Ephemeral habitat supports high fish α-diversity and β-diversity during drought in a subtropical semiarid wetland
Associate Editor: Eleanor Slade
Handling Editor: Jennifer Powers
ABSTRACT
Globally, the number of rivers with intermittent flow is increasing due to climate change and water abstraction for human consumption. Currently, our understanding of how hydrology in subtropical rivers with intermittent flow affects the structure and dynamics of aquatic communities is poorly understood. Here, we investigated how fish α- and β-diversity patterns in intermittent channels in the lower reaches of the Okavango Delta respond to seasonal flooding and drought. Under low-flow conditions, ephemeral habitat had higher α-diversity, and this was influenced by a combination of fish aggregation and apparent transient legacy effects as habitat patches became smaller and more isolated. Investigation of changes in fish assemblage structure across different hydrological periods and habitat types showed significant species turnover when water levels fell, suggesting a strong influence from species sorting. During high-water periods, species assemblages were homogenized both at local and regional scales, suggesting a greater influence of mass effects. Our findings support hydrology as a major factor regulating diversity patterns in intermittent rivers of a major wetland in a semiarid region of subtropical Africa. We infer from these findings that maintenance of a relatively natural flow regime will be necessary for conserving aquatic ecosystem structure and function in this system.
1 INTRODUCTION
Shifts between flowing water, standing water, and terrestrial habitats in intermittent rivers lead to increased habitat complexity such that community diversity may result from multiple processes acting jointly across space and through time. During the dry season, when the habitat is fragmented into standing pools of water, dispersal limitation may increase diversity and allow coexistence of ecologically similar species (Leibold & Chase, 2018). However, desiccation of intermittent river reaches often causes local extirpation of some species. After flow resumption, species recolonize these patches from their dry season refugia, either by active or passive dispersal (Winemiller et al. 2010). Recruitment may also occur within-patches as a result of complex life cycles (Lancaster & Belyea, 1997; Winemiller et al. 2010) such as estivation (Fishman et al. 1986; Johnels & Svensson, 1955) and embryonic diapause (Polačik & Podrabsky, 2015; Watters, 2009) that allow organisms to withstand complete desiccation. However, periodic cycles of wetting and drying over long periods may lead to enhanced functional acclimatization due to legacy effects that in turn may result in unexpected responses to local environmental variation (Hawkes & Keitt, 2015; Vass & Langenheder, 2017).
Within a river reach, total biodiversity (γ-diversity) depends on average biodiversity within habitats (α-diversity) and differences in biodiversity composition among habitats (β-diversity) (Peláez & Pavanelli, 2018). Depending on the strength of local ecological filters, variation in species composition within similar habitats may result from deterministic factors such as priority effects, competition, and predation, or stochastic factors including random dispersal and random changes in species abundance (ecological drift) (Chase, 2007; Leibold & Chase, 2018; O'Neill, 2016). In intermittent rivers, α, β, and γ-diversity may exhibit dynamic variation along spatial hydrological gradients (Datry et al. 2017). For example, α-diversity may significantly vary between permanent habitats and ephemeral habitats (Beesley & Prince, 2010; Datry et al. 2017; Katz et al. 2012; Stromberg et al. 2005). Therefore, biodiversity conservation in intermittent rivers requires a further understanding of how communities respond to disturbances, such as flooding and drying.
β-diversity is affected by compositional heterogeneity due to different species identities within and among sites (turnover) and ordered species loss where the species present in sites with poor species richness are proper subsets of the species in sites that have higher species richness (nestedness) (Anderson et al. 2011; Baselga, 2010; Legendre, 2014). In intermittent rivers, compositional heterogeneity and differences in species richness within and among aquatic habitats are higher with increasing fragmentation and environmental harshness (Bogan et al. 2013; Datry et al. 2014; Miyazono & Taylor, 2015; Ruhí et al. 2015). For example, fish and invertebrate assemblage composition may show significant turnover during the last stages of drought, and spatial heterogeneity often declines during and immediately after flooding (Buendia et al. 2014; Fernandes et al. 2009). Species nestedness may dominate along intermittence gradients owing to a greater loss of species in reaches with short water residence time when compared to perennial reaches due to physiological and environmental constraints (Datry, Larned, Fritz, et al., 2014). In addition to being influenced by the temporal dynamics and spatial scale of habitat disturbance, species turnover or nestedness are both influenced by interspecific variation in mobility (Tonkin et al. 2015) and landscape features (Datry et al. 2017).
Here, we provide additional insights into the drivers of fish community diversity in naturally functioning intermittent rivers. Flows in the Boro, Thamalakane, and Boteti rivers in the lower reaches of the Okavango Delta are intermittent in response to a sustained flood pulse that occurs each year from mid-winter (June) to mid-autumn (March). Part of the delta's fluvial network flows through the town of Maun, a rapidly growing human population that depends on water and aquatic resources from the delta. We obtained fish samples from four sites representing two different habitat types (ephemeral and permanent).
Surveys were conducted during the dry and wet seasons. We tested three hypotheses: (a) Fish α-diversity was expected to be relatively lower during the flood peak compared with the low-water phase of the annual hydrologic cycle. The lower α-diversity results from high dispersal and low densities of most species within expanded and well-connected channel habitats. (b) β-diversity was expected to be higher during the dry season due to habitat fragmentation and isolation that increase the strength of environmental filtering and ecological drift. (c) Fish assemblages were expected to be homogenized during the high-water period due to increased aquatic habitat connectivity and greater fish dispersal resulting in higher between-habitat similarity of assemblage structure.
2 METHODS
2.1 Study area
The Okavango Delta (19°17′ S, 22° 54′ E) in northern Botswana is an inland delta comprised of a complex network of channels that creates habitat heterogeneity and supports ecosystem productivity within an arid subtropical region. The total area of the delta varies between 12,000 km2 during the wet season and 3000 km2 during base flow conditions (Gumbricht et al. 2004). The delta is classified as a natural ecosystem with relatively high biodiversity, especially for large mammals (Mladenov et al. 2005). Species richness in the Okavango Delta is estimated to include multiple taxonomic groups. Among them are 71 fishes, 33 amphibians, 64 reptiles, 444 birds, 122 mammals, and approximately 1300 plants (Ramberg et al. 2006). The magnitude and duration of the annual flood pulse depend on the amount of rain falling in the Angolan highlands where headwaters originate. The flood pulse in the lower delta is not correlated with local rainfall (Ringrose et al. 1988) and peaks typically between August and October (McCarthy et al. 1998).
2.2 Sampling scheme
Surveys were conducted at four sites in intermittent rivers (Boro, Thamalakane, and Boteti) of the lower reaches of the Okavango Delta (Figure S1). One site (Borojunction, BJN; 19° 55ʹ 01.0ʺS; 023° 30ʹ 52.0ʺE) was located where the Boro River joins the Thamalakane River. Approximately 4 km upstream of the junction on the Boro River was a second survey site (Botswana Defence Force Camp, BDF; 19° 52ʹ 28.1ʺS; 023° 26ʹ 45.0ʺE). Two additional sites were located downstream: on the Thamalakane River at Dikgathong (DKN) (20° 08ʹ 27.9ʺS; 023° 22ʹ 38.5ʺE) and on the Boteti River at Chanoga (CHN) (20° 10ʹ 02.4ʺS; 023° 39ʹ 33.6ʺE). At DKN, the river branches. One channel flows southwest toward Lake Ngami, and the other channel forms Boteti River that flows southeast through CHN, terminating in Makgadikgadi salt pans.
Water levels were high at all sites from August to November, and this period was classified as the high-water season (Figure S2). From December to February, water levels had fallen, and this survey period was classified as the low-water season. During the low-water season, the four sites encompassed a range of hydrological conditions. Compared to other sites, CHN and DKN retained water for extended periods. These sites remained wet and connected even after nearby river reaches had dried and therefore were classified as permanent habitat. CHN maintained a sizeable deep pool (ca. 400 m length, 50 m width, 2.5 m depth) throughout the dry season (named Chanoga lagoon in Merron & Bruton, 1995). Dikgathong had a vast floodplain that got inundated during high-water season (Figure S3d). Phragmites and hippos were always spotted at the site. BDF and BJN completely dried out and became isolated by the end of the low-water season, and therefore were classified as ephemeral habitat. During February, the site at BDF only retained water in a small stretch of aquatic habitat (ca. 5 m × 70 m, 0.6 m depth) along the channel margin, and the site at BJN was mostly dry except for some shallow areas (<20 cm depth) and a moderately deep stretch of aquatic habitat (ca. 10 m × 90 m, 0.9 m depth) within the Thamalakane channel.
We sampled fish bi-monthly between August 2017 and February 2018 using a nylon multifilament gillnet. The gillnet consisted of eleven panels. Each panel had a length of 10 m and a depth of 2.4 m. The panels comprised of different mesh sizes: 12, 16, 22, 28, 35, 45, 57, 73, 93, 118, and 150 mm. We randomly tied the panels together to form one multipanel gill net. We set the gill net once at each site during each survey period for approximately 12 h between 1800 hr and 0600 hr the following day. We surveyed each site four times (two times during the high-water season and two times during the low-water season), resulting in a total of 16 gill net samples. Although gillnets are passive gears that can select for fishes with specific morphologies and behavioral tendencies, the long duration of sets and multiple mesh sizes was effective in capturing a multitude of sizes, from small (<5 cm total length, TL) to large (>80 cm TL) individuals of the species that are documented to be common in this system (Merron & Bruton, 1995). Due to limitations of the sampling gear, small fishes were underrepresented in samples, and some species were absent. Specimens were identified to species level based on keys and illustrations in Skelton (2001) and measured for total length to the nearest 1 mm. Voucher specimens were euthanized (TAMU AUP IACUC 2017–0069), fixed in formalin, preserved in ethanol, and archived in the Biodiversity Research and Teaching Collection at Texas A&M University, College Station.
2.3 Data analysis
To test our hypotheses, we investigated two diversity aspects of the fish assemblage. We used α-diversity to describe the number of species at a local scale by characterizing the number of species in a gillnet sample. We characterized β-diversity by quantifying overall variation in species composition between samples obtained from distinct aquatic habitat types during different hydrological seasons (e.g., ephemeral habitat during low-water season [henceforth: habitat-by-season samples]). We calculated each metric as follows:
2.3.1 α-diversity
Because differences in the number of sampled individuals can bias estimates of α-diversity, we rarefied the data (sensu Gotelli & Colwell, 2001) before comparing α-diversity between samples. We estimated α-diversity after bootstrapping the data: a resampling procedure that allows for generation of samples with equal number of individuals. We generated a pooled sample of species abundance by summing the abundance of each species in all gillnet samples in each habitat-by-season. From the pooled sample, we randomly resampled and replaced 93 individuals. This sample size represented the smallest number of fish captured by a gill net from all seasons and habitat types during the study period. We repeated this procedure to produce a collection of 9999 bootstrap samples and calculated α-diversity in each of them. This generated a sampling distribution of 9999 α-diversity values for each habitat-by-season sample which were used for hypotheses testing. Using sampling distributions for statistical inference is more appropriate than using raw data if the number of samples is small, but the size of each sample is large and representative of the population (e.g., Bickel & Freedman, 1984). This was the case with our data set, which had a total of 8289 fishes distributed in 16 gillnet samples. This data set represented the diversity of the sampled sites with species accumulation curves approaching horizontal asymptotes (see species accumulation curves of habitat-by-season samples; Figures S4 and S5).
However, using sampling distributions does not allow for the application of conventional tests like ANOVA due to violation of the assumption of independence. For this reason, we chose to use bootstrapping techniques to test whether α-diversity differed between pairs of habitat-by-season samples. From two continuous distributions of α-diversity positioned along the x-axis, we randomly and repeatedly sampled a pair of samples and subtracted the sample of the first distribution from the sample of the second distribution. This produced a sampling distribution of differences (SDD) comprising of 9999 differences in α-diversity. We tested if SDD differed from zero (Chernick & LaBudde, 2011; Dixon et al., 1987). SDD comprised of exclusively positive differences when the two distributions did not overlap, and this indicated that all α-diversity values from the first distribution were smaller than values from the second distribution. Negative differences occurred when samples from the first distribution were larger than those from the second distribution, indicating that the distributions overlapped. To estimate the p-value, we calculated the proportion of negative differences in SDD and doubled this proportion to comply with a two-tailed test (Chernick & LaBudde, 2011). The null hypothesis was that the two sampling distributions have similar α-diversity. We did not reject the alternative hypothesis that α-diversity differs between two sampling distributions if P-values were less than or equal to 0.05.
2.3.2 β-diversity
We tested for variation in β-diversity across seasons and habitat types using resampling procedures. For each habitat type and season, we generated a pooled habitat-by-season sample and performed bootstrapping by repeatedly resampling and replacing 93 individuals from the pooled sample. This produced a collection of 9999 bootstrap samples for each habitat-by-season. We combined all habitat-by-season samples to form a large collection of 39,996 bootstrap samples. From the large collection, we generated a dissimilarity matrix using turnover and nestedness metrics described in Podani et al. (2013). We used the dissimilarity matrix in metric multidimensional scaling (MDS) and produced ordinations containing 39,996 scores (i.e., 9999 scores for each sample). The first two axes of the ordination accounted for most of the variation in turnover and nestedness. Therefore, we only considered these axes for further analysis. We used scores from these axes to produce bidimensional sampling distributions that were used to test for species turnover and nestedness across habitat-by-season samples.
We tested whether there was turnover or nestedness between pairs of samples by evaluating the overlap between two bidimensional distributions. This was performed using a bidimensional extension of the bootstrap method used in the previous analysis. From two sampling distributions positioned along the x-axis and y-axis, we repeatedly resampled a pair of bootstrap samples and calculated the differences in the MDS scores. Differences were calculated by subtracting x- and y-scores of the first distribution from the x- and y-scores of the second distribution. This produced a collection of 9999 differences constituting a bidimensional distribution of the differences in MDS scores (dMDS). We plotted dMDS and described its major direction by fitting a vector starting at the origin and cutting across the cloud of points (a no-intercept regression line).
We rotated dMDS by radially moving it around the origin to align its direction with the x-axis and tested if it comprised of differences that differed from the origin. All differences were positive along the x-axis if all the MDS scores sampled from the first distribution were closer to the origin than all the MDS scores sampled from the second distribution. Positive differences indicated no overlap between the two distributions. Differences in MDS scores were negative along the x-axis when the following conditions were met: (a) Samples from the two distributions had a similar direction from the origin to the MDS plot, and (b) the MDS scores sampled from the first distribution were farther from the origin than the MDS scores sampled from the second distribution. Negative differences indicated that the two distributions overlapped. To estimate the p-value, we calculated the proportion of negative differences in dMDS and doubled the proportion to comply with a two-tailed test (Chernick & LaBudde, 2011). The null hypothesis was that the two sampling distributions have similar β-diversity. We did not reject the alternative hypothesis that β-diversity differs between two sampling distributions if p-values were less than or equal to 0.05. All analyses were carried out in R (R Core Team, 2019).
3 RESULTS
3.1 α-diversity
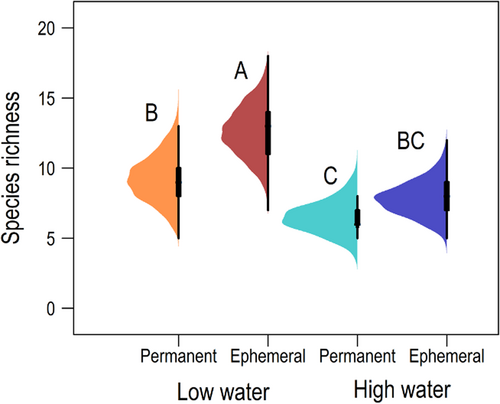
A total of 8289 fishes representing 30 species were collected during the study (Table S1). Species accumulation curves approached horizontal asymptotes for a collection of all samples (Figure S4) and for samples obtained from distinct habitat types and seasons (Figure S5), indicating high sampling sufficiency for documenting α-diversity. Generally, α-diversity was higher in samples collected during the low-water season compared with the high-water season (Figure 1). Samples collected from permanent habitats had approximately three more species during the low-water season compared with the high-water season (p = 0.04) and those collected from ephemeral habitats had approximately five more species during the low-water season compared with the high-water season (p = 0.002). α-diversity was marginally insignificant among samples during the low-water season, with samples collected from ephemeral habitats having approximately four more species compared with those from permanent habitats (p = 0.06). α-diversity was not significantly different among samples during the high-water season (p = 0.13).
3.2 β-diversity
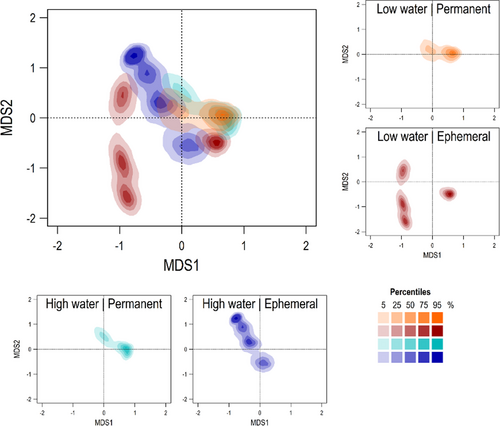
Assemblage composition significantly differentiated among samples during the low-water season (p = 0.02). Turnover in the sample collected from ephemeral habitats occupied a region of assemblage ordination space distinct from other samples during the low-water season (Figure 2). There was no significant difference in turnover among samples from different habitat types during the high-water season (p = 0.07), and among seasons in either permanent (p = 0.40) or ephemeral habitats (p = 0.22). Nestedness was not significantly different among all habitat-by-season samples (Figure S6).
4 DISCUSSION
Analysis of α-diversity and β-diversity showed that hydrology and habitat type play critical roles in maintaining biodiversity in intermittent habitats of the lower Okavango Delta. Within sampled habitats, α-diversity was lowest during the high-water period, and highest during low flows, suggesting that greater connectivity enhanced fish dispersal during the wet season, while habitat loss resulted in crowding within shrinking habitats during the dry season. This partially agrees with Merron and Bruton’s (1995) finding that water persistence and hydroperiod stage were significant determinants of fish diversity patterns in the Thamalakane and Boteti river systems. However, Merron and Bruton (1995) were concerned only with species richness at a broader scale, and they found higher α-diversity in the permanent swamps of the upper delta, compared to the intermittent rivers in the lower delta. By analyzing multiple components of fish diversity, our results suggest potential additional insights into diversity-disturbance relationships in the lower Okavango Delta. Our results showed that β-diversity for the sampled habitats was generally higher during the dry season compared with the wet season. This suggests greater potential for demographic and environmental stochasticity within and among habitat types during this period.
During the wet season, river-floodplain systems are characterized by increased connectivity in three dimensions (longitudinal, lateral, and vertical) which facilitates dispersal of biota, nutrients, and other material among habitats (Fullerton et al. 2010). Fish dispersal allows species to exploit newly available resources and to escape competition and predation in newly created habitat that has low fish densities (Bokhutlo et al. 2016; Junk, 1999; Shimadzu et al. 2013; Winemiller & Jepsen, 1998). In the Okavango Delta, species such as Schilbe intermedius, Clarias gariepinus, and Marcusenius altisambesi usually leave the main river channel during the flood to seek prey and spawn in the flooded area (Merron, 1993; Merron & Mann, 1995). Low per-unit-area densities of fishes in newly expanded habitats may partially explain the lower α-diversity in samples collected during the high-water period.
In perennial river-floodplain systems, during the initial stages of drying, aquatic habitat shrinks in the floodplains, resulting in higher fish densities within the main river channel, and high α-diversity may be encountered within restricted habitat (Grubh & Winemiller, 2018; Merron, 1993; Winemiller & Jepsen, 1998). This could explain the relatively high α-diversity in samples collected from permanent habitats during low flows. In ephemeral habitats, drying generally is accompanied by a rapid decline in species richness due to deterioration of abiotic environmental conditions, even to the point of local extirpation (Benton et al. 2003; Fahrig, 2003; O'Neill, 2016). Therefore, sustained high α-diversity in the sampled ephemeral habitats during low flows could suggest a legacy effect whereby species persist under suboptimal environmental conditions for a limited time, but these levels of α-diversity would not be sustainable if these conditions were chronic (Vass & Langenheder, 2017).
Centuries of seasonal wetting and drying might promote the evolution of adaptive traits that allow fish to cope with environmental stress or disperse within a dynamic habitat mosaic. Physiological and behavioral adaptations might lessen the influence of species sorting during community assembly (Boersma et al. 2014; Brendonck, 1996; Chase, 2003; Loeuille & Leibold, 2008). Such historical contingencies influence demographic responses to disturbances at local scales (Hawkes & Keitt, 2015). In the sampled ephemeral habitats, legacy effects may be strongest during the early portion of the dry season when communities are in a transitional stage. With a prolonged drying and habitat degradation, local fish assemblages are strongly influenced by current conditions. Consequently, greatest changes in community structure should be observed during late stages of the dry season (Boersma et al. 2014; Bogan et al. 2017). Except for species that estivate during drought conditions, aquatic organisms must disperse or else perish when their aquatic habitat dries entirely (Bonada et al. 2006; O'Neill, 2016).
There was significant turnover in species composition across the sampled habitats during the low-water period. This means that during the dry season, biodiversity in different habitats was subjected to different ecological selection pressures or local filters, leading to the divergence of local assemblages and increased β-diversity (Myers et al. 2015; O'Neill, 2016). This is consistent with the patch dynamics and neutral theory metacommunity paradigms (Winemiller et al. 2010) and may indicate the importance of dispersal limitation due to habitat fragmentation (Arrington & Winemiller, 2006; Chase, 2010; Datry et al. 2016; O'Neill, 2016; Tonkin et al. 2017). It could also be due to increased effects of species sorting as a result of intermediate levels of dispersal as the water gradually dried up before complete loss of connectivity (Heino et al. 2015; Leibold & Chase, 2018; Leibold et al. 2004). On the other hand, fish diversity was homogenized across habitats during the wet season due to increased connectivity among habitats, suggesting a strong mass effect in community assembly (Amoros & Bornette, 2002; Bower et al. 2019; Leibold & Chase, 2018; Leibold et al. 2004; Ng et al. 2009; Thomaz et al. 2007).
Analysis of fish diversity patterns for the sampled sites in the lower reaches of the Okavango Delta supports the idea that hydrology plays a crucial role in structuring aquatic communities in intermittent rivers. However, it is important to note that our findings could be a result of sampling artifacts as a result of using gill nets in habitats that differed in size during low-water conditions of the dry season. Gillnets are passive gears that may not obtain a representative sample of the species composition in larger permanent habitats compared with smaller ephemeral habitats where fishes have a greater chance of encountering the net. Therefore, we cannot discount the possibility that some of the variation in our response variables could have been influenced by sampling bias. This could partially explain the markedly higher alpha diversity in ephemeral habitats when compared to permanent habitats during low-water conditions. Further, although our samples were large, collections were made within a restricted area of the landscape, and additional sites might have revealed additional spatial heterogeneity. Thus, extrapolation of the outcomes of our study to regional scales should be done with caution.
Globally, fish in intermittent rivers are threatened by habitat fragmentation, invasion by alien species, groundwater extraction, and climate change (Kerezsy et al. 2017). In the Okavango Delta, significant threats to biodiversity include potential large-scale water abstraction for irrigation schemes, hydropower generation, human consumption, and climate change (Mitchell, 2013). These threats may lead to declines in the fishery, resulting in reduced food security, and diminished social and recreational services with negative consequences on the tourism industry and the economic security of riparian communities. Therefore, annual flow pulses of sufficient magnitude and duration are essential for the maintenance of ecosystem services in this system. This study expands our understanding of intermittent river ecology during a time when the hydrology of these ecosystems is being altered by climate change and water use by humans (Acuña et al. 2014; Ruhí et al. 2016). To further understand the mechanisms that structure aquatic communities in intermittent rivers, future research should examine species functional traits and methods capable of revealing the role of biotic interactions.
ACKNOWLEDGMENTS
We thank the Department of Wildlife and National Parks (DWNP) in Maun for logistical support and assistance in the field during the first sampling in August 2017. This study was supported by the Botswana International University of Science and Technology, through a graduate fellowship awarded to Thethela Bokhutlo. We thank the DWNP for issuing a director’s permit no. 00000654A that made data collection possible.
Open Research
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are openly available in Dryad Digital Repository: https://doi.org/10.5061/dryad.cjsxksn57 (Bokhutlo et al. 2021).




