules downstream of the APC gene product can be considered as targets for pharmacological intervention. Traf2 and Nck-interacting protein kinase (TNIK) was identified as a regulatory component of the β-catenin and T-cell factor-4 (TCF-4) transcriptional complex. Several small-molecule compounds targeting this protein kinase have been shown to have anti-tumor effects against various cancers. An anthelmintic agent, mebendazole, was recently identified as a selective inhibitor of TNIK and is under clinical evaluation. TNIK regulates Wnt signaling in the most downstream part of the pathway, and its pharmacological inhibition seems to be a promising therapeutic approach. We demonstrated the feasibility of this approach by developing a small-molecule TNIK inhibitor, NCB-0846.
Carcinoma of the colon and rectum is a major health problem worldwide, accounting for over 700 000 deaths annually.1 The majority of colorectal cancer patients without lymph node or distant organ metastasis can be readily cured by surgical resection alone,2 but the outcome of patients with distant metastasis remains unsatisfactory. Recent advances in combinational chemotherapy and molecular therapeutics directed against vascular endothelial growth factor (VEGF) (bevacizumab) and the epidermal growth factor receptor (EGFR) (cetuximab and panitumumab) have significantly prolonged the survival of patients with metastatic colorectal cancer, and the median overall survival time of such patients now exceeds 30 months. However, cure is still exceptional, and the 5-year survival rate of patients with stage IV colorectal cancer is around 15%.3, 4
In a recent international phase 3 trial named CORRECT, a multikinase inhibitor, regorafenib (BAY 73-4506), was shown to provide significant survival benefits for patients with metastatic colorectal cancer that was refractory to all other preceding conventional chemotherapies.5 Although the survival benefits were statistically significant, prolongation of the median overall survival period was limited to only 1.4 months. Another more recent phase 3 trial (RECOURSE) employing TAS-102 (a combination of the nucleoside analogue trifluridine and tipiracil hydrochloride, a thymidine phosphorylase inhibitor) showed significant survival benefit in patients with previously treated metastatic colorectal cancer; however, only minimal prolongation of the median overall survival period, which increased from 5.3 months (placebo group) to 7.1 months (TAS-102 group), was observed.6 Therefore, it is necessary to identify molecules that are essential for colorectal carcinogenesis as new drug targets, and to develop therapeutics that target them.
Genomics of Colorectal cancer
The genetic and epigenetic alterations of colorectal cancer have been studied extensively over the past few decades.7, 8 The most notable finding is that the great majority of colorectal cancers carry mutations in genes that are involved in the canonical Wnt/β-catenin signaling pathway; more than 80% of colorectal cancers have mutations in the APC tumor suppressor gene (Fig. 1). The genes encoding β-catenin (CTNNB1), frizzled 10 (FZD10), T-cell factors 3 and 4 (TCF3/4) (TCF7L1/2), axis inhibitor 2 (AXIN2), and APC membrane recruitment protein 1 (AMER1, WTX or FAM123B) are also mutated recurrently in colorectal cancer.9 In total, more than 90% of sporadic colorectal cancers carry mutations in at least one Wnt signaling gene. These findings suggest that Wnt signaling is a major driving force of colorectal carcinogenesis and a potential therapeutic target. However, most Wnt signaling genes mutated in colorectal cancer, including APC, are tumor suppressors and cannot be directly targeted for therapeutic purposes. β-Catenin is a proto-oncogene product, but it is a ubiquitously expressed cell adhesion molecule and, therefore, cannot be used as a drug target.

Druggable Targets in the Wnt Signaling Pathway
There are a few technically “druggable” molecules (secreted proteins, cell surface receptor proteins and kinases/enzymes) in the Wnt signaling pathway (Table1). Therapeutics targeting porcupine (LGK974, Novartis Pharmaceuticals, Basel, Switzerland)10 and frizzled (FZD) receptors (OMP-18R5 and OMP-54F28, OncoMed Pharmaceuticals Redwood City, CA)11, 12 have been developed, and their safety and toxicities have been evaluated in phase 1 clinical trials.13 Porcupine is a membrane-bound O-acyltransferase enzyme that is essential for the extracellular secretion of Wnt proteins. LGK974 is a small-molecule compound that inhibits the enzyme activity of porcupine. OMP-18R5 (vanituctumab) is a fully humanized monoclonal antibody that recognizes an epitope that is conserved across the extracellular domains of five FZD receptors (1, 2, 5, 7 and 8). OMP-54F28 is a recombinant FZD8 protein that is fused to the Fc portion of immunoglobulin. OMP-54F28 acts as a decoy receptor by competing with endogenous FZD8 for binding to its ligand.12
| Target molecule | Localization | Agent | Clinical development |
|---|---|---|---|
| Porcupine | Extracellular | LGK974/Wnt974 | Phase 1 |
| Wnt5a | Extracellular | Foxy-5 (Wnt5a mimic peptide) | Phase 1 |
| FZD receptor 8 | Extracellular | OMP-54F28 (decoy receptor) | Phase 1 |
| FZD receptors (1, 2, 5, 7, 8) | Membrane | OMP-18R5 (vanituctumab) | Phase 1 |
| LRP6 co-receptor | Membrane | Salinomycin | Preclinical |
| Niclosamide | Phase 1 | ||
| Silibinin | Phase 2 | ||
| Rottlerin | Preclinical | ||
| Salinomycin | Preclinical | ||
| Niclosamide | Phase 1 | ||
| Dvl | Cytoplasm | NSC668036 | Preclinical |
| FJ9 | Preclinical | ||
| 3289-8625 | Preclinical | ||
| TNKS1/2 | Cytoplasm | XAV939 | Preclinical |
| NVP-TNK656 | Preclinical | ||
| JW55 | Preclinical | ||
| CK1α | Cytoplasm | Pyrvinium | Preclinical |
| CK1δ/ɛ | Cytoplasm | TAK-715 | Preclinical |
| AMG-548 | Preclinical | ||
| PPI between β-catenin and CBP | Nucleus | ICG-001 | Preclinical |
| PRI-724 | Phase 2 | ||
| TNIK | Nucleus/cytoplasm | NCB-0846 | Preclinical |
- CBP, cAMP response element binding protein (CREB)-binding protein; CK, casein kinase; Dvl, disheveled; FZD, frizzled; LRP6, low-density lipoprotein receptor-related protein 6; PPI, protein-protein interaction; TNIK, Traf2 and Nck-interacting protein kinase; TNKS, tankyrase.
At present, these anti-Wnt therapeutics appear to be clinically safe, and no long-feared adverse effects in the gastrointestinal tract have been observed.13 However, in the great majority of colorectal cancers, Wnt signaling is activated by loss of function of the APC tumor suppressor gene, which means that it will be necessary to block Wnt signaling in the pathway downstream of APC. Unfortunately, LGK974, OMP-18R5 and OMP-54F28 are presumed to block Wnt signaling by inhibiting the binding of secreted Wnt ligands to FZD receptors and, therefore, these agents cannot be used for the treatment of such colorectal cancers.
XAV939 has been shown to target the enzymes tankyrase 1 and 2 (TNKS1/2)14 that poly-ADP-ribosylate axins (axin-1 and axin-2). Poly (ADP-ribosylated) axins are subjected to ubiquitination and subsequent degradation. The inhibition of tankyrases results in the stabilization of axins and blocks Wnt signaling. XAV939 inhibited the proliferation of APC-deficient colorectal cancer cells. A more selective TNKS inhibitor, NVP-TNKS656, which was identified through structure-based optimization of XAV939,15 was orally available, and its early clinical application is anticipated.
Targeting Wnt Signaling Inside the Nucleus
As mentioned earlier, restoration of the loss-of-function mutation of the APC gene in colorectal cancer cells does not seem to be a realistic therapeutic approach, and only signaling molecules downstream of the APC gene product can be considered as therapeutic targets. The T-cell factor (TCF)/lymphoid enhancer factor (LEF) and β-catenin transcriptional complex is the most downstream effector of Wnt signaling. Nuclear proteins associated with the transcriptional complex seem to be feasible targets for molecular therapy against colorectal cancer. Groucho/transducin-like enhancer (TLE) protein,16 C-terminal binding protein-1 (CtBP),17, 18 CREB-binding protein (CBP)/p300,19, 20 smads,21 NEMO-like kinase (NLK),22 chibby23 and other proteins24-40 have been reported to interact with the TCF/LEF and β-catenin nuclear complexes and modulate their transcriptional activity.
Of these proteins, CBP and its closely related homolog p300 participate in the TCF/LEF and β-catenin complex as transcriptional coactivators.41 A peptide mimetic small-molecule compound, ICG-001,42 has been shown to selectively inhibit the protein-protein interaction (PPI) between β-catenin and CBP and induce apoptosis of colorectal cancer cells. The second generation CBP/β-catenin PPI inhibitor, PRI-724,43 has been shown to have an acceptable safety profile in early-phase clinical trials and its evaluation in a phase 2 trial of metastatic colorectal cancer is planned (ClinicalTrials.gov Identifier: NCT02413853).
Identification of TNIK as a Druggable Target of Wnt Signaling
The TCF/LEF transcription factor family comprises LEF1 (LEF1), TCF-1 (TCF7), TCF-3 (TCF7L1) and TCF-4 (TCF7L2), of which only TCF-4 is ubiquitously expressed in colorectal cancer cells.44 Induction of dominant-negative TCF-4 restored the epithelial cell polarity of a colorectal cancer cell line and converted the cell line into a single layer of columnar epithelium.45 We have, therefore, been searching for druggable molecules in the TCF-4 and β-catenin transcriptional complex. Through comprehensive mass spectrometry analyses we identified fusion/translocated in liposarcoma (FUS/TLS),46 poly(ADP-ribose) polymerase-1 (PARP-1),47 Ku70 (70-kD thyroid autoantigen),48 Ku80,48 DNA topoisomerase IIα (Topo IIα),49 splicing factor-1 (SF1),50 ras-related nuclear protein (Ran),51 Ran-binding protein-2 (RanBP2),51 Ran GTPase-activating protein-1 (RanGAP1),51 promyelocytic leukemia (PML) protein52 and TNIK51 as putative regulatory components of the TCF-4 and β-catenin transcriptional complex.
Among these identified proteins, TNIK attracted our interest as a potential drug target because various ATP-competitive small-molecule kinase inhibitors have been applied successfully to cancer treatment. Mahmoundi et al.53 also identified TNIK as a protein that interacts with Tcf-4 in the mouse intestinal crypt. In the mouse system, TNIK was found to be a component of the Tcf-4 and β-catenin transcriptional complex and was essential for the expression of Wnt target genes.
Regulation of Wnt Signaling by TNIK
We identified TNIK by analyzing the composition of proteins that were immunoprecipitated from two colorectal cancer cell lines with an anti-TCF4 antibody using highly tuned liquid chromatography and mass spectrometry (LC-MS).51 TNIK was originally identified as a new member of the Germinal Center Kinase (GCK) family by Fu et al.54 It is known that TNIK regulates the c-Jun N-terminal kinase (JNK) pathway through its C-terminus55 and the nuclear factor-κB (NF-κB) signaling pathways through its N-terminal kinase domain (Fig. 2).55 In addition, TNIK has been shown to regulate the filamentous-actin (F-actin) cytoskeleton.56

TCF-4, β-catenin and TNIK proteins form a complex in colorectal cancer cells. TNIK phosphorylates the TCF-4 protein at the conserved serine 154. This phosphorylation is essential for full activation of Wnt signaling. Knockdown of TNIK decreased the transcriptional activity of the TCF-4 and β-catenin complex and inhibited the growth of colorectal cancer cells and xenografts (Fig. 3). This growth inhibition was abrogated by expression of the catalytic domain of TNIK.57 A recent clinical study showed that increased expression of the TNIK protein was significantly associated with the poor postsurgical outcome of patients with stage 2 and 3 colorectal cancer.58

The regulation of Wnt signaling by TNIK is conserved across species. Xenopus TNIK lacks the C-terminal regulatory portion that is present in human TNIK, but the kinase domain is conserved. Xenopus TNIK is also essential for β-catenin-mediated determination of the dorsal axis.59
Development of a TNIK Inhibitor
Wnt signaling is a major force driving colorectal carcinogenesis. TNIK is an essential regulatory component of Wnt signaling, and colorectal cancer cells are highly dependent upon the expression and catalytic activity of TNIK for proliferation. Targeting of TNIK for pharmacological intervention was, thus, anticipated to inhibit Wnt signaling and suppress the growth of colorectal cancer cells.60
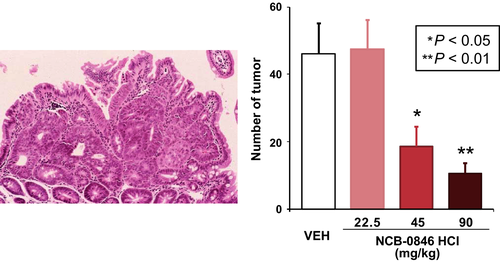
We screened a compound library in collaboration with Carna Biosciences (Kobe, Japan) and identified a series of quinazoline analogues with high TNIK enzyme-inhibitory activity.61 Subsequent lead optimization resulted in identification of the novel compound NCB-0846 [cis-4-(2-(3H-benzo[d]imidazol-5-ylamino)quinazolin-8-yloxy)cyclohexanol]. NCB-0846 inhibited the Wnt signaling of HCT116 (carrying a CTNNB1 mutation) and DLD-1 (carrying an APC mutation) colorectal cancer cells. NCB-0846 reduced the expression of Wnt target genes such as AXIN2 and MYC, suppressed Wnt-driven intestinal tumorigenesis in Apcmin/+ mice (Fig. 4) and the stemness (sphere formation and tumorigenicity) of colorectal cancer cells. NCB-0846 was orally administrable and suppressed the growth of different kinds of patient-derived colorectal cancer xenografts.
The ATP-binding pocket of TNIK is structurally similar to that of other protein kinases.62 NCB-0846 also inhibited several other oncogenic proteins, including FMS-like tyrosine kinase 3 (FLT3), platelet derived growth factor-α (PDGFRα) and cyclin-dependent kinase 2 (CDK2)/cyclin A2 (CycA2). However, inhibition of other kinases is a common feature of every ATP-directed kinase inhibitor and is not problematic for its clinical development. By carefully including a diastereomer (named NCB-0970) with 13-fold weaker TNIK enzyme-inhibitory activity as a TNIK-specific negative control, we excluded any off-target effects of NCB-0846.
Other TNIK Inhibitors
Various TNIK inhibitors with different chemical structures have been developed by several pharmaceutical companies, including Compound 3 (Celon Pharma, Lomianki, Poland),63 PF-794 (Pfizer)64 and ON-1081050/ON-108600 (Onconova Therapeutics, Newtown, PA). A series of 4-phenyl-2-phenylaminopyridine analogs with potent activity against TNIK have also been reported by Astex Phamaceutical (Cambridge, England).65 A CK2 (casein kinase-2)/TNIK dual inhibitor, ON108600, has been shown to target cancer stem cells and induce apoptosis of paclitaxel-resistant triple-negative breast cancer cells.66 Furthermore, TNIK was identified as being involved in the anti-cancer mechanism of a benzimidazole-quinolinone compound, dovitinib, in multiple myeloma.67
An FDA-approved anthelmintic drug, mebendazole, was recently identified as a selective inhibitor of TNIK.62 Mebendazole showed anti-tumor effects in a broad range of pre-clinical studies across a number of different cancer types, including colorectal cancer,68 and the combination of mebendazole with a non-steroidal anti-inflammatory drug reportedly reduced tumor initiation in ApcMin/+ mice.69, 70 Remarkable tumor regression by the administration of mebendazole was observed in a patient with drug-refractory metastatic colorectal cancer.71 Based on promising preclinical efficacy data,72 mebendazole is currently under clinical evaluation in adult and pediatric brain tumors (ClinicalTrials.gov Identifier: NCT01729260 and NCT01837862). Medulloblastoma, a pediatric brain tumor of the cerebellum, is known to harbor mutations in the CTNNB1 and AXIN1 genes.73, 74
Conclusion
The genetics of colorectal cancer has been extensively studied over the past few decades, and frequent mutations of Wnt signaling genes have been recognized since the 1990s. Several inhibitors have been developed against various components of the Wnt pathway, but so far none of them have been incorporated into oncology practice. Normal intestinal epithelium and colorectal cancer cells have distinct Wnt pathways. However, Wnt signaling can be blocked by targeting nuclear components. We demonstrated the feasibility of this therapeutic approach by developing a small molecule inhibitor of TNIK.61 TNIK is essential for Wnt signaling and colorectal cancer growth, and its inhibition is a promising therapeutic approach. The clinical development of TNIK inhibitors is warranted.
Acknowledgments
This study was supported by the National Cancer Center Research and Development Fund (26-A-13 and 26-A-5 to TY) and the Acceleration Transformative Research for Medical Innovation (ACT-MS) program of the Japan Agency for Medical Research and Development (AMED) (16im0210804h0001 to TY).
Disclosure Statement
T. Y. received a research grant from Carna BioSciences (Kobe, Japan). The authors have no other conflicts of interest to declare.




