Patterns of variation in fleshy diaspore size and abundance from Late Triassic–Oligocene
ABSTRACT
Vertebrate-mediated seed dispersal is a common attribute of many living plants, and variation in the size and abundance of fleshy diaspores is influenced by regional climate and by the nature of vertebrate seed dispersers among present-day floras. However, potential drivers of large-scale variation in the abundance and size distributions of fleshy diaspores through geological time, and the importance of geographic variation, are incompletely known. This knowledge gap is important because fleshy diaspores are a key mechanism of energy transfer from photosynthesis to animals and may in part explain the diversification of major groups within birds and mammals. Various hypotheses have been proposed to explain variation in the abundance and size distribution of fleshy diaspores through time, including plant–frugivore co-evolution, angiosperm diversification, and changes in vegetational structure and climate. We present a new data set of more than 800 georeferenced fossil diaspore occurrences spanning the Triassic–Oligocene, across low to mid- to high palaeolatitudes. We use this to quantify patterns of long-term change in fleshy diaspores, examining the timing and geographical context of important shifts as a test of the potential evolutionary and climatic explanations. We find that the fleshy fruit sizes of angiosperms increased for much of the Cretaceous, during the early diversification of angiosperms from herbaceous ancestors with small fruits. Nevertheless, this did not cause a substantial net change in the fleshy diaspore size distributions across seed plants, because gymnosperms had achieved a similar size distribution by at least the Late Triassic. Furthermore, gymnosperm-dominated Mesozoic ecosystems were mostly open, and harboured low proportions of specialised frugivores until the latest Cretaceous, suggesting that changes in vegetation structure and plant–frugivore co-evolution were probably not important drivers of fleshy diaspore size distributions over long timescales. Instead, fleshy diaspore size distributions may be largely constrained by physical or life-history limits that are shared among groups and diversify as a plant group expands into different growth forms/sizes, habitats, and climate regimes. Mesozoic gymnosperm floras had a low abundance of fleshy diaspores (<50% fleshy diaspore taxa), that was surpassed by some low-latitude angiosperm floras in the Cretaceous. Eocene angiosperm floras show a mid- to high latitude peak in fleshy fruit abundance, with very high proportions of fleshy fruits that even exceed those seen at low latitudes both in the Eocene and today. Mid- to high latitude proportions of fleshy fruits declined substantially over the Eocene–Oligocene transition, resulting in a shift to more modern-like geographic distributions with the highest proportion of fleshy fruits occurring in low-latitude tropical assemblages. This shift was coincident with global cooling and the onset of Southern Hemisphere glaciation, suggesting that rapid cooling at mid- and high latitudes caused a decrease in availability of the climate conditions most favourable for fleshy fruits in angiosperms. Future research could be focused on examining the environmental niches of modern fleshy fruits, and the potential effects of climate change on fleshy fruit and frugivore diversity.
I. INTRODUCTION
The plant–frugivore mutualism, wherein vertebrate frugivores consume fleshy diaspores (or the fleshy part of the diaspore) and transport their seeds away from the parent plant, is a widespread phenomenon, and is especially prevalent in the tropics (Jordano, 2014). In this ecological interaction, frugivores gain nutrition and energy while aiding the dispersal and, sometimes, the germination of seeds (Fuzessy et al., 2016; Dugger et al., 2019; Fricke et al., 2019). Fleshy diaspores provide an important food source for vertebrates (Jordano, 2014), potentially enabling higher species richness of frugivorous birds, bats and primates in terrestrial ecosystems, and may in part explain the evolutionary diversification of those groups (Fleming & Kress, 2011). The importance of this mutualistic plant–animal interaction has been widely studied on short ecological timescales. For example, primate-, bird- and bat-mediated dispersal facilitates the maintenance and expansion of hyperdiverse tropical rainforests (Jordano, 2014; Dent & Estrada-Villegas, 2021; Muscarella & Fleming, 2007). Plant–frugivore associations also influence the physical and chemical attributes of the fleshy diaspores themselves, for example with a correlation between fruit nutritional quality and frugivore preference (Sorensen, 1984), and between frugivore body size and plant fruit size (Moran & Catterall, 2010; Burns, 2013). A latitudinal gradient in the proportion of fleshy fruits is evident today, with low-latitudes harbouring a greater proportion of fleshy-fruited plant species than mid- to high latitudes (Chen et al., 2017a), suggesting important climatic influences on the production of fleshy diaspores (Zhao et al., 2018). Nevertheless, the potential impacts of these present-day drivers on fleshy diaspore size and abundance through deep time are not well understood.
Direct evidence of frugivory is rare in the fossil record of terrestrial vertebrates, since it relies on well-preserved coprolites and consumulites containing undamaged seeds. Such records include gut remains from the Early Jurassic–Early Cretaceous ornithischians Isaberrysaura (Salgado et al., 2017), and Kunbarrasaurus (Leahey et al., 2015; Molnar & Clifford, 2000), from the Early Cretaceous birds (= Avialae) Jeholornis and Sapeornis (O'Connor et al., 2018; Hu et al., 2022; O'Connor, 2019), and from many placental mammals and birds in the Paleogene (Collinson & Hooker, 1991). More commonly, fossils of seed plant reproductive structures themselves provide indirect evidence of plant–frugivore mutualism. For example, fleshy tissue is often preserved in permineralised, petrified, and compression fossils of fruits and seeds, from which the mode of seed dispersal (i.e. whether they were frugivore dispersed) can be inferred (Hayes et al., 2018; Wilf et al., 2017; Collinson, Manchester & Wilde, 2012; Atkinson, Stockey & Rothwell, 2018). Frugivory can also be inferred indirectly from fossilised endocarps (the stony layer of the fruit wall, which in modern fruits is often covered by an outer fleshy layer), or from fossilised seeds using the Nearest Living Relative approach for those groups for which the fruit type is conserved, e.g. endocarps of Menispermaceae, Icacinaceae, etc. (Doria, Jaramillo & Herrera, 2008; Pigg, Manchester & DeVore, 2008b) and seeds of Vitaceae (Herrera, Manchester & Jaramillo, 2012).
Fleshy seeds first evolved in pteridosperms (‘seed ferns’) as early as the Carboniferous (Tiffney, 1986). Since then, functionally analogous fleshy dispersal units (herein referred to as ‘diaspores’) have also been produced by various groups of gymnosperms and angiosperms (Tiffney, 2004). Seed plants as a whole show considerable variation in the morphology and development of their reproductive structures and the fleshy diaspores of different plant groups are often not homologous. For example, fleshy tissue can be derived from the seed coat, floral parts or extra-floral parts, including the receptacle, aril, interseminal scales and cupule (Bobrov & Romanov, 2019; Cleal & Thomas, 2019). In angiosperms, they are predominantly derived from the ovary.
The mode of seed dispersal is correlated with seed size in living plants [without accounting for phylogeny (Westoby, Leishman & Lord, 1996; Moles et al., 2007)] such that larger seeds are more likely to be dispersed by frugivores. Therefore, previous investigations into the history of frugivore-mediated seed dispersal have focused on analysing changes in seed size through time [see Tiffney (1984), Wing & Tiffney (1987) and Eriksson, Friis & Lofgren (2000) for angiosperms and Tiffney (1986) for non-angiosperms]. Tiffney (1986) showed that pteridosperms and gymnosperms in the late Paleozoic and Mesozoic had average seed volumes that were two or three orders of magnitude greater than Cretaceous angiosperms. Further investigations (Wing & Tiffney, 1987; Tiffney, 1984; Eriksson et al., 2000) have consistently recovered a pattern of small seed size in Cretaceous angiosperms during the early phases of their diversification. Small seed size in early angiosperms, especially in the Early Cretaceous, was followed by a substantial increase in average size and in size disparity from the Late Cretaceous through a sustained period up to the early Cenozoic (Paleocene–Eocene). Studies have also documented the origin and early radiation of important modern frugivores including primates, rodents and mousebirds in the Paleocene–Eocene (Eriksson, 2016; Mayr & Peters, 1998). This was followed by the diversification of frugivorous bats, and the origin of some living groups of frugivorous birds such as trogons and oilbirds by the Oligocene (Eriksson, 2016; Mayr, 2009). Prior to this, the consumption of fruits and fleshy diaspores may primarily have been on a facultative or opportunistic basis by vertebrate herbivores. However, determining the presence of frugivory in extinct groups is difficult, and candidates include Late Cretaceous cimolodontan multituberculates (Chen, Strömberg & Wilson, 2019; Wilson et al., 2012), eutherians, metatherians, meridiolestid dryolestoids (Grossnickle, Smith & Wilson, 2019) and some Mesozoic birds (Hu et al., 2022).
Various hypotheses have been put forward to explain the large-scale patterns of plant diaspore evolution through time, often invoking the importance of co-evolutionary interactions between plants and vertebrate frugivores. The co-evolution hypothesis proposes that the early Paleogene increase in angiosperm seed size resulted from co-evolution between angiosperms and frugivores, which in turn facilitated the development of closed-canopy forests in the early Paleogene (Wing & Tiffney, 1987). The recruitment hypothesis, by contrast, states that the establishment of closed-canopy forests in the early Paleogene enforced a selective pressure towards larger seed sizes, which in turn led to diversification of mammal and bird frugivores (Eriksson et al., 2000; Eriksson, 2008). Either of these hypotheses may explain the long-term diversification of angiosperm diaspore sizes and frugivore diversity since the origin of angiosperms. However, neither hypothesis explains the gymnosperm pattern shown by Tiffney (1986) – that seed size disparity in Cretaceous gymnosperms was higher than that of coeval angiosperms – because neither abundant frugivores (Tiffney, 1986) nor closed-canopy forests (Rees et al., 1999) existed during that time. Furthermore, previous studies lack analytical treatment of palaeogeographic variation in seed (or fruit) sizes, making it difficult to evaluate the effects of climate and geographic variation separately from the effects of major events in the evolution of either plants or frugivores.
Data for extant plants suggest that seed size might be influenced by not only the dispersal mode (e.g. frugivore-mediated), but also by other plant life-history and environmental traits including plant growth form, lifespan, seedling survival rate and time to maturity (Zheng, Guo & Wang, 2017; Moles et al., 2005; Moles & Westoby, 2006), vegetation type and net primary productivity (Moles et al., 2007), and latitude (Moles & Westoby, 2003; Moles et al., 2007; Leslie, Beaulieu & Mathews, 2017). For example, average seed size is greater in the tropics than at mid-latitudes today, and also greater in closed-canopy rainforests than in open grasslands (Moles et al., 2007). Similarly, plants with greater photosynthetic capacity (including large plants) produce larger seeds (Moles et al., 2005). Interestingly, the increase in angiosperm fruit size during the Late Cretaceous occurred in parallel with a rapid expansion of leaf vein density (Boyce et al., 2009; Feild et al., 2011). Leaf vein density is related to photosynthetic capacity (indicating plant productivity), and therefore might have been an important factor influencing energy investment in larger diaspore sizes during the Late Cretaceous phase of angiosperm diversification. This raises the possibility that the expansion of seed size at large phylogenetic scales is driven by a combination of life-history and environmental factors, and that seed size may not be well explained by hypotheses related to plant–frugivore interactions alone.
Due to the incompleteness of the fossil record of diaspores and historical sampling bias towards mid-latitude regions, databases of previous studies that looked at diaspore size through time were limited by their geographical extent, and by the absence of more recent fossil discoveries from low-latitude palaeofloras, e.g. floras from the Paleocene of South America and Oligocene of South China (Doria et al., 2008; Wing et al., 2009; Chen & Manchester, 2015; Dong et al., 2015; Chen et al., 2017b; Liufu, Chen & Wang, 2017; Hu et al., 2017). We present a new data set including more than 800 georeferenced occurrences of fossil diaspores to quantify patterns of fleshy diaspore size and abundance through time, at both low and mid- to high palaeolatitudes. We use this to evaluate various hypotheses previously put forward to explain the variation in fleshy diaspore size and their relative abundance through geological time, and to propose new hypotheses for novel findings. The analyses presented in this study go beyond previous work in that they focus on fleshy diaspore size (rather than on all types of diaspores) and cover a large geographical range to quantify spatial variation between low- and mid- to high latitude regions.
II. METHODS
(1) Palaeobotanical data
We compiled a new global database of georeferenced fossil diaspore occurrences. We included both fleshy and non-fleshy diaspores of angiosperms (used to analyse the proportion of fleshy diaspores in angiosperms through time), as well as fleshy diaspores of gymnosperms throughout the fossil record (used in our analyses of fleshy diaspore size distributions through time), and both fleshy and non-fleshy diaspores of gymnosperms from well-sampled palaeofloras (used in our analyses of the proportion of fleshy diaspores in well-sampled assemblages through time). These data allowed us to assess the impact of climate, frugivore proportions and plant diversification on fleshy diaspore sizes and the proportion of fleshy diaspores within palaeofloras through time and space. Our data set spans from the Late Triassic to Oligocene, therefore spanning from the first appearance of ‘modern’ fleshy gymnosperm groups (e.g. Cycadales, Ginkgoales) in the Late Triassic, through the Cretaceous origin and diversification of angiosperms and their fruits, up to the change in floral composition associated with the onset of icehouse conditions at the Eocene–Oligocene boundary. Occurrences span from low to mid- to high palaeolatitudes, allowing characterisation of latitudinal variation in fleshy diaspore size distributions and abundance, and how they have varied through time. Each record also has information on the geological formation bearing the plant remains, including age and location (co-ordinates in decimal degrees) as well as palaeocoordinates calculated with the function ‘reconstruct’ from the R package chronosphere using the PaleoMAP PaleoAtlas database (Scotese, 2016). These palaeocoordinates were used in analyses to group records as ‘low latitude’ (absolute palaeolatitudes of 0–35°), or ‘mid- to high latitude’ (>35°). These latitude bins were used since most of the data are spread between 20° and 50°. To ensure congruence in taxonomic assignment, we followed the gymnosperm classification in Anderson, Anderson & Cleal (2007) and the angiosperm classification in APG IV (Chase et al., 2016).
The term ‘diaspore’ here refers to the unit of dispersal. It may be an isolated seed (where the organ is dehiscent) or the entire seed-bearing organ itself (where it is indehiscent). Diaspore trait data were compiled from published descriptions of whole floras [e.g. the Middle Jurassic Yorkshire flora (Harris, Millington & Miller, 1961); Early Cretaceous Torres Vedras flora (Friis, Crane & Pedersen, 2019c); the early Eocene London Clay flora (Collinson, 1983)], and from individual reports of seeds, fruits and ovulate organs [e.g. isolated Jurassic records of ginkgoalean seeds (Zheng & Zhou, 2004); caytonialean seeds (Elgorriaga, Escapa & Cúneo, 2019); cycadalean seed (Spencer et al., 2017); Maastrichtian Vitaceae fruits (Manchester, Kapgate & Wen, 2013); and various Paleogene fruits (Pigg, DeVore & Wojciechowski, 2008a; Hu et al., 2017; Han et al., 2018a). The complete data set, with sources listed, is available as online supporting information (Table S1).
(2) Fossil diaspore size
Three measurements of diaspore size were assembled from the literature: length, breadth, and thickness in millimetres. Wherever breadth and thickness were not reported, we used the shape of the diaspore to estimate their values. For example, the breadth and thickness of a spherical diaspore are the same as its length, the ‘diameter’ of an ellipsoid diaspore is the same as its breadth and thickness, but not length. Where only length and breadth were mentioned with no clear reference to the shape of the diaspore, its thickness was assumed to be 66% of the shorter measurement (either length or breadth), after Tiffney (1984). Where no measurements were provided but a scaled image was available, length and breadth were measured manually using the image-processing software ImageJ v1.53e (Schneider, Rasband & Eliceiri, 2012). Volume of the diaspore in mm3 was calculated as (4/3) × π × length × breadth × thickness, after Tiffney (1984). Records that had only measurements of length were not used for volumetric calculation.
(3) Fossil diaspore dispersal mode
Along with diaspore size, we recorded other morphological traits, based on published descriptions and interpretations, including diaspore type (i.e. drupe, berry, follicle, achene, nut, capsule, cupulate seed, multi-ovulate organ), complexity of the fruit (for angiosperms only), presence of fleshy tissue and presence of wings. Each diaspore record was categorised as frugivore dispersed (i.e. the diaspore or its fleshy tissue is consumed, and undamaged seeds are transported by vertebrate frugivores) or non-frugivore dispersed, based on its morphological attributes as described or interpreted by the original author(s). We classified a diaspore fossil as frugivore dispersed when it was preserved with evidence of fleshy tissue in permineralised, silicified or coalified remains, irrespective of where the tissue was derived from (e.g. from the seed coat, outer fruit wall or accessory tissues). We corroborated this categorisation using information from descriptions given in publications (e.g. descriptions including the terms ‘fleshy’, ‘carnose’, ‘sarcotesta’ were used to infer fleshiness). Further, we classified a diaspore as endozoochorous when it was preserved as an endocarp (stony outer layer of fruit wall which is generally enclosed by a fleshy layer). Wherever the diaspore was badly preserved, or its published description lacked information on the mode of dispersal, we referred to its most recent taxonomic assignment, e.g. isolated seeds assigned to the grape family Vitaceae (Herrera et al., 2012). We then used the Nearest Living Relative approach to infer its mode of dispersal (e.g. for fossil endocarps/seeds belonging to Menispermaceae, Vitaceae, Lauraceae, Icacinaceae, Sabiaceae and the genus Sabal (Areaceae), which are very similar to modern taxa and have fleshy drupes/berries). We did not use this approach for taxa in which the dispersal syndrome is very plastic (e.g. Rutaceae, Rosaceae, Anacardiaceae, etc). If no taxonomic or morphological information could be used to infer dispersal mode, then the record was excluded from our analyses.
Our analyses of diaspore size through time used data from fleshy diaspores only, and are intended to provide a test of the importance of hypothesised plant–frugivore interactions on fleshy diaspore distributions through time by comparison to the fossil record of frugivore diversity.
(4) Fleshy fruit abundance
We used within-assemblage proportions of morphotypes with fleshy diaspores (for gymnosperm and angiosperm floras) and within-interval/latitudinal zone proportions of morphotypes with fleshy diaspores (for angiosperms only) as indices for the abundance of fleshy diaspores within ecosystems through time. We used proportions of fleshy diaspore morphotypes instead of total counts because variation in the count of fleshy diaspore morphotypes will be influenced by variation in the species/morphotype counts, which underwent large increases during the diversification of angiosperms (Niklas, Tiffney & Knoll, 1983, 2014; Knoll, 1986), and by other biases related to collection and sampling methods. Proportions provide a measure of the relative abundance of fleshy diaspores, independent of the overall species count.
Our analyses of fleshy diaspore proportions through time were conducted at two scales. First we analysed the proportion of fleshy diaspores among angiosperms through time, and secondly we analysed the proportion of fleshy and non-fleshy diaspores within well-sampled palaeofloras, for both angiosperms and gymnosperms (e.g. the Middle Jurassic Yorkshire flora and the Late Triassic Kap-Stewart flora (Harris et al., 1961; Harris, 1926). Exclusion of gymnosperms from analyses of fleshy diaspore proportions through time was pragmatic, because it would require tabulating large counts of gymnosperm diaspore morphotypes. Nevertheless, gymnosperm data were included in our analyses of well-sampled floras, as they may enable a better understanding of the proportions of taxa within assemblages comparable to present-day floras.
Broader-scale analyses (within-intervals/latitudinal zones rather than assemblages) were restricted to angiosperms because isolated dry diaspore records that are needed to calculate this ratio are only available for angiosperms in our data set. Proportions were calculated as the richness of fleshy diaspore species divided by the total species richness of diaspores within the given flora or within the given time interval and region. For regional analyses, data were parsed into two latitude zones: low latitude (0–35°), and mid- to high latitude (>35°).
(5) Modern fruit size data
Diaspore size data for modern tropical and temperate fleshy diaspores was compiled from selected studies on fruit–frugivore networks, including both gymnosperm diaspores and angiosperm fruits. We include six data sets of fleshy diaspore size covering low to middle latitudes from both hemispheres: Neotropical rainforest (Wheelwright et al., 1984; Bello et al., 2017), Afrotropical rainforest (Dowsett-Lemaire, 1988), Paleotropical rainforest (Kitamura et al., 2002), Mediterranean shrubland (Herrera, 1987), and cool and warm temperate forests (Nakanishi, 1996). Since the Atlantic Forest data (Bello et al., 2017) cover a large area of the eastern coast of equatorial South America, inclusion of the whole data set might pose the problem of spatial autocorrelation. Hence, we used a subset of these data to include records from only the Parque Estadual de Intervales state park. We chose this area because the relevant data come from recent ecological network studies including observations of avian and mammalian frugivores (Silva et al., 2002; Izar, 2008). These data are available in Table S2.
(6) Modern fleshy fruit proportions
Presence-only occurrence data for angiosperms and gymnosperms was downloaded from GBIF (https://www.gbif.org/) using the R package rgbif. The species occurrences were then cleaned to exclude duplicated and non-terrestrial records using the function cc_sea and cc_dupl from the R package CoordinateCleaner v. 2.0.2. Thereafter, fruit-trait data (frugivore dispersed or not frugivore dispersed) were imported from plant trait databases (Bello et al., 2017; Kattge et al., 2020; Jordano, 2008; Maitner et al., 2018; Tovar et al., 2020; Hawes et al., 2020; Falster et al., 2021) and matched with species occurrence data (see Table S3). The proportion of frugivore-dispersed species was calculated for low and mid- to high latitudes separately. All analyses were performed in R version 4.1.3. The R code for species-level fruit trait synonymization and original fruit trait data sets are available on request to the corresponding author.
(7) Statistical tests of diaspore size differences
We evaluated differences in global fleshy diaspore size distributions through time for well-sampled intervals of the Mesozoic and Cenozoic, including the Late Triassic, Early Jurassic, Middle Jurassic, Early Cretaceous, mid-Cretaceous (Albian–Cenomanian), individual stages during the Late Cretaceous, the Paleocene, Ypresian, Lutetian, Bartonian–Priabonian, Oligocene and modern. These intervals were identified as being relatively well sampled based on the number of fleshy diaspore species available in the fossil record (the minimum was set at seven species, to include some distributions in the Mesozoic; Table 1). Medians and inter-quantile ranges of size distributions with temporal binning and latitudinal binning (low and mid- to high latitude) were calculated for visualisation purposes and for qualitative comparisons. Pairwise differences were investigated using non-parametric Mann–Whitney Wilcoxon rank-sum tests, with and without Bonferroni correction. This specifically investigates whether size data might have been drawn from the same distribution, independent of sample size (i.e. does not evaluate differences in species richness). Diaspore size distributions are generally not parametric so we used non-parameteric statistical tests, and summary statistics such as the median and inter-quantile ranges that are subject to smaller outlier-bias effects.
(8) Statistical test of the effects of preservation type on diaspore size
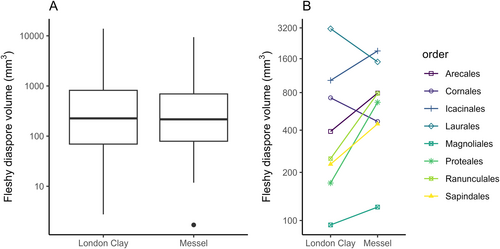
To assess the effects of preservation mode on diaspore size, we compared the distributions in two European Eocene floras: (i) the mid-Eocene Messel Lagerstätte flora from Germany; and (ii) the Ypresian London Clay flora from the UK. We selected these floras because both show uniformity in effect of preservation across all specimens (pyritisation in London Clay and compression in Messel Lagerstatte). The two distributions were statistically similar (two sample t-test on log-transformed diaspore size, P = 0.84; see Fig. 1). The distinct preservation modes of these environments thus do not result in systematic differences in reported fleshy diaspore size.
(9) Proportion of frugivorous taxa in vertebrate groups
We identified the potential for fruit consumption amongst terrestrial dinosaurs, birds and mammals and used their occurrence records to document the maximum proportion of frugivores amongst these vertebrate groups as an index for the frequency of vertebrate fruit consumption through time. Occurrence records for these tetrapod groups, with genus-level or higher assignments, were downloaded from the Paleobiology Database (http://paleodb.org). The data were spatially categorised into low latitude and mid- to high zones using the same approach used for diaspores (see Section II.1). This data set is available in Table S4.
To categorise the potential for fruit consumption among birds, non-avian dinosaurs, and mammals, we first referred to direct evidence of seed consumption based on the fossil record of their gut contents, with the assumption that such evidence may indicate fruit consumption.
(a) Non-avialan dinosaurs
Various groups of herbivorous dinosaurs existed from the Jurassic up to the end of the Cretaceous period. These include sauropods, ornithischians and several groups within Theropoda (Barrett, 2014). Of these, megaherbivorous ornithischians, i.e. ankylosaurs, hadrosaurs stegosaurs, iguanodonts (Early Jurassic–Cretaceous) were widespread and have a good fossil record, whereas small-bodied herbivorous ornithischians including pachycephalosaurs, leptoceratopsids (Late Cretaceous), and heterodontosaurids are rarer as fossils (Butler et al., 2009a,b, 2010; Barrett, 2014). Preserved gymnosperm seeds in small-bodied iguanodontian (Isaberrysaura) and ankylosaurian (Kunbarrasaurus) gut contents provide direct evidence of at least opportunistic fruit consumption by herbivorous dinosaurs (Salgado et al., 2017; Leahey et al., 2015; Molnar & Clifford, 2000). Here, we hypothesised that megaherbivores that browsed at low heights, primarily small-sized sauropods, ceratopsians, ankylosaurs, hadrosaurids and iguanodonts (Mallon et al., 2013), were likely to feed upon fleshy diaspores of Caytoniales, Bennettitales, Cycadales and angiosperms. These are grouped as ‘other small-sized ornithischians’. Further, we categorised small-bodied herbivores such as hypsilophodonts, heterodontosaurids, pachycephalosaurs and leptoceratopsids as potential frugivores. Cretaceous non-avialan theropod clades are also thought to have been plant-eaters that browsed at low heights, e.g. oviraptorosaurids, ornithomimimosaurids, and therizinosaurs (Zanno & Makovicky, 2011; Barrett, 2014; O'Connor, 2019), and hence are categorised here as frugivorous.
(b) Birds
Birds (Avialae) first appeared in the Late Jurassic and became taxonomically diverse by the time of the Early Cretaceous Lagerstätten of the Chinese Jehol Biota. Early-diverging avialans like the Jeholornithidae, Sapeornithidae and Confuciusornithidae from the Jehol Biota are preserved with consumulites containing intact gymnospermous seeds (O'Connor, 2019). Most specimens containing seeds also possess gastroliths, indicating a possibly granivorous diet, but a few are preserved without gastroliths, making fruit consumption more likely as an explanation for seeds within gut contents (Ksepka, Grande & Mayr, 2019; Hu et al., 2022). Although the degree of importance of fruit consumption in Mesozoic stem birds is not known (Miller & Pittman, 2020) its existence cannot be ruled out and there is direct evidence of fruit consumption at least in the Early Cretaceous bird Jeholornis (Hu et al., 2022). Hence, we include these groups as potential fruit consumers in our analyses.
Modern bird groups with strictly frugivorous species include curassows and guans (Crassidae), cassowaries (Casuariidae), mousebirds (Coliidae), touracos (Musophagidae), trogons (Trogonidae), oilbirds (Steatornithidae), parrots (Psittacidae), toucans (Ramphastidae), hornbills (Bucerotidae), and various passerine families (e.g. Cotingidae, Euylaimidae, Ptilonorhynchidae). We classified extinct species on the stem lineages of these groups as potential frugivores (including opportunistic frugivores) using the following approaches. (i) We used the Nearest Living Relative approach, where there is evidence of little to no change in the morphology of an extant frugivorous group since the Paleogene, including Casuariiformes (cassowaries and emus) and Trogoniformes (trogons). The Trogonidae and Casuariidae – which include important Neotropical and Paleotropical frugivores today – have undergone little morphological change in the last 50 million years (Mayr, 2009), and hence their Eocene ancestors are likely also to have been frugivorous. (ii) Previous inference from beak morphology or gastroliths suggesting frugivory, including Turnicidae (buttonquails), Gallinuloididae and Quercymegapodiidae (Galliformes), and Psittacopedidae (stem parrots) (Mayr, 2009). (iii) Direct evidence of seeds or fruits in gut contents, including Messelornis (Messelornithidae; stem rails and cranes), Primobucco and Eocoracias (Primobucconidae and Eocoracidae; stem rollers), Eoglaucidium pallas (Sandcoleidae; stem mousebirds), Selmes absurdipes and Masillacolius brevidactylus (Coliiformes; crown mousebirds), Primozygodactylus major (Zygodactylidae; stem passerines) (Mayr & Peters, 1998; Mayr, 1998, 2015, 2018; Peters, 1999; Mayr & Mourer-Chauviré, 2000; Morlo, 2004). Although the gut contents of these species are preserved with seeds, the general consensus is that they probably engaged in opportunistic rather than high-frequency frugivory (Mayr, 2009). There are six species of mousebirds today, all of which exclusively consume fruits. Gut contents of Paleogene stem and crown Colliformes indicate that they are likely to have been omnivores, and not specialist frugivores, unlike their modern relatives. Extant parrots prominently consume seeds, fruit pulp, and flowers, while extant passerines show a wide spectrum of dietary adaptations, ranging from obligate frugivory to insectivory. We classified the Psittacopedidae and Zygodactylidae as frugivores, considering the possibility that they may have fed on fruits opportunistically. In addition to these, two genera of arboreal rollers (Primobucco and Eocoracias) and one stem-crane (Messelornis) from Messel also fed on fruits (Mayr, 2009). Living descendants of Eocene rollers and cranes are insectivorous and piscivorous respectively, but it is possible that they acquired this habit recently. Hence, we classified them as being potentially frugivorous.
(c) Mammals
Mammaliaforms originated in the Late Triassic and crown-group mammals (including monotremes, therians, their most recent common ancestor and all of its descendants) diversified in the Middle Jurassic. Recent advances in mammal ecomorphology have demonstrated a Late Cretaceous diversification in mammalian dietary strategies (Grossnickle & Polly, 2013; Grossnickle & Newham, 2016; Wilson et al., 2012). Among stem-group therian mammals, the gondwanatherians (Campanian–Maastrichtian), cimolodontan multituberculates (Late Cretaceous–Eocene), and mesungulatoids (Late Cretaceous) are known to have engaged in a high degree of plant-based omnivory and may have been opportunistic consumers of fruit (Schultz et al., 2014; Wilson et al., 2012). Further, among eutherians, ‘archaic ungulates’ are possible candidates for plant-dominated omnivory (indicating the potential for opportunistic fruit consumption) during the latest Cretaceous (Harper, Parras & Rougier, 2019; Wilson Mantilla et al., 2021; Grossnickle & Newham, 2016). We therefore categorised these groups as potential fruit consumers.
The early Paleogene fossil record of South American land mammals shows dominance of ground-dwelling metatherians that occupied the folivore–frugivore, insectivore–frugivore and obligate frugivore niches (Woodburne et al., 2014). The early Eocene Itaborai fauna from tropical Brazil contained many possible frugivorous species belonging to the Protodidelphidae, Bonapartheriidae (Polydolopimorphia) and Caroloameghiniidae, which are known mainly from their bunodont teeth (Oliveira & Goin, 2011). Further, comparative models based on extant marsupial ecomorphological variation and the isolated postcranial fossil material from Itaborai point towards an abundance of arboreality in the extinct metatherians (Szalay & Sargis, 2001). Out of the 11 morphotypes of ulnae studied, eight were classified as scansorial/arboreal. This evidence suggests that Eocene marsupials from the Itaborai fauna might predominantly have been arboreal fruit-eaters, and hence we classify these taxa as being potential consumers of fruit.
Around the same time, the northern mid-latitude mammalian communities were dominated by placentals, some of which were clearly arboreal herbivores (Franzen & Wilde, 2003; Franzen, 2006; Franzen & Habersetzer, 2017; Smith, Schaal & Habersetzer, 2018; Collinson & Hooker, 1991). Species containing seeds in their gut mass from the German middle Eocene Messel Lagerstätte fauna include the herbivorous ground-dwelling perissodactyls (Eurohippus, Propalaeotherium), artiodactyls (Aumelasia, Messelobunodon), an arboreal rodent Eogliravus and an arboreal primate Darwinius. Some insectivorous and piscivorous species experimenting with opportunistic frugivory also show seeds in their gut, e.g. Buxolestes and Kopidodon (Schaal, Ziegler & Shaffer-Fehre, 1992). The primate Darwinius masilae, with an intact seed in its gut, shows adaptation for life in the canopy and an entirely plant-based diet (Franzen & Wilde, 2003). The diverse herbivore fauna at Messel would have engaged in different levels of fruit consumption. For this study, we categorised the perissodactyls, artiodactyls, rodents and primates – i.e. major present-day fruit-consuming groups for which fossilised gut material containing seeds is also known – as potential frugivores in the early Paleogene (Franzen & Wilde, 2003; Franzen, 2006; Schaal et al., 1992).
The final data set of potentially frugivorous mammal, bird and non-avialan dinosaur occurrences is available in Table S4.
III. RESULTS
(1) Fleshy diaspore richness through time based on existing data in the Paleobiology database
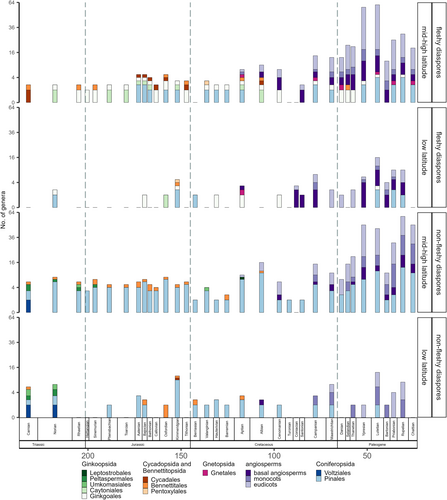
Figure 2 shows the genus richness of seed plants (Spermatophyta) separately for genera with fleshy and non-fleshy diaspores from Late Triassic to Oligocene, based on existing data in the Paleobiology Database (http://paleobiodb.org; see Table S5 for the data used to create this figure). Richness for fleshy and non-fleshy genera was calculated for each geological stage, across mid- to high latitudes and low latitudes. Plant classification follows Anderson et al. (2007).
(2) Diaspore size through time and between well-sampled intervals
There is no evidence for a large-scale change in the size distribution of gymnosperm fleshy diaspores from the Late Triassic up to the present day (Fig. 3). By contrast, fleshy fruit sizes of angiosperms were initially small in the early Cretaceous, increased substantially after the mid-Cretaceous up to the Campanian, and thereafter underwent little change.
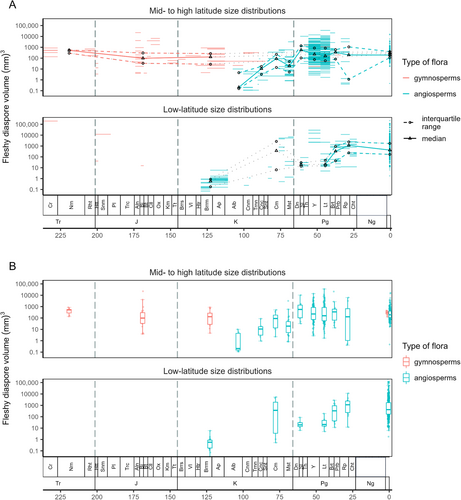
Fleshy diaspore size distributions of gymnosperms from the Late Triassic (median 524 mm3, interquartile range 325–697 mm3), Early Jurassic (41, 23–166 mm3; note small sample size, N = 7) and Middle Jurassic (89, 34–279 mm3) are statistically similar to those of the Early Cretaceous (130, 28–265 mm3) (Tables 1 and 2; Mann–Whitney pairwise comparisons of well-sampled time intervals, Bonferroni-adjusted and unadjusted P values). All of these Mesozoic gymnosperm assemblages are from mid- to high latitudes; we lack knowledge of their low-latitude size distribution (Fig. 3). The size distributions of fleshy diaspores in Mesozoic gymnosperms are similar to those of angiosperms from the Campanian (although note the small Campanian sample size, N = 14), Maastrichtian, Paleocene, Eocene, Oligocene and present day (Fig. 3; Table 2, adjusted P values). Tiffney (1986) also reported that the diaspore size range of Mesozoic gymnosperms (and also Paleozoic pteridosperms) was similar to that of Cenozoic angiosperms. Mesozoic median gymnosperm diaspore size (Fig. 3B, Tables 1 and 2) was larger than that of the Early Cretaceous angiosperms.
Early and mid-Cretaceous angiosperms produced very small fleshy diaspores at low and mid- to high latitudes (0.58, 0.18–0.87; 0.21, and 0.16–4.6 mm3, respectively; Fig. 3, Table 1; see Eriksson et al., 2000; Wing & Tiffney, 1987). The global Early Cretaceous Barremian–Aptian distribution is significantly different from those of most other angiosperm and gymnosperm distributions (Table 2). Angiosperm fleshy diaspore sizes increased after the Cenomanian (Fig. 3), reaching a median of 11 mm3 in the Coniacian and 90 mm3 in the Campanian, at mid- to high latitudes. At low latitudes, median size in the Campanian was 365 mm3 (Table 1, but note the low sample size, N = 7). The global Campanian angiosperm fleshy diaspore size distribution is significantly different from the Early Cretaceous angiosperm distribution, but not from the Mesozoic gymnosperm distributions and Paleogene angiosperm distributions (Table 2). The global Maastrichtian angiosperm distribution is different from Early Cretaceous angiosperms, and most Cenozoic angiosperms (Table 2).
In the Cenozoic, the only significant difference in global fleshy diaspore distributions is between Lutetian (median 150 mm3) and modern global angiosperm distribution (median 288 mm3) (Table 2). The potentially smaller diaspore size of low-latitude Lutetian angiosperms compared to modern angiosperms occurred during a period in which there were widespread closed-canopy rainforests, which are habitats with the largest seed sizes today (Moles et al., 2007).
(3) Proportions of fleshy diaspores through time
(a) Within assemblages
Well-sampled Mesozoic assemblages preserving fleshy diaspores are known from a wide range of palaeolatitudes, although for low-latitude assemblages the earliest data are available only from the Early Cretaceous (Fig. 4). Mid- to high latitude assemblages of the Mesozoic generally have low proportions of fleshy diaspores (<50%; Fig. 4). These include gymnosperm diaspore assemblages from the Late Triassic to Early Cretaceous (Late Triassic Molteno Formation, Triassic–Jurassic Kap Stewart Formation, Middle Jurassic Yorkshire flora, Early Cretaceous Rajmahal Formation), angiosperm diaspore assemblages from the mid-Cretaceous (Shet-Irgiz Formation in Kazhakhstan), the Coniacian–Campanian (Klikov, Asen and lower Heidelberg Formations in central Europe), the Maastrichtian (Deccan Intertrappean beds in India), and mixed assemblages from Barremian–Aptian (Crato formation in Brazil and Yixian Formation in China). In most of these Mesozoic ecosystems, a large proportion of diaspores consisted of dry diaspores that were not dispersed by frugivores, as also reported by Wing & Tiffney (1987).
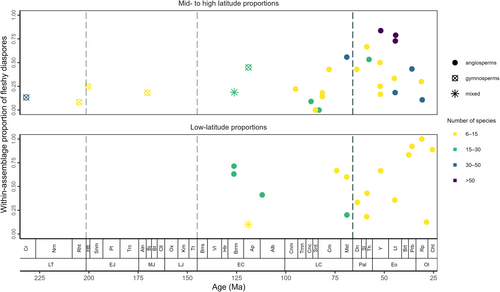
Some well-sampled assemblages of the Cretaceous show higher abundances of fleshy diaspores (>50%), including low-latitude assemblages in southern Europe (Barremian–Albian, Figueira da Foz and Almargem formations which consist of basal angiosperms that grew in disturbed alluvial floodplain areas; Friis et al., 2018a), but despite the greater abundance of fleshy fruits in these floras, their sizes remained very small (0.1–10 mm3), e.g. in eastern Europe (Maastrichtian; Densus-Ciula Formation; Lindfors et al., 2010), central America [Campanian; Cerro del Pueblo Formation (Rodríguez-de la Rosa & Cevallos-Ferriz, 1994; Cevallos-Ferriz, Estrada-Ruiz & Perez-Hernandez, 2008; Cevallos-Ferriz & Vazquez-Rueda, 2016; Hayes et al., 2018)], and the mid-latitudes of central Europe (Maastrichtian; upper Heidelberg Formation; Knobloch & Mai, 1986).
(b) Across assemblages
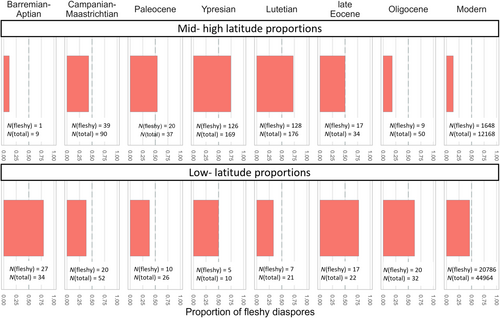
The relative abundance of fleshy fruits across all angiosperm fossil diaspore occurrences was low at mid- to high latitudes during the Cretaceous (~11% in the Barremian–Aptian and 43% in Campanian–Maastrichtian), and then increased in the Eocene (Ypresian 74%; Lutetian 72%) (Fig. 5). Some of the highest proportions of fleshy diaspores/fruits are observed at mid- to high latitudes during the early-mid-Eocene, substantially different to the present-day pattern in which fleshy fruits are considerably more abundant at low latitudes. Proportions of fleshy fruits at mid- to high latitudes then decreased through the late Eocene (50%), to reach very low proportions in the Oligocene (18%). Well-sampled low-latitude carpofloras are rare, but the existing data suggest that the fleshy fruit abundance increased from the Paleocene (38%) to the late Eocene (77%), followed by only a slight decrease in the Oligocene (63%). The Eocene–Oligocene transition thus saw a substantial decrease in fleshy fruit proportions at mid-latitudes, but only a slight decrease at low-latitudes. This results in an overall pattern of decreasing fleshy fruit abundance with latitude in the Oligocene, similar to that of present-day floras (Fig. 5).
(4) Frugivore abundance through time
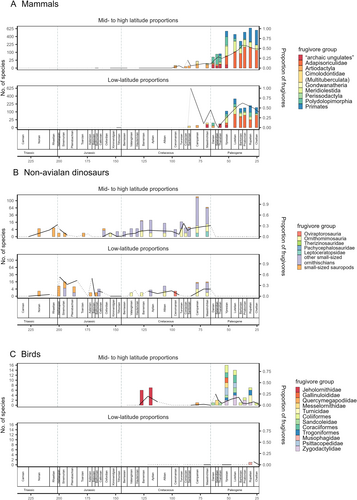
During the Mesozoic, the proportions of potentially frugivorous non-avialan dinosaurs appear to increase slightly during the Early Cretaceous, in both low and mid- to high latitude regions (Fig. 6B). At mid- to high latitudes, this is coincident with increasing diversity within Ornithopoda (Butler et al., 2009a). There was also an increase in the abundance of dinosaur and mammal frugivores in the Late Cretaceous (Fig. 6A,B). The Campanian–Maastrichtian increase in the proportional abundance of potentially frugivorous non-avialan dinosaurs in mid- to high latitudes is coincident with increased diversity within the Ornithomimosauridae, Oviraptorosauridae, Pachycephalosauridae and Leptoceratopsidae. In mammals, increasing species richness of potentially frugivorous metatherians, eutherians and multituberculates is likely responsible for the increase in frugivore proportions from the Turonian to the Danian. After a brief mid-Paleocene dip, this pattern of increase continues from the Thanetian into the late Eocene (Bartonian) both in low (from 12% to 64%) and mid- to high latitudes. Frugivore proportions across mammals decrease from the Bartonian to the Chattian at low and mid- to high latitudes (Fig. 6A).
IV. DISCUSSION
(1) Comparisons with previous studies
Our results differ from previous analyses (e.g. Huegele & Manchester, 2020; Manchester et al., 2013; Kapgate, Manchester & Stuppy, 2017; Matsunaga et al., 2018, 2019; Smith et al., 2021; Hayes et al., 2018; Atkinson, Stockey & Rothwell, 2016; Stockey, Nishida & Atkinson, 2016) in several aspects, partly due to addition of new data and partly due to the expanded spatial scope of this study, which allows comparison of patterns at mid- to high palaeolatitudes with those from low palaeolatitudes. Firstly, we find that angiosperm fleshy fruit size increased sharply after the mid-Cretaceous, followed by an extended interval of relatively little change in median fleshy fruit size and interquartile range from the Campanian onwards (Tables 1 and 2, Fig. 3). This differs from a previous hypothesis of a more protracted and continuous increase in fruit size from the Late Cretaceous to the Eocene (Eriksson et al., 2000). Second, previous studies recovered a post-Eocene pattern of decrease in angiosperm fruit size coinciding with a climate shift that saw mid-northern latitudes transition from a hot and humid climate to a cool temperate regime (Eriksson et al., 2000). Interestingly, our findings suggest an opposite pattern for low latitudes, where the median size and size variation of fleshy fruits may have increased from the late Eocene to the Oligocene (Fig. 3). Third, we find a shift in the latitudinal distribution of fleshy fruit abundance between the Eocene and Oligocene. Fleshy fruits were most abundant in mid- to high latitude floras during the Paleocene and early to mid-Eocene (Fig. 5). This contrasts with Oligocene and present-day floras, in which fleshy fruits are more abundant in the tropics than at mid- to high latitudes. This latitudinal shift in peak abundance of fleshy fruits results from a decrease in their relative abundance at mid- to high latitudes during the Eocene/Oligocene transition (Fig. 4).
| Latitude | Plant group | Mean age (million years) | Time interval | Minimum (mm3) | Q1 (mm3) | Median (mm3) | Q3 (mm3) | Maximum (mm3) | Median absolute deviation (mm3) | Sample size |
|---|---|---|---|---|---|---|---|---|---|---|
| Global | Gymnosperms | 219.15 | Late Triassic | 135.5 | 324.6 | 523.6 | 696.9 | 199051.3 | 295.1 | 9 |
| Global | Gymnosperms | 187.7 | Early Jurassic | 3.5 | 22.9 | 41.5 | 166.3 | 12031.5 | 56.4 | 7 |
| Global | Gymnosperms | 168.8 | Middle Jurassic | 4.0 | 33.5 | 88.7 | 279.1 | 22449.3 | 108.4 | 23 |
| Global | Gymnosperms | 122.75 | Early Cretaceous | 4.7 | 27.6 | 129.5 | 264.8 | 881.7 | 160.7 | 13 |
| Global | Angiosperms | 122.75 | Early Cretaceous | 0.0 | 0.2 | 0.6 | 0.9 | 5.9 | 0.5 | 24 |
| Global | Angiosperms | 103.45 | Mid-Cretaceous | 0.1 | 0.2 | 0.6 | 2.5 | 10.1 | 0.7 | 12 |
| Global | Angiosperms | 88.05 | Coniacian | 0.9 | 3.3 | 10.7 | 17.1 | 87.1 | 11.8 | 7 |
| Global | Angiosperms | 77.85 | Campanian | 0.5 | 11.1 | 90.2 | 480.0 | 5393.0 | 132.5 | 14 |
| Global | Angiosperms | 69.05 | Maastrichtian | 0.8 | 6.0 | 19.7 | 57.7 | 6927.9 | 25.3 | 29 |
| Global | Angiosperms | 61 | Paleocene | 6.4 | 25.0 | 86.6 | 575.4 | 10295.4 | 106.5 | 19 |
| Global | Angiosperms | 51.9 | Ypresian | 2.7 | 80.2 | 244.2 | 1069.2 | 28730.9 | 324.8 | 114 |
| Global | Angiosperms | 44.5 | Lutetian | 1.7 | 49.6 | 149.7 | 737.4 | 35734.9 | 182.7 | 115 |
| Global | Angiosperms | 37.5 | Late Eocene | 11.4 | 43.8 | 330.0 | 763.9 | 21232.1 | 435.8 | 27 |
| Global | Angiosperms | 28.465 | Oligocene | 0.4 | 140.9 | 673.0 | 2390.3 | 11501.4 | 996.4 | 24 |
| Global | Gymnosperms | 0.5 | Modern | 124.8 | 288.0 | 384.5 | 490.9 | 8181.2 | 160.7 | 10 |
| Global | Angiosperms | 0.5 | Modern | 0.5 | 133.3 | 288.0 | 904.8 | 214639.2 | 314.6 | 826 |
| Mid-high | Gymnosperms | 219.15 | Late Triassic | 135.5 | 285.8 | 521.0 | 566.9 | 863.9 | 276.0 | 8 |
| Mid-high | Gymnosperms | 168.8 | Middle Jurassic | 4.0 | 33.5 | 98.3 | 297.0 | 22449.3 | 129.1 | 22 |
| Low | Angiosperms | 122.75 | Early Cretaceous | 0.0 | 0.2 | 0.6 | 0.9 | 5.9 | 0.5 | 24 |
| Mid-high | Gymnosperms | 122.75 | Early Cretaceous | 4.7 | 27.6 | 129.5 | 264.8 | 881.7 | 160.7 | 13 |
| Mid-high | Angiosperms | 103.45 | Mid-Cretaceous | 0.1 | 0.2 | 0.2 | 4.6 | 10.1 | 0.1 | 7 |
| Mid-high | Angiosperms | 88.05 | Coniacian | 0.9 | 3.3 | 10.7 | 17.1 | 87.1 | 11.8 | 7 |
| Low | Angiosperms | 77.85 | Campanian | 0.5 | 6.4 | 364.9 | 2653.6 | 5393.0 | 540.3 | 7 |
| Mid-high | Angiosperms | 77.85 | Campanian | 2.3 | 13.9 | 89.8 | 222.2 | 518.4 | 117.0 | 7 |
| Mid-high | Angiosperms | 69.05 | Maastrichtian | 0.8 | 5.4 | 19.4 | 47.9 | 3190.6 | 24.3 | 24 |
| Low | Angiosperms | 61 | Paleocene | 6.4 | 13.9 | 19.3 | 29.9 | 86.6 | 13.6 | 8 |
| Mid-high | Angiosperms | 61 | Paleocene | 32.3 | 104.6 | 565.5 | 1368.8 | 10295.4 | 765.8 | 11 |
| Mid-high | Angiosperms | 51.9 | Ypresian | 2.7 | 74.1 | 225.8 | 848.6 | 13887.3 | 295.1 | 109 |
| Low | Angiosperms | 44.5 | Lutetian | 5.0 | 15.0 | 20.8 | 48.5 | 190.1 | 23.4 | 7 |
| Mid-high | Angiosperms | 44.5 | Lutetian | 1.7 | 58.0 | 157.2 | 839.2 | 35734.9 | 186.7 | 108 |
| Low | Angiosperms | 37.5 | Late Eocene | 11.4 | 43.2 | 330.0 | 923.6 | 2807.2 | 472.4 | 13 |
| Mid-high | Angiosperms | 37.5 | Late Eocene | 13.7 | 82.4 | 347.5 | 599.9 | 21232.1 | 429.7 | 14 |
| Low | Angiosperms | 28.465 | Oligocene | 12.1 | 235.4 | 1156.8 | 2390.3 | 11501.4 | 1466.0 | 16 |
| Mid-high | Angiosperms | 28.465 | Oligocene | 0.4 | 1.1 | 195.8 | 1082.7 | 6263.6 | 289.0 | 8 |
| Low | Angiosperms | 0.5 | Modern | 1.8 | 169.3 | 422.5 | 1719.8 | 214639.2 | 479.5 | 560 |
| Mid-high | Gymnosperms | 0.5 | Modern | 124.8 | 232.8 | 297.1 | 384.5 | 458.2 | 150.9 | 7 |
| Mid-high | Angiosperms | 0.5 | Modern | 0.5 | 111.1 | 192.1 | 398.9 | 16424.3 | 160.3 | 266 |
| Late Triassic gymnosperms | Early Jurassic gymnosperms | Middle Jurassic gymnosperms | Early Cretaceous gymnosperms | Early Cretaceous angiosperms | Mid Cretaceous angiosperms | Coniacian angiosperms | Campanian angiosperms | Maastrichtian angiosperms | Paleocene angiosperms | Ypresian angiosperms | Lutetian angiosperms | Late Eocene angiosperms | Oligocene angiosperms | Modern angiosperms | Modern gymnosperms | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Late Triassic gymnosperms | 1 | 1 | 1 | 0.24 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 0.84 | 1 | 0 | 0.12 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Early Jurassic gymnosperms | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Middle Jurassic gymnosperms | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 0.84 | 1 | 1 | 0 | 1 | 1 | 1 | 0.48 | 1 | 1 | 1 | 1 | 1 | 0.12 | 1 | ||
| Early Cretaceous gymnosperms | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Early Cretaceous gymnosperms | 0.24 | 1 | 0 | 0 | 1 | 1 | 0.12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Mid Cretaceous angiosperms | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.12 | 1 | |
| 0.12 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.48 | 0 | 0 | 0 | 0.12 | 0 | 0.48 | ||
| Coniacian angiosperms | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0.48 | 0.48 | 0.96 | 1 | 0.12 | 1 | ||
| Campanian angiosperms | 1 | 1 | 1 | 1 | 0.12 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Maastrichtian angiosperms | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0.6 | 1 | 0 | 1 | |
| 0 | 1 | 0.48 | 1 | 0 | 1 | 1 | 1 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0.12 | ||
| Paleocene angiosperms | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 0 | 0.48 | 1 | 1 | 0.6 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Ypresian angiosperms | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 0 | 0 | 0.48 | 1 | 0 | 1 | 1 | 1 | 1 | 0.6 | 1 | ||
| Lutetian angiosperms | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0.12 | 1 | |
| 1 | 1 | 1 | 1 | 0 | 0 | 0.48 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | ||
| Late Eocene angiosperms | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0.6 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 0 | 0 | 0.96 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Oligocene angiosperms | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 0 | 0.12 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Modern angiosperms | 1 | 1 | 1 | 1 | 0 | 0.12 | 1 | 1 | 0 | 1 | 1 | 0.12 | 1 | 1 | 1 | |
| 1 | 1 | 0.12 | 1 | 0 | 0 | 0.12 | 1 | 0 | 1 | 0.6 | 0 | 1 | 1 | 1 | ||
| Modern gymnosperms | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 0 | 0.48 | 1 | 1 | 0.12 | 1 | 1 | 1 | 1 | 1 | 1 |
(2) Differences in fleshy diaspore distributions between well-sampled intervals
Our analyses suggest that Mesozoic size distributions of fleshy diaspores are not statistically different from those of Campanian and early Cenozoic angiosperm assemblages. This comparison involved well-sampled gymnosperm assemblages from the Late Triassic, Early Jurassic, Middle Jurassic and the Early Cretaceous and angiosperm-dominated floras of the Campanian, Palaeocene and early–mid-Eocene. Therefore, by the Campanian, Late Mesozoic angiosperm fleshy fruits had a similar size distribution to that present much earlier in gymnosperm fleshy diaspores (Fig. 3; Table 1).
The similarity of Mesozoic gymnosperm diaspore size distributions to those of late Mesozoic and early Cenozoic angiosperms is surprising, considering that: (i) Middle Jurassic and Early Cretaceous fleshy gymnosperms were less diverse than early Cenozoic fleshy angiosperms; and (ii) most present-day fleshy diaspore-bearing gymnosperms are restricted to mid-latitude open-canopy forests, whereas the early Cenozoic mid-latitude angiosperms occupied closed-canopy forests (hypothesised to facilitate larger fruit sizes; Eriksson, 2008). This prompts re-evaluation of the hypotheses reviewed by Eriksson (2008) that attempt to explain increases in angiosperm seed size during the late Mesozoic and early Cenozoic, including (i) selection for larger seeds resulted from co-evolution with frugivores (the co-evolution hypothesis; Wing & Tiffney, 1987), (ii) selection for larger seeds resulted from the establishment of dense, closed-canopy forests (the recruitment hypothesis; Eriksson et al., 2000), and (iii) a larger plant growth form necessitated the development of larger seeds (the life-form hypothesis; Moles et al., 2005).
(a) Co-evolution hypothesis
Our results indicate that potential frugivores co-existed with fleshy gymnosperms throughout the Mesozoic. During the Barremian–Aptian and at the end of the Cretaceous, there are increases in proportion of frugivores (Fig. 6), in agreement with the available data on avian and non-avialan dinosaur and mammal feeding ecology, indicating the presence of granivory in Early Cretaceous avialans (Hu et al., 2022), increased herbivory in Late Cretaceous non-avialans (Zanno & Makovicky, 2011; Barrett, 2014; O'Connor, 2019), and a Late Cretaceous origin of specialised herbivory in multituberculate mammals (Grossnickle & Newham, 2016; Grossnickle et al., 2019; Wilson et al., 2012; Chen et al., 2019). In spite of large changes in potential frugivore assemblages, size distributions of fleshy gymnosperm diaspores of the Mesozoic are similar to Paleocene–Eocene angiosperm fruit size distributions. This suggests that, although plant–frugivore interactions must have occurred throughout most of the evolutionary history of seed plants, fleshy diaspore size distributions were not driven directly by changes in the relative abundance of frugivory.
(b) Recruitment hypothesis
The recruitment hypothesis argues that the establishment of multi-stratal, closed-canopy forests in the early Cenozoic resulted in selection for larger seed sizes, enabling the radiation of vertebrate frugivores (Eriksson, 2008). This hypothesis includes two distinct components: firstly, that large seed size in angiosperms was enabled by the establishment of closed-canopy forests, and secondly that vertebrate frugivores diversified after the diversification of fleshy diaspores sizes. The patterns shown here are consistent with the recruitment hypotheses for Cenozoic angiosperms. However, the environmental component of the recruitment hypothesis does not provide an explanation for the occurrence of the large maximum fleshy diaspore sizes of Mesozoic gymnosperms (Fig. 3). Although gymnosperm-dominated wet forests existed in the Triassic–Jurassic, there is little evidence that these forests had a multi-stratal canopy structure. A hot and humid, ‘tropical’ climate in the Triassic–Jurassic is thought to have been restricted to small equatorial areas (Ziegler et al., 2003; Rees et al., 1999), although a recent study suggests that the extent of ‘tropical rainforest’ climate might have increased in the Cretaceous (Burgener et al., 2023). However, both leaf vein length per area (VLA) and carbon isotope analyses have shown that even Late Cretaceous tropical rainforests lacked a multistratal, closed-canopy structure similar to that of modern tropical rainforests (Carvalho et al., 2021; Graham et al., 2019). Hence, the large fleshy diaspore sizes of Mesozoic gymnosperms can not be explained by selection in a closed-canopy environment. It is of course possible that gymnosperm-dominated semi-open environments of the Mesozoic had different seed recruitment dynamics from present-day, angiosperm-dominated open environments, such that large seed size was favoured in Mesozoic open-canopy gymnosperms, but this was not the case in the Cenozoic.
(c) Life-form hypothesis
Previous studies have documented an increasing disparity of angiosperm fruit sizes from the mid-Cretaceous to the Paleocene that co-occurred with evolutionary diversification of angiosperms into different environments (Coiffard et al., 2012), growth forms and habits (Isnard et al., 2012), and productivity levels (Boyce et al., 2009; Feild et al., 2011). This pattern was also evident for angiosperms in the present study. Earlier events of plant diversification, for example of Paleozoic pteridosperms, had also resulted in diverse fleshy seed sizes in the late Carboniferous (Tiffney, 1986; Sims, 2000), concurrent with their expansion into diverse habitats and growth forms (DiMichele et al., 2009). Moles et al. (2005) showed that divergence in seed size within the Spermatophyta (seed plants) resulted from divergence in growth form and hypothesised that seed size and growth form are part of a spectrum of intercorrelated life-history traits, which also include plant size, plant lifespan, time to first reproduction and seedling survival. Thus, one possibility is that similarities between the size distributions of Middle Jurassic and Early Cretaceous gymnosperms and those of Paleocene–Eocene angiosperms may be explained by shared physical or life-history limitations. Our data support the hypothesis that at large phylogenetic and temporal scales, expansion of diaspore sizes within major plant groups is governed by the diversification of plant height, growth form, habitat and net productivity as the plant group undergoes an initial phase of radiation. However, this does not provide an explanation for within-group variation in fleshy diaspore size distributions on Earth today, for which climate variation (Moles et al., 2007) or co-evolution with frugivores (Eriksson, 2016) may have a role. The small but significant differences between the diaspore size distributions of Lutetian and present-day angiosperms (Tables 1 and 2) suggest that other ecophysiological factors, in conjunction with climate, could have enforced an upper limit on diaspore size in the Lutetian. These factors could potentially be related to the existence of hothouse conditions in the Eocene (Holz, 2015; Lowenstein & Demicco, 2006).
(3) Late Cretaceous increase in angiosperm fleshy fruit size
Our results identify a pattern of sharp increase in angiosperm fleshy diaspore size during the Late Cretaceous followed by little net change until the Oligocene, although we note that the sample size of fossil diaspores known from some periods (e.g. the Campanian) is small (Table 1). While the Late Cretaceous increase may be explained by an expansion of life-history traits associated with angiosperm diversification (see Section IV.2.c), it could also be related to the diversification of leaf hydraulic capacity – an ecophysiological trait that underwent rapid evolution in Late Cretaceous angiosperms to reach levels four times as high as those in contemporaneous gymnosperms (Brodribb & Feild, 2010; Feild et al., 2011). Leaf hydraulic capacity is related to photosynthetic capacity (or primary productivity), which may partly control the ecological range of the plant (and potentially also climatic feedback mechanisms; Boyce et al., 2009). This suite of traits may have allowed angiosperms to move out of their exclusively dark and disturbed environments in the Barremian–Aptian (Feild et al., 2004; Coiffard, Gomez & Thevenard, 2007; Coiffard et al., 2012, 2006) to occupy more stable floodplain areas, by the Turonian.
Moles et al. (2005) documented weak associations between seed size and a variety of factors, including net primary productivity, leaf area index, precipitation, and temperature. While life-history traits such as size, lifespan, time to first reproduction and seedling survival are relevant to all plants, the expansion in photosynthetic capacity is unique to angiosperms, and could be associated with the Late Cretaceous increase in fleshy fruit size.
(4) Fleshy diaspore abundance through time
We used within-assemblage and whole-assemblage proportions of fleshy diaspores as indices of the abundance of fleshy fruits through time. There is a well-known present-day latitudinal gradient in the proportion of fleshy fruits, i.e. fleshy fruits are more abundant at low latitudes than at high latitudes (Chen et al., 2017b). However, the evolutionary drivers for this pattern remain unclear. Our results document three phases of fleshy diaspore abundance: (i) while Mesozoic data are more scarce, they suggest a potential increase in the abundance of fleshy diaspores at low latitudes after the Late Cretaceous (Figs 4 and 5); (ii) the proportion of fleshy diaspores appears to increase at both low and mid- to high latitudes during Paleocene–Eocene hothouse conditions, resulting in a higher abundance of fleshy diaspores at mid- to high latitudes than at low latitudes (i.e. a mid- to high latitude peak of fleshy diaspore abundance); (iii) a large decrease in proportion of fleshy diaspores at mid- to high latitudes occurred during the Eocene–Oligocene transition to icehouse climates, coupled with only a minor decrease in low-latitude proportion. These changes gave rise to the present-day pattern of higher abundance at low latitudes than at mid- to high latitudes (i.e. higher fleshy fruit abundance in the tropics).
Previous studies have proposed that a high abundance of fleshy fruits is related to strict co-evolution with frugivores and to multistratal, closed-type vegetation (Wing & Tiffney, 1987; Eriksson, 2016, 2008), but such forests did not evolve until the Paleocene (Carvalho et al., 2021; Graham et al., 2019). Therefore, the low proportions of fleshy diaspores seen in the Mesozoic, both within and across assemblages (Figs 4 and 5) could be explained by the scarcity of closed-canopy forests in the Mesozoic. Our results also document that relatively few frugivores were present until the Late Cretaceous (Fig. 6), raising the possibility of a long period in which fleshy-diaspore–frugivore interactions were scarce. Nevertheless, a few Cretaceous angiosperm-dominated assemblages do show a proportion of fleshy diaspores exceeding 50% [Almargem Formation (low latitude, 63%) and Figueira da Foz Formation (low latitude, 71%) in the Early Cretaceous; Cerro del Pueblo Formation in the Campanian (low latitude, 67%) and Densus-Ciula Formation (low latitude, 60%) and Heidelberg Formation in the Maastrichtian (56%); Fig. 4], suggesting the potential for higher abundance of fleshy diaspores in some Mesozoic floras. More data are needed to discern a clear spatiotemporal pattern in this index.
Both within-assemblage and across-assemblage proportions of fleshy fruits increased in the Paleocene, more so at mid- to high latitudes than at low latitudes, and these levels were maintained until the Lutetian (Figs 4 and 5). Our results also document a large, sustained increase in the proportion of frugivorous mammals from the Selandian to the Bartonian (Fig. 6A), which lags substantially behind the Paleocene increase in fleshy fruit abundance and thus is more consistent with the recruitment hypothesis than with the co-evolution hypothesis. This suggests that the Paleocene increase in fleshy diaspore abundance may be related to the Palaeocene–Eocene Thermal Maximum and the establishment of closed-canopy forests (Jaramillo et al., 2010) rather than to changes in the frugivore assemblage.
Our results suggest a substantial reduction in the proportion of fleshy fruits within temperate regions during the Eocene–Oligocene transition, accompanied by only a minor increase in tropical-subtropical regions (Figs 4 and 5). The Eocene–Oligocene transition is characterised by a shift from a hothouse to icehouse climate, and the onset of a modern climatic gradient (Liu et al., 2009; Hren et al., 2013). Analyses of leaf architecture, including the leaf climate analysis multivariate program (CLAMP) and the leaf margin analysis and coexistence approach, to estimate mid-latitude palaeoclimates and vegetation in the late Eocene–Oligocene point towards the emergence of a cool and dry climate at mid-latitudes and a wet subtropical climate with low seasonality at low latitudes (Sheldon et al., 2009; Collinson, 2014; Steinthorsdottir et al., 2016; Herman et al., 2017; Su et al., 2019; DeVore & Pigg, 2010; Li et al., 2019b). Studies of floristic changes suggest that modern tropical and sub-tropical taxa (many of which are fleshy-fruited) retreated from a more latitudinally widespread distribution in the Eocene to a latitudinally restricted tropical distribution in the Oligocene [e.g. Icacinaceae (Del Rio & De Franceschi, 2020; Del Rio et al., 2021b), Menispermaceae (Han et al., 2018b), Annonaceae (Li et al., 2019a), tribe Spondiadoidea (Fu et al., 2017)]. This pattern could reflect the contraction of favourable warm, wet climate conditions for fleshy-fruited and mixed-fruited groups.
Climate may have primarily driven the pattern in proportion of fleshy fruits either directly (by affecting the composition of existing vegetation) or indirectly (by causing range shifts of frugivores from cool to warm regions, in turn facilitating range shifts of fleshy angiosperms to low latitudes). However, low-latitude proportions of mammalian frugivores decreased from 64% in the Bartonian (late Eocene) to 43% in the Chattian (late Oligocene), similar to the reduction in their mid- to high latitude proportion from 57% to 46% (Fig. 6A). This suggests there were regional extinctions of frugivores, more consistent with a hypothesis in which the shift in fleshy fruit abundance was directly climate driven rather than animal driven. Data on Paleogene frugivorous birds are too scarce to observe clear trends in their diversity (Fig. 6C). However, considering that birds can cover larger geographical ranges faster than most mammals, and that tropical birds constitute the largest component of modern frugivorous vertebrates, it is likely that they were more influential than mammals in the dispersal of fleshy-fruited angiosperms at low latitudes from the end of the Paleogene.
V. CONCLUSIONS
- (1)
There has been no net change in fleshy diaspore size distributions since at least the Late Triassic, despite extensive turnover between gymnosperm-dominated and angiosperm-dominated floras. The independent evolution of similar fleshy diaspore size distributions in gymnosperms and angiosperms supports the hypothesis that the fleshy diaspore size ranges of major plant groups are largely determined by shared physical or life-history limits along a spectrum of intercorrelated life-history traits, which also include size, lifespan, time to first reproduction and seedling survival (i.e. the life-form hypothesis).
- (2)
Against this background, individual plant groups show a pattern of increases in diaspore size and size disparity during early radiations. This is shown here for angiosperms [see also Tiffney (1984) and Eriksson et al. (2000)]. This suggests that expansion into different terrestrial environments and climate regimes led to diversification of life-history traits. Such adaptive radiations in pteridosperms (DiMichele et al., 2010, 2009; DiMichele, Gastaldo & Pfefferkorn, 2005; DiMichele, Pfefferkorn & Gastaldo, 2001), early gymnosperms (Spalletti, Artabe & Morel, 2003), and early angiosperms repeatedly coincided with expansions in their diaspore sizes (Tiffney, 1986).
- (3)
Angiosperm fleshy fruit size and size disparity increased in the Late Cretaceous, and thereafter underwent little to no change up to the Eocene.
- (4)
Local and regional abundance of fleshy fruits was less than or equal to 50% from the Late Triassic to the Late Cretaceous, with a few exceptions provided by low-latitude angiosperm-dominated assemblages of the Barremian–Aptian and Maastrichtian. Fleshy fruits became more abundant in angiosperm-dominated forests of the early Cenozoic, at mid- to high latitudes, and perhaps achieved peak abundance in the Ypresian. Abundance of potential frugivores also increased from the Danian, but unlike fleshy fruit abundance, it peaked in Bartonian (Late Eocene), when fleshy fruits were becoming less abundant at mid- to high latitudes. Thus the evolution of frugivores may have followed patterns of variation in fleshy fruit abundance, and not vice versa.
- (5)
Peak abundance of fleshy diaspores (mainly angiosperm fruits) occurred at mid latitudes during the early Cenozoic. A shift to the present-day pattern of peak abundances in low-latitude, tropical floras occurred during the Eocene–Oligocene transition, caused by large decreases at mid- to high latitudes, coupled with smaller decreases at low latitudes. This shift is co-incident with a climatic change from a hothouse to icehouse environment suggesting a role for global cooling in driving the present-day distribution of fleshy fruit abundance.
ACKNOWLEDGEMENTS
We thank Paul Kenrick (NHM London), Margaret Collinson (Royal Holloway), Steven Manchester (Florida Museum of Natural History), Indah Huegele (Florida Museum of Natural History) and Dashrath Kapgate for their comments.
Open Research
DATA AVAILABILITY STATEMENT
Tables S1–S5 are available online at https://osf.io/24x7d/?view_only=68a9a7d490254d23a4840bfe72f6a06b. Raw data from the TRY database (Kattge et al., 2020) and R codes are available upon request to the corresponding author.




