Leaf litter decomposition in tropical freshwater swamp forests is slower in swamp than non-swamp conditions
Associate Editor: Jennifer Powers
Handling Editor: Ann Russell
Abstract
Decomposition is a key ecosystem function, and the rate of decomposition in forests affects their carbon storage potentials. Processes and factors determining leaf litter decomposition rates in dry-land and temperate forests are well understood, but these are generally poorly studied in tropical wetland forests, especially freshwater swamp forests (FSF). The home-field advantage (HFA) hypothesis predicts that soil microbes specialize in decomposing leaf litter produced by the tree species in their immediate vicinity. However, empirical support for the HFA is equivocal, and the HFA has never been tested in the highly heterogeneous and biodiverse ecosystems of tropical FSFs. We collected leaf litter from swamp and non-swamp tree species in a tropical FSF in Singapore and monitored the decomposition rates of these in swamp and non-swamp plots for a period of eight months. Leaf litter decomposed 3.7 times more slowly in swamp plots. Leaf litter from swamp tree species were significantly poorer in quality (higher C:N ratio) than those of non-swamp FSF tree species, but this had only a weak effect on decomposition rates. There was also only weak evidence for the HFA and only in non-swamp conditions. Our results show that while the leaf litter of tropical FSF swamp and non-swamp tree species differ significantly in chemical traits, litter decomposition rate is ultimately determined by local abiotic conditions, such as hydrology. Additionally, the high FSF tree diversity may prevent decomposer communities from specializing on any group of leaf litter types and thus limit the extent of HFA observed in such heterogeneous forests.
1 INTRODUCTION
Decomposition is a key ecosystem function and is critical to the maintenance of the carbon cycle globally (Aerts, 1997). The rate at which decomposition occurs in forests both determines the productivity and affects the carbon-sequestering ability of systems (Chapin et al., 2002). At the global scale, climate is one of the strongest determinants of leaf litter decomposition rate, with decomposition rates more rapid with higher actual evapotranspiration (Aerts, 1997; Meentemeyer, 1978). However, because decomposition rates in different forest types can differ greatly even within a single climatic zone (Elliott et al., 1993), the carbon storage/sequestration of forests may be poorly estimated if only climatic variables are considered. A key goal of decomposition studies is thus to determine how biotic and abiotic factors interact within specific forest communities to determine decomposition rates.
Leaf traits – particularly those associated with leaf litter quality – are also important predictors of decomposition rates and are even more important determinants of decomposition rates than climate in environments that do not experience extreme temperature or drought stress (Couˆteaux et al., 1995; Meentemeyer, 1978). Leaves with traits associated with low leaf litter quality (e.g., high C:N ratio, high lignin content) generally decompose more slowly because specialized enzymes are required to breakdown recalcitrant carbon forms in them (Chapin et al., 2002; Freschet et al., 2012; Gholz et al., 2000). Most of these studies have been conducted in well-drained or “dry-land” forests, especially those of temperate regions. However, some studies have shown that hydrological conditions are likely to interact with litter quality to determine litter decomposition rates in waterlogged swamp forests. Yule and Gomez (2009) showed that the high-quality leaf litter of a generalist non-swamp tree species, Macaranga tanarius, decomposed faster in pools as compared to on dry areas in tropical PSFs, but the low-quality leaf litter of the congeneric swamp specialist, Macaranga pruinosa, decomposed at similar rates in pools and dry areas of the same habitat. However, such an interaction has remained poorly studied.
For waterlogged habitats such as temperate peat bogs, coastal mangrove forests, and tropical swamp forests, it is generally understood that burial within peat and/or submersion below water table levels slows decomposition through anoxia and acidification (Laiho, 2006). The rate of accumulation of organic matter that is higher than the loss rate through decomposition and runoff leads to the accumulation of a deep layer rich in organic matter known as peat and makes such habitats important sinks of belowground carbon (Chapin et al., 2002; Moore & Basiliko, 2006). The understanding that waterlogging retards decomposition rates is gained from multiple observations, including that drained peat bogs emit large amounts of carbon dioxide due to accelerated decomposition rates immediately post-draining (Chimner & Cooper, 2003; Clarkson et al., 2014; Silvola et al., 1996). Also, buried leaf litter often decomposes slower than leaf litter placed on exposed substrates (Clarkson et al., 2014; Moore et al., 2007; Straková et al., 2012), and litter decomposes faster aerobically than anaerobically under laboratory conditions (Bragazza et al., 2013). In addition, coastal wetlands store significantly greater amounts of carbon (“blue carbon”) per unit area than terrestrial forests (Mcleod et al., 2011). However, this seemingly intuitive understanding is not without problems. A significant number of studies actually show that decomposition rates are not always slower in waterlogged habitats than in climatically similar well-drained habitats (Moore & Basiliko, 2006), and that, in the long term, the drainage of peatlands can sometimes lead to increased carbon storage (Laiho, 2006; Minkkinen et al., 2002; Minkkinen & Laine, 1998). Although anoxia and acidification caused by submersion in waterlogged peat substrates generally reduce decomposition rates, the complex interactions between litter quality, decomposer communities, and environmental conditions such as substrate pH, temperature, and nutrients make it impossible to infer general patterns of decomposition across all types of waterlogged habitats (Laiho, 2006).
There exists a great diversity of swamp habitat subtypes around the globe (Junk et al., 2011) for which our knowledge of ecological processes such as decomposition remains superficial. In the tropics, inland swamp forests can generally be categorized into one of two types: peat swamp forests (PSF) or freshwater swamp forests (FSF). Both tropical PSFs and FSFs occur on permanently waterlogged soils, and many tree species found in them (for which a moderate degree of taxonomic overlap exists) display adaptations to anoxia and unstable substrates (Corner, 1978). However, PSFs are almost exclusively rain-fed (ombrogenous) and thus highly nutrient-limited, whereas FSFs receive dissolved mineral nutrients from rivers and streams, and are thus often nutrient-rich (Page et al. 2006, Chong et al. unpublished data). Several studies have investigated decomposition rates in tropical PSFs (Ong et al., 2017; Yule & Gomez, 2009); but to the best of our knowledge, there are no published studies on decomposition in tropical FSFs.
General patterns of decomposition are important for understanding the process of nutrient cycling. However, the extrapolation of generalizations from the better studied systems of dry-land forests, temperate, and/or coastal mangrove peatlands to tropical inland swamp forests has proven to be difficult (Bragazza et al., 2013; Yule & Gomez, 2009), and differences between the biogeochemical processes within each of these swamp habitat subtypes greatly limit the transferability of findings between them (Laiho, 2006). Even between tropical PSFs and FSFs, despite many similarities in climate, vegetation physiognomy, and biogeochemical properties, differences in soil nutrient levels are still expected to limit the transferability of general patterns of decomposition between the two forest types. Soil and/or substrate nutrient levels are known to interact with leaf litter quality to alter the decomposition rate of leaf litter (Bonanomi et al., 2017; Freschet et al., 2012). Soils of PSFs are very nutrient deficient (Page et al. 2006), and leaf litter quality of PSF species is often very low (Ong et al., 2015; Ong et al., 2017; Yule & Gomez, 2009), but the soils of FSFs are often richer in nutrients (Chong et al. unpublished data), thus species may produce higher-quality leaf litter. Hence, there is a need for studies of decomposition in FSFs, and a need for such studies to investigate the possible interactions between hydrological conditions and litter quality on decomposition rates.
Many studies in dry-land forests have also shown that leaf litter decomposition is faster when leaves decompose at the sites or habitats from which they originate—a phenomenon often referred to as the “home-field advantage” (HFA) (Gholz et al., 2000, Ayres et al., 2009; Ayres, Steltzer, Simmons, et al. 2009; Gießelmann et al., 2011, Freschet et al., 2012; Gao, et al., 2016, Sun & Zhao, 2016; Yu et al. 2015). The likely reason for this is that soil microbes specialize in decomposing the mix of leaves produced by the plant communities at the site (Ayres, Steltzer, Berg, et al., 2009) and/or leaf litter that is most similar in chemical composition/quality to that of the leaf litter matrix (Freschet et al., 2012). Additionally, specialization in particular litter types may develop because both litter production and the decomposition abilities of soil microbes are constrained by local environmental conditions such as nutrient availability (Hoyos-Santillan et al., 2018). However, the HFA is not conclusively demonstrated in all well-drained forests (Gao et al., 2016; Gießelmann et al., 2011; Sun & Zhao, 2016; Veen et al., 2015) and has not been tested extensively in waterlogged habitats. Hoyos-Santillan et al. (2018) found a strong HFA between a high-nutrient, palm-dominated area and a low-nutrient mixed forest area within a neotropical peat swamp forest, but Clarkson et al. (2014) found no evidence of it between restiad peatlands of differing successional stages in New Zealand.
In this study, we aimed to estimate the leaf litter decomposition rates within a catchment containing a tropical freshwater swamp forest and to examine factors affecting these decay rates to understand how leaf litter decomposition responds to different biotic and abiotic conditions of this forest type. Tropical FSFs are spatially heterogeneous in hydrological and edaphic conditions and are thus composed of sub-habitats and sub-communities that are typical of both well-drained and waterlogged forests (K.Y. Chong et al. unpublished data). To our knowledge, there are no published studies on decomposition in tropical freshwater swamp forests. Our findings may be useful for deducing how the ecology of this forest system may change under global change. We tested the following hypotheses: (a) that there is a difference in leaf litter decomposition rates between the well-drained (non-swamp) and waterlogged (swamp) areas within tropical freshwater swamp forests; (b) that litter quality is a good predictor of decomposition rates; and (c) that decomposition rates are affected by the home-field advantage. Hypothesis (a) tests the assumption that the submersion of leaf litter in water slows down decomposition; hypothesis (b) tests whether factors other than litter quality are important in determining decomposition rates of the swamp forests; and hypothesis (c) tests whether microbe communities may be differentiated between swamp and non-swamp local site conditions and/or specialize in decompose the leaf litter produced by trees in such conditions.
2 METHODS
2.1 Study areas
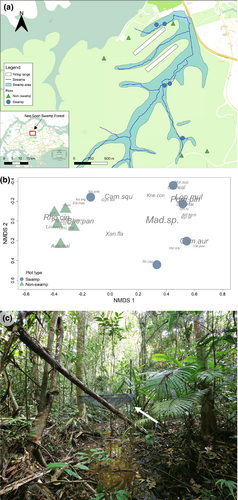
The study was conducted in ten 20 × 20 m vegetation plots (Figure 1a) in the Nee Soon catchment in Singapore (1°23'27.3"N, 103°48'35.5"E). The Nee Soon catchment contains Singapore's last substantial, relatively intact tract of freshwater swamp forest. Five of these plots (hereafter “swamp plots”) occurred in this swamp, with surface water within the plots, frequently waterlogged soil, and a water table that was <1 m below the soil surface level, while the remaining five plots (known hereafter as non-swamp plots) occurred in well-drained areas within the same catchment and did not have any surface water within them. The 10 plots had been established earlier as a part of a larger study involving 40 plots and are used for the monitoring of forest tree communities in the Nee Soon freshwater swamp forest (Chong et al., 2016; 2018). They had been established through a random sampling procedure stratified between all available swamp-type and non-swamp-type areas within the watershed that constitutes the Nee Soon freshwater swamp forest. Tree communities in the five swamp and five non-swamp plots are clearly different (Figure 1b), and litterfall composition between them similarly reflect this difference (Lam et al. unpublished data).
2.2 Litterfall collection and processing
Litterfall was collected between September 2018 and June 2019 at fortnightly intervals from four 1 × 1 m leaf litter traps established in each of these plots. Leaves collected from the five plots of each hydrological condition (swamp or non-swamp) were pooled, sorted by morphospecies, and thereafter dried for 7–21 days at room temperature in a box containing silica gel beads. At every collection, large numbers of species would be represented in litterfall (18.7 ± 12.1 [mean ± SD] species in non-swamp plots; and 14.8 ± 10.9 species in swamp; Lam et al. unpublished data), but the bulk of the litter would be consistently and typically dominated by species that were also dominant in the plant community within that hydrological condition. Leaf litter was accumulated over multiple collections until enough litter was available for the deployment of 60 leaf litter bags, that is, six bags per plot (hereafter referred to as a “batch”). In order to maintain compositional similarity between the litter bag contents for plots of the same hydrological condition while ensuring that they preserved the diversity of leaf litter accumulating with the different plot conditions, leaves of each morphospecies were distributed across all bags within the batch, starting first with the most dominant litter morphospecies of each batch, until the leaf litter in each bag had a combined mass of 6.00–7.00 g.
Litter bags were made from high-density polyethylene netting with 3-mm mesh size to allow entry by mesofaunal detritivores and measured 20 × 23 cm. They were sealed along the edges of the bags using wax from a glue gun after leaves had been packed into them. Litter bags were then randomly assigned to a hydrological condition (swamp or non-swamp) that was either the same (the “home field”) or different (the “away field”) from the one from which they had originated. They were tied to the legs of leaf litter traps using 1-meter long nylon strings for ease of retrieval and left in the field for zero, one, two, four, or eight months. A zero-month (i.e., immediate retrieval) time interval was used to control for potential leaf mass loss caused by handling. A total of 560 litter bags (not including 108 litter bags which were deployed for zero-month time intervals) were deployed and were evenly distributed across plots, plot conditions, and experimental durations.
Decomposition bags were deployed in batches between September 2018 and February 2020, and were planted such that replicates for the shorter-duration retrievals were deployed over the whole period to control for potential effects of weather variations (but our study site, being in the equatorial tropics, is considered relatively aseasonal). After retrieval, the contents of each litter bag were washed and brushed gently over a 2-mm sieve to remove mud and other non-organic debris. They were then dried in an oven at 60°C for six days before being weighed.
2.3 Leaf chemical analyses
To examine the effect of leaf litter quality on decomposition rates, and the possible association between leaf litter quality and hydrological condition, elemental analyses were performed on leaves from the zero-month time interval. Leaves were ground into fine powder using a Rommelsbacher EGK 200 coffee grinder. Elemental carbon and nitrogen composition of these powdered samples were then determined using a vario MICRO cube elemental analyser (Elementar), and the C:N ratio obtained from this. Chemical analyses were performed on seven litter bags from each hydrological condition, with six replicates for each litter bag, resulting in a total of 84 samples.
2.4 Statistical analyses
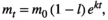 (1)
(1)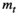 is the dry mass of leaf litter at time
is the dry mass of leaf litter at time 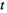 , the number of days that litter was left to decompose in the field;
, the number of days that litter was left to decompose in the field; 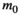 is the initial mass at deployment; l is the proportion of mass lost due to handling;
is the initial mass at deployment; l is the proportion of mass lost due to handling; 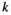 is the decay rate. Equation 1 may be linearized by taking the natural logarithm on both sides:
is the decay rate. Equation 1 may be linearized by taking the natural logarithm on both sides:
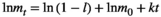 (2)
(2)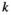 may be conveniently estimated when the log-transformed remaining dry weight of leaf litter is regressed against the duration of field deployment with log-transformed
may be conveniently estimated when the log-transformed remaining dry weight of leaf litter is regressed against the duration of field deployment with log-transformed 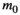 as a covariate. The log-transformed proportion of mass remaining after initial handling
as a covariate. The log-transformed proportion of mass remaining after initial handling 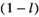 is therefore estimated as the intercept. Furthermore, it was possible to include additional variables in such a model to estimate their potential effects on the rate of decay:
is therefore estimated as the intercept. Furthermore, it was possible to include additional variables in such a model to estimate their potential effects on the rate of decay:
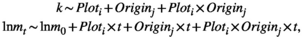 (3)
(3)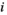 and
and 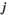 indicate the plot type (swamp or non-swamp) in which decomposition occurred and the origin of the litter (swamp or non-swamp species), respectively. The three-way interaction (
indicate the plot type (swamp or non-swamp) in which decomposition occurred and the origin of the litter (swamp or non-swamp species), respectively. The three-way interaction (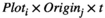 ) is a first but not the only condition to determine whether there is HFA; HFA is considered to manifest whether there is an increase in
) is a first but not the only condition to determine whether there is HFA; HFA is considered to manifest whether there is an increase in 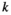 when
when 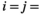 swamp and/or
swamp and/or 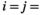 non-swamp. Importantly, this definition of the HFA deviates from the conventional HFA index (Ayres et al., 2009b) by estimating potential asymmetry in the phenomenon (e.g., litter of non-swamp species decompose faster in non-swamp conditions, but litter of swamp species do not decompose faster in swamp conditions).
non-swamp. Importantly, this definition of the HFA deviates from the conventional HFA index (Ayres et al., 2009b) by estimating potential asymmetry in the phenomenon (e.g., litter of non-swamp species decompose faster in non-swamp conditions, but litter of swamp species do not decompose faster in swamp conditions).The model (Equation 3) was fitted using linear mixed-effects models with the R package “nlme” (Pinheiro et al., 2019) and an exponential variance function. The exponential variance function was chosen because the log transformation of the original equation of decomposition (Equation 1) results in errors which scale with remaining litter mass; thus, they are not normally distributed (Adair et al., 2010). The litter batch and the plot where the bags were deployed were included as crossed random effects in the model to account for potential non-independence for the same batch or plot of deployment. Model selection was performed according to Burnham and Anderson (2002) using the R package “MuMIn” (Bartoń, 2016). All models were made to include an intercept (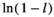 in Equation 2) and the initial mass parameter (
in Equation 2) and the initial mass parameter (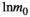 in Equations 2 and 3) as recommended by Adair et al. (2010). Sum-of-variable weights—that is, the sum of the Akaike model weights of all models containing a given variable—were calculated for each variable as an indicator of its importance relative to other variables. All statistical analyses were conducted in the R v3.6.1 statistical programming environment (R Core Team, 2018).
in Equations 2 and 3) as recommended by Adair et al. (2010). Sum-of-variable weights—that is, the sum of the Akaike model weights of all models containing a given variable—were calculated for each variable as an indicator of its importance relative to other variables. All statistical analyses were conducted in the R v3.6.1 statistical programming environment (R Core Team, 2018).
In order to visualize the data and examine the effect of litter origin on decomposition while excluding the overwhelming effects of local site conditions resulting from plot type differences, bootstrapping was performed on the proportion of remaining leaf litter over time. We calculated the value of remaining litter proportion of non-swamp tree species minus that of swamp tree species—a difference value which would be positive if litter from non-swamp tree species decomposed more than that of swamp tree species, and negative if reversed. This value was calculated for each batch of leaf litter and resampled with replacement 9,999 times within each combination of plot type (swamp and non-swamp) and decomposition duration in months (i.e., zero, one, two, four, or eight months). Bootstrapped medians and 95% confidence intervals (CIs) were obtained from this sample.
3 RESULTS
Leaf litter decomposition in our experiments was well approximated by the exponential decay function (Equation 1), with greater mass losses occurring earlier in experiments. All top (ΔAICc < 2) models analyzed had high R2 values of approximately 73% (Table 1). Leaf litter also decomposed much slower in swamp plots than in non-swamp ones. On average, leaves which were left to decompose in swamp plots lost 47% of their dry mass by the end of eight months (the longest experimental duration used in this study), but this value was approximately 80% for leaves left to decompose in non-swamp plots.
| Model rank | Prop. remain (intercept) | Initial mass | Duration | Duration × Plot | Duration × Origin | Duration × Plot ×Origin | df | ΔAICc | Weight |
|
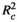
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.603 (±1.066) | 0.549 (±0.586) | −0.0066 (±0.0005) | 0.0048 (±0.0006) | – | – | 8 | 0.000 | 0.427 | 0.729 | 0.729 |
| 2 | 0.679 (±1.068) | 0.508 (±0.587) | −0.0068 (±0.0006) | 0.0048 (±0.0006) | 0.0004 (±0.0006) | – | 9 | 0.456 | 0.340 | 0.730 | 0.730 |
| 3 | 0.688 (±1.062) | 0.503 (±0.583) | −0.0070 (±0.0006) | 0.0052 (±0.0008) | 0.0007 (±0.0008) | −0.0007 (±0.0012) | 10 | 1.217 | 0.233 | 0.731 | 0.731 |
| 4 | 0.794 (±1.138) | 0.445 (±0.624) | −0.0043 (±0.0005) | – | – | – | 7 | 145.460 | <0.001 | 0.636 | 0.636 |
| 5 | 0.885 (±1.142) | 0.396 (±0.626) | −0.0045 (±0.0006) | – | 0.0005 (±0.0007) | – | 8 | 145.628 | <0.001 | 0.637 | 0.637 |
| 6 | 0.766 (±1.337) | 0.370 (±0.734) | – | – | – | – | 6 | 380.779 | <0.001 | 0.415 | 0.415 |
Note
-
The interaction terms containing Plot and Origin estimate the differences in the response when the plot type or litter origin are swamp plots or swamp tree species, instead of dry plots or non-swamp tree species, respectively. Prop. remain = the logarithm of the proportion of remaining mass after accounting for handling loss;
 = marginal R2, the explanatory power of only the fixed-effects terms of the models;
= marginal R2, the explanatory power of only the fixed-effects terms of the models; 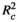 = conditional R2, the explanatory power of both the fixed- and random-effects terms of the models.
= conditional R2, the explanatory power of both the fixed- and random-effects terms of the models.
Decomposition duration (i.e. time 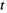 in Equation 1), and the interaction between plot type and decomposition duration were the most important predictors of remaining litter dry mass in retrieved litter bags (Table 1; Figure 2). These two variables each had sum-of-weights values that were close to 1 (Figure 2a) and strong estimated effects in all of the top models with ∆AICc < 2 (Table 1). Thus, the top three models all contained both these two terms – duration and its interaction with plot type—while the remaining three models, which did not contain the interaction term between duration and plot type, had negligible support from the data, with model weights that were approximately zero (Table 1). We thus consider only the models ranked first to third.
in Equation 1), and the interaction between plot type and decomposition duration were the most important predictors of remaining litter dry mass in retrieved litter bags (Table 1; Figure 2). These two variables each had sum-of-weights values that were close to 1 (Figure 2a) and strong estimated effects in all of the top models with ∆AICc < 2 (Table 1). Thus, the top three models all contained both these two terms – duration and its interaction with plot type—while the remaining three models, which did not contain the interaction term between duration and plot type, had negligible support from the data, with model weights that were approximately zero (Table 1). We thus consider only the models ranked first to third.
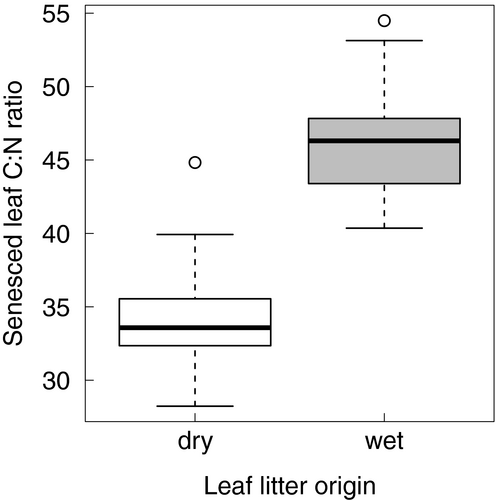
The first-ranked and most parsimonious model of remaining litter dry mass estimated the coefficient of experimental duration to be − 0.0066 (95% CI = [–0.0071, –0.0061]) in non-swamp plots and –0.0018 (95% CI = [–0.0023, –0.0011]) in swamp plots. In other words, the instantaneous rate of litter mass loss was 3.7 × higher in non-swamp than swamp plots. The model ranked second (ΔAICc = 0.456) contained the same variables as the first as well as the interaction between experimental duration and leaf litter origin (Table 1). This model estimated that leaf litter originating from swamp tree species decomposed 0.0004 day−1 more slowly on average than litter originating from non-swamp tree species. The leaf litter origin × decomposition duration term had a sum-of-weights value of almost 60% (Figure 2a), indicating some evidence in support for it as a predictor of remaining litter dry mass. However, the effect was weak, with the bootstrapped 95% CI ranging from 0.0010 day−1 slower to 0.0002 day−1 faster, that is, 95% CIs intercepted with zero when decomposition duration and plot type were controlled for (Figure 2c). Litter quality was evidently different between the types of leaf litter origin, as C:N ratio of litterfall in swamp plots was found to be significantly higher than that in non-swamp plots (t82 = 17.9, p-value < 0.001; Figure 3).
On the whole, there was only weak support for the HFA from the data. The model ranked third (ΔAICc = 1.217) was similar to the first two in all respects, albeit with slight differences in parameter estimates, and the inclusion of the three-way interaction term between experimental duration, leaf litter, and plot type (Table 1) that is the first statistical condition of the HFA. However, bootstrap median estimates of proportion of remaining litter of non-swamp tree versus swamp tree species exhibited the expected hump-shape described by Ayres, Steltzer, Berg, et al. (2009) only for non-swamp litter decomposing in non-swamp plots, albeit with confidence intervals both of model coefficients and bootstrap estimates overlapping zeroes (Table 1; Figure 2c). Leaf litter originating from swamp tree species decomposed 0.0007 day−1 slower on average (95% CI = [0.00, 0.16]) than litter originating from non-swamp tree species for leaf litter decomposing in non-swamp plot types. When leaf litter decomposed in swamp plot types, the model estimated that litter originating from non-swamp and swamp tree species decomposed at rates which are approximately equal (Figure 2c).
4 DISCUSSION
In this study, we examined the decomposition of leaf litter from two groups of co-occurring but functionally divergent tree species (swamp and non-swamp tree species) in contrasting local site conditions (swamp and non-swamp conditions; Figure 1a) in the Nee Soon FSF in Singapore. Decomposition rates in swamp plots were found to be much slower than decomposition rates in non-swamp plots. C:N ratio of leaf litter shed by swamp tree species was also found to be significantly higher (i.e., of a lower quality) than that of non-swamp tree species. But contrary to expectation, there was only weak evidence to suggest that leaf litter origin affected decomposition rate of litter and that leaf litter originating from trees specialized to non-swamp conditions decomposed faster in non-swamp conditions, that is, the HFA. Taken together, these findings suggest that, in FSFs, decomposition rates are overwhelmingly determined, not by traits of the decomposing leaves or by the biotic effects associated with the HFA, but by abiotic—here, hydrological–conditions at the sites of decomposition.
Decomposition is a key process in the global carbon cycle, which has been extensively studied in well-drained forests and more recently in PSFs but remains unexplored in FSFs. We show here that decomposition rates are highly dependent upon local hydrological conditions within sites, with the most parsimonious model estimating decomposition rate (the 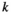 value of Equation 1) to be 0.0066 day−1 in non-swamp conditions, and 0.0018 day−1 in swamp conditions, the latter of which is very close to that estimated by Sulistiyanto (2005), Hoyos-Santillan et al. (2015), and Yule and Gomez (2009) in PSFs. Our findings agree with the general observation that buried or submerged leaf litter in swamps decompose slower than unsubmerged or more exposed leaf litter (Clarkson et al., 2014; Moore et al., 2007; Straková et al., 2012). This observation contrasts starkly with that which is observed in streams and riparian habitats, where submersion often leads to higher decomposition rates (Junk and Furch, 1991; McArthur et al., 1994; Nakajima et al., 2006), possibly because streams are well oxygenated in comparison with the anoxic substrates of swamps. The findings of Yule and Gomez (2009) that leaf litter decomposes faster submerged in pools than in the terrestrial environment of tropical PSFs are anomalous in this regard and may have been caused by unique local site effects. In our study, leaf litter bags from non-swamp plots were often penetrated by plant roots, but this was never observed in leaf litter bags from the swamp plots. It is likely that the mycorrhiza associated with these plant roots would have accelerated decomposition in these litter bags from non-swamp plots. Flooding is known to inhibit both fine root development and mycorrhiza proliferation (Kozlowski, 1997), and this is one possible mechanism for the retardation of decomposition rate under swamp conditions.
value of Equation 1) to be 0.0066 day−1 in non-swamp conditions, and 0.0018 day−1 in swamp conditions, the latter of which is very close to that estimated by Sulistiyanto (2005), Hoyos-Santillan et al. (2015), and Yule and Gomez (2009) in PSFs. Our findings agree with the general observation that buried or submerged leaf litter in swamps decompose slower than unsubmerged or more exposed leaf litter (Clarkson et al., 2014; Moore et al., 2007; Straková et al., 2012). This observation contrasts starkly with that which is observed in streams and riparian habitats, where submersion often leads to higher decomposition rates (Junk and Furch, 1991; McArthur et al., 1994; Nakajima et al., 2006), possibly because streams are well oxygenated in comparison with the anoxic substrates of swamps. The findings of Yule and Gomez (2009) that leaf litter decomposes faster submerged in pools than in the terrestrial environment of tropical PSFs are anomalous in this regard and may have been caused by unique local site effects. In our study, leaf litter bags from non-swamp plots were often penetrated by plant roots, but this was never observed in leaf litter bags from the swamp plots. It is likely that the mycorrhiza associated with these plant roots would have accelerated decomposition in these litter bags from non-swamp plots. Flooding is known to inhibit both fine root development and mycorrhiza proliferation (Kozlowski, 1997), and this is one possible mechanism for the retardation of decomposition rate under swamp conditions.
This information provided by our study also has crucial implications for the management of tropical PSFs and FSFs. Surrounding land use changes (e.g., drainage for agricultural or urban development) and climate change (e.g., decreased or irregular rainfall) can significantly alter ground water table depths in FSFs even while forest cover is retained. Our study shows that such changes can result in more than threefold differences in the potential carbon loss rates from the litterfall of such habitats. Thus, carbon-monitoring programs such as REDD + must evaluate the possibility of hydrological forest degradation when evaluating the state and carbon storage potential of tropical forests (Mertz et al., 2012). Wetland habitats are increasingly being recognized as globally important carbon sinks, with slow decomposition rates being a common attribute shared between these habitats and contributing to their ability to accumulate large reservoirs of belowground organic matter (Iqbal & Shang, 2020; Lovelock & Duarte, 2019; Mcleod et al., 2011).
4.1 Litter quality and the home-field advantage (HFA) in FSFs
Our study also found only a weak effect of traits and HFA on litter decomposition in the Nee Soon FSF in Singapore. Leaves with low C:N ratio contain high amounts of proteins and nucleic acids (nitrogen-containing molecules) relative to the amount of structural tissues such as lignin or cellulose (carbon-rich molecules, which do not contain nitrogen) and are thus considered to be of a high quality. Studies of leaf litter decomposition in well-drained forests have repeatedly shown that high quality leaves with low C:N ratios decompose faster than low-quality leaves with high C:N ratios (Aerts, 1997). However, despite significant differences in C:N ratios between leaf litter of swamp and non-swamp tree species (a finding which agrees with those of Yule and Gomez [2009]), we found only a weak effect of leaf litter origin on decomposition rates. Rather than a simple relationship between C:N ratio and decomposition rate, we argue that other leaf traits such leaf chemistry (e.g., the presence and concentrations of secondary metabolites (Chomel et al., 2016)) may interact with leaf C:N ratios to determine decomposition rates (Ong et al., 2015, 2017). Given the varying ecophysiological stressors affecting trees in dry-land forests, PSFs, and FSFs, and the effect that these stressors may have on plant survival strategies, it seems unlikely that leaf C:N ratios alone may be used to predict decomposition rates across diverse tropical forest types.
Regarding the HFA, our experiments found only very weak evidence supporting this and only in terms of leaves from non-swamp tree species decomposing in non-swamp conditions. Microbial communities are known to play important roles in the decomposition of leaf litter in tropical swamp forests (Too et al., 2018) and were likely to be the main agents of decomposition in our experiments, given that macroinvertebrates had been excluded by the 3-mm mesh size of our leaf litter bags. That a strong HFA was not observed thus suggests that litter-decomposing microbe communities do not segregate clearly between swamp and non-swamp areas in our study site to specialize on breaking down the litters of only the tree species more abundant in these respective areas. The simplest possible explanation for this observation is that the high tropical plant diversity present even between plots sharing the same hydrological conditions prevents decomposer communities from specializing on any group of leaf litter types. This hypothesis is supported by the observation that non-swamp plots were more convergent in canopy tree community composition than swamp plots (Figure 1b) and that the HFA was therefore more evident – even if still extremely weak – in non-swamp conditions. If this is indeed so, then our study suggests that, in highly diverse communities, the “home field” for microbe decomposer communities occurs at the micro scale rather than at the plot or landscape spatial scales. Unfortunately, the design of our study only permitted the testing of whether HFA was observed or not, but did not allow for the testing of various mechanistic theories of the HFA. For example, we neither examined the leaf litter matrix in which leaf litter bags were buried, nor investigated the microbe communities responsible for early- or late-stage decomposition of the litters.
4.2 Limitations and future directions
This study was conducted within the relatively small area of the Nee Soon FSF in Singapore, and the extrapolation of our findings to all FSFs globally should thus be made with caution. Differences in history (e.g., legacies from exploitation), geography (e.g., topography), and biology (e.g., tree species identities) may limit the generalizability of some aspects of our study (e.g., the negligible HFA effect). Nevertheless, we argue that the main finding of this study – that hydrological conditions are the main determinants of leaf litter decomposition in tropical FSFs – represents a phenomenon that is likely to be consistent across all tropical FSFs because it is both pronounced in our study and brought about by the very biochemical processes that define tropical FSFs.
Decomposition rates estimated using litter bag experiments often overestimate decomposition rates slightly, because retrieval and washing of partially to extensively decomposed leaves often result in the loss of small leaf fragments through litter bag meshes. Our study suffers from a similar weakness, and it is thus possible that the k values reported here were slightly higher than they are in reality. Nevertheless, the small mesh sizes (3 mm) used in our study means that this overestimation is likely to be kept to a minimum, since most leaf fragments in even the most extensively decomposed samples were still significantly larger than this. On the other hand, the use of the 3-mm mesh effectively excludes many meso- and macro-detritivores, such the reticulated swamp crab that is endemic to this catchment (Ng, 1990), which may play important roles in the breakdown of litter especially in swamps and other freshwater habitats. Finally, we used a simple exponential model which assumed constant proportional decay rates, but the litter decomposition dynamics are more complex and more labile components would be lost first (Grossman et al., 2020).
Local site abiotic (hydrological, edaphic, and anoxic) conditions vary along a continuum, as do the degrees to which tree species in FSFs specialize or associate with local site conditions (Chong et al. unpublished data). However, we chose to describe both local site conditions and leaf litter origins as binary variables: swamp versus non-swamp. Furthermore, leaf litter in this study was too diverse to carry out a study of differences between species, and we were thus unable to correlate species decomposition rates to species leaf litter qualities and/or species–site condition specialization/association. Examining such correlations may reveal the subtler effects of leaf litter chemistry on decomposition rates and may allow the investigation of the HFA at micro-spatial resolutions (e.g., in the immediate vicinity of parent trees).
ACKNOWLEDGMENTS
This project was initiated as JJL's undergraduate Final Year Project examined by Ryan Chisholm and Hugh Tan who provided helpful feedback and encouragement. We also thank Stuart W. Smith for the constructive comments given on an earlier manuscript draft, and Leng Lee Eng for the technical assistance in leaf elemental analysis. This study was partially supported by the projects “An Estimation and Assessment of Carbon Stocks in the Nee Soon Swamp Forest” and “Ecological Modelling of Plant Responses to Hydrological Changes in the Nee Soon Swamp Forest” funded by National Parks Board, Singapore, and was conducted under the National Parks research permit number NP/RP18-002.
CONFLICT OF INTEREST
We declare we have no competing interests.
AUTHOR CONTRIBUTION
JJL, KYC, SJR, and KSHP designed the study; JJL, PJC, YYT, RC, NER, LWAT, QYH, and WNL collected the data; WNL and JJL analyzed the data; WNL wrote the first manuscript draft; all authors contributed to subsequent revisions and gave final approval of the manuscript for submission.
Open Research
DATA AVAILABILITY STATEMENT
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.hhmgqnkfr (Lam et al., 2020).




