In This Issue
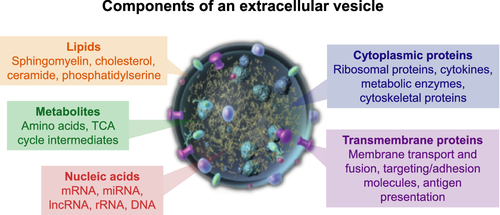
Extracellular vesicles as transgenomic agents: Emerging roles in disease and evolution
Page 824–30
The transfer of genetic information from a cell to its progeny through cellular division is part of the central dogma of cellular biology. In unique scenarios, DNA may be transferred by pathogens, but gene transfer through pockets of lipids, termed extracellular vesicles (EVs), is a mysterious phenomenon that may have wide-reaching scientific and clinical implications. To shed light on the diverse research into EVs, Kawamura and colleagues summarize a growing body of published work and synthesize complex scientific progress into a highly accessible review. The authors begin with basic questions and delve into the practical implications of horizontal genetic connectivity between cells. The implications of such a process may be difficult to grasp, but the authors provide tenable examples of how EVs could impact biologic processes with the result being an exciting and thought-provoking scientific review. doi: 10.1111/cas.13222
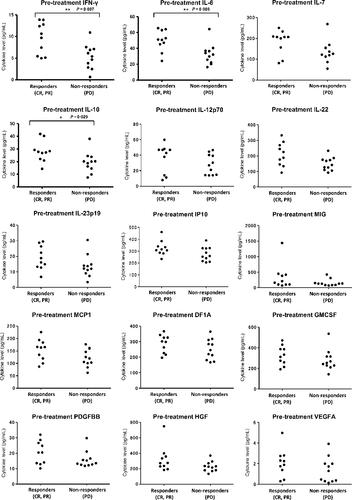
Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase II study for advanced melanoma
Page 1022–31
This phase II study investigated the response of 37 Japanese patients with metastatic melanoma to nivolumab, a fully humanized IgG4 inhibitor antibody against the programmed death-1 protein. The results indicate that nivolumab is associated with significant antitumor responses with a tolerable safety profile, with 31.4% of patients experiencing grade 3 to 4 adverse effects. The overall 1- and 2-year survival rates were 54.3% and 42.9%, respectively, which are highly encouraging and in line with the larger international experience with immunotherapeutic approaches to metastatic melanoma. doi: 10.1111/cas.13226
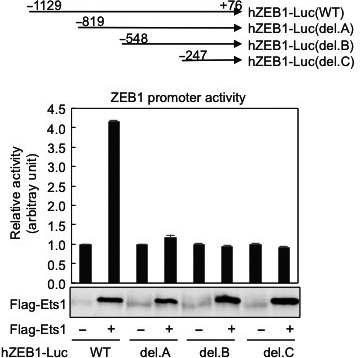
Ets1 and ESE1 reciprocally regulate expression of ZEB1/ZEB2, dependent on ERK1/2 activity, in breast cancer cells
Page 952–60
Breast cancer metastasis progression involves the epithelial–mesenchymal transition. In previous studies, Sinh et al. have shown that activation of ZEB1/δEF1 and ZEB2/SIP1 are positively correlated with the epithelial–mesenchymal transition phenotype and aggressiveness of the breast cancer. However, it was previously unknown how these proteins are controlled. This study reveals that Ets1 acts to induce ZEB1, and this activation can be suppressed by epithelium-specific ETS transcription factor 1 (ESE1). By activating ESE1 and thereby reducing ZEB1 downstream, it is possible to perhaps decrease the malignancy of the cells and offer patients a more favorable prognosis. doi: 10.1111/cas.13214