The fitness consequences of wildlife conservation translocations: a meta-analysis
ABSTRACT
Conservation translocation is a common strategy to offset mounting rates of population declines through the transfer of captive- or wild-origin organisms into areas where conspecific populations are imperilled or completely extirpated. Translocations that supplement existing populations are referred to as reinforcements and can be conducted using captive-origin animals [ex situ reinforcement (ESR)] or wild-origin animals without any captive ancestry [in situ reinforcement (ISR)]. These programs have been criticized for low success rates and husbandry practices that produce individuals with genetic and performance deficits, but the post-release performance of captive-origin or wild-origin translocated groups has not been systematically reviewed to quantify success relative to wild-resident control groups. To assess the disparity in post-release performance of translocated organisms relative to wild-resident conspecifics and examine the association of performance disparity with organismal and methodological factors across studies, we conducted a systematic review and meta-analysis of 821 performance comparisons from 171 studies representing nine animal classes (101 species). We found that translocated organisms have 64% decreased odds of out-performing their wild-resident counterparts, supporting claims of systemic issues hampering conservation translocations. To help identify translocation practices that could maximize program success in the future, we further quantified the impact of broad organismal and methodological factors on the disparity between translocated and wild-resident conspecific performance. Pre-release animal enrichment significantly reduced performance disparities, whereas our results suggest no overall effects of taxonomic group, sex, captive generation time, or the type of fitness surrogate measured. This work is the most comprehensive systematic review to date of animal conservation translocations in which wild conspecifics were used as comparators, thereby facilitating an evaluation of the overall impact of this conservation strategy and identifying specific actions to increase success. Our review highlights the need for conservation managers to include both sympatric and allopatric wild-reference groups to ensure the post-release performance of translocated animals can be evaluated. Further, our analyses identify pre-release animal enrichment as a particular strategy for improving the outcomes of animal conservation translocations, and demonstrate how meta-analysis can be used to identify implementation choices that maximize translocated animal contributions to recipient population growth and viability.
I. INTRODUCTION
Rates of floral and faunal species loss in the new Anthropocene signal the onset of a sixth global mass extinction (Barnosky et al., 2011; Pimm et al., 2014). Among vertebrates, extinction rates in the last century increased 100 times above pre-industrial levels (Ceballos et al., 2015) and recorded population size declines have averaged 68% in the last 50 years (WWF, 2020). Continued species loss will have irreversible consequences for ecosystem services and resiliency. Slowing the rate of species loss will require eliminating existing threats (e.g. disease, poaching; Heppell, Crowder & Crouse, 1996; Snyder et al., 1996) and extensive habitat preservation and restoration (Bouzat et al., 2009; Newmark et al., 2017). While environmental threats are being addressed, supportive breeding and translocation programs have been proposed to maintain species and their ecosystem roles (Ford, 2002; Seddon et al., 2014; Hufbauer et al., 2015).
Animal conservation translocations (ACTs) are a common strategy intended to directly offset species extinction or provide beneficial ecosystem functions through the supplementation of threatened populations and the repatriation of extirpated populations (Ceballos, Ehrlich & Dirzo, 2017; Ceballos, Ehrlich & Raven, 2020). ACT programs supplementing extant populations in their indigenous range are called reinforcements, and can be grouped into two categories (Seddon, Armstrong & Maloney, 2007; IUCN/SCC, 2013). First, in situ reinforcements (ISRs) collect and transport wild organisms to another natural area for the benefit of the recipient conspecific population. Second, ex situ reinforcements (ESRs) incorporate a captive phase lasting from less than one generation (i.e. head-starting) up to several generations (i.e. captive breeding) before release. Despite immense efforts across a wide array of taxa to employ ACTs and document their efficacy in the literature, a major unresolved question is whether translocated individuals are as capable as their wild conspecifics at contributing to population viability and preventing extinctions.
To date, ACTs have had many celebrated results and a quantifiable impact on global extinction rates (Hoffmann et al., 2010; Jachowski et al., 2011; Hess et al., 2012; Jensen et al., 2018; Bolam et al., 2021). Nevertheless, ACTs are often considered a halfway technology with logistical and systemic shortcomings that result in failure to achieve pre-defined conservation goals (Snyder et al., 1996; Fischer & Lindenmayer, 2000; Pérez et al., 2012; Germano et al., 2015; Sullivan, Nowak & Kwiatkowski, 2015). In particular, multiple studies indicate that ACTs might produce individuals with inherent genetic and performance-related deficits that compromise ACT program goals and reduce recipient population viability (Frankham, 2008; Satake & Araki, 2012; Farquharson, Hogg & Grueber, 2018, 2021). These deficits are most severe in ESRs involving multiple captive generations since the capture of ESR founders acts as a bottleneck on genetic diversity. Even with a stringent captive breeding structure, factors inherent to husbandry practices (e.g. small effective population size, inbreeding depression, relaxed purifying selection) increase the likelihood of fixation of undesirable alleles and a loss of allelic heterozygosity (Lynch & O'Hely, 2001; Ford, 2002; Araki et al., 2008; Furlan et al., 2020). A large influx of captive-born individuals into the wild with such genetic properties can swamp genetic diversity of locally adapted populations and reduce effective population sizes despite an overall increase in numbers (Ryman & Laikre, 1991; Alleaume-Benharira, Pen & Ronce, 2006; Laikre et al., 2010; Ford, Murdoch & Howard, 2012; Waples et al., 2016; Pinter, Epifanio & Unfer, 2019). For example, Klütsch et al. (2019) examined how stocking of captive-bred brown trout (Salmo trutta) influenced the genetic diversity of wild sub-populations isolated by hydroelectric dams. They found that historically stocked sections of the system exhibited genetic signs of bottleneck events indicative of genetic swamping by the released captive-bred fish. However, such genetic deficits require multiple generations of captive breeding to accumulate, which suggests that alternative factors contribute to fitness reductions observed in ESRs lasting a generation or less (Araki, Cooper & Blouin, 2007; Farquharson et al., 2018).
A fundamental criticism of ESRs is that non-natural conditions present in captivity can influence individual phenotypes, diminishing post-release performance relative to wild conspecifics (Araki et al., 2008; Stuparyk et al., 2018; Tetzlaff, Sperry & DeGregorio, 2019a; Crates, Stojanovic & Heinsohn, 2022), where animal performance is defined as a measure of some critical life-history character (e.g. growth rate, survival, fecundity) that correlates with individual fitness (Thoday, 1953; Hendry et al., 2018). In a variety of taxa, ESRs lasting a generation or less negatively influence post-release performance metrics such as anti-predator behaviour (Kraaijeveld-Smit et al., 2006; Melstrom, Salau & Shanafelt, 2019), dispersal (Lehrer et al., 2016; DeGregorio et al., 2017; McCallen et al., 2018), growth (Horreo et al., 2018), survival (McCleery et al., 2013; Vanderwerf et al., 2014), and reproductive success (Araki et al., 2007; Knudsen et al., 2008; Ford et al., 2016). Such phenotypic mismatches can arise via domestication selection, where trait values that are selected for in captivity are maladaptive when expressed in a natural environment (Heath et al., 2003; Stringwell et al., 2014).
The unintentional selection for domesticated phenotypes in artificial environments is likely a major contributor to reduced performance of ESRs, since it can alter phenotype distributions within a single generation (Araki et al., 2007; Horreo et al., 2018). For example, Araki et al. (2007) showed that captive-bred steelhead trout (Oncorhynchus mykiss) exhibited a 40% reduction in fecundity relative to their wild counterparts for every additional generation of captive ancestry. In the absence of domestication selection, phenotypic plasticity during less than one generation of captive development and rearing can lead to similar deviations from the wildtype in anatomical, physiological, and behavioural traits (Huntingford, 2004; Stamps & Swaisgood, 2007; Stringwell et al., 2014). Moreover, certain plastic phenotypic deviations have an epigenetic basis, thereby potentially influencing performance of future generations regardless of their developmental environment (Day & Bonduriansky, 2011; Evans et al., 2014b).
By virtue of the use of wild-born organisms and lack of a captive component, ISRs are lauded as a less intrusive and a generally more successful translocation strategy than ESRs (Griffith et al., 1989; Wolf et al., 1996; Fischer & Lindenmayer, 2000; Kingsbury & Attum, 2009; Rummel et al., 2016). However, failure rates for ISRs are still notably high and potentially a consequence of similar issues that affect ESRs. At the population level, supplementing existing populations with conspecifics from other wild populations can lead to maladaptive gene flow or outbreeding depression of the recipient population and a reduction in meta-population genetic diversity (Lenormand, 2002; Moritz, 2002; Ficetola & De Bernardi, 2005). At the individual level, ISRs can impact post-release performance either directly through transportation-related stress (Dickens, Delehanty & Michael Romero, 2010) or indirectly through energetic costs of establishment (Armstrong & Seddon, 2008), errant dispersal and homing behaviours (Germano & Bishop, 2009), diminished social structure (Goldenberg et al., 2019), and phenotypic mismatches within the novel ecosystem (Stamps & Swaisgood, 2007; Turlure et al., 2013).
Exposing captive organisms to some form of enrichment is the most intuitive strategy to counteract phenotypic mismatch, and a reasonable compromise when ISRs are not possible. We use the term enrichment broadly to refer to a variety of supportive measures (Fischer & Lindenmayer, 2000) employed by ACTs to offset any maladaptive behaviour, physiology, or physical injury incurred during captivity and/or translocation (Reading, Miller & Shepherdson, 2013; Goldenberg et al., 2019; Tetzlaff et al., 2019a). Some examples include fostering pre-release organisms with experienced conspecifics (Lumsden & Drever, 2002; Shier & Owings, 2007), simulating in situ environments with naturalistic enclosures or antipredator training (Brown, Davidson & Laland, 2003; Nazar & Marin, 2011; Roe, Frank & Kingsbury, 2015; Tetzlaff, Sperry & DeGregorio, 2018; Zhu et al., 2022), implementing a soft-release phase where supplemental feeding and/or shelter are provided (Tuberville et al., 2005; Brown et al., 2006), or simply reducing the length of the captive phase (DeGregorio et al., 2017, 2013; but see Tetzlaff et al., 2019b). Literature reviews suggest that enriched ESR cohorts are more successful post-release compared to unenriched cohorts (Fischer & Lindenmayer, 2000; Tetzlaff et al., 2019a), but the lack of a wild-reference group in the synthesized findings makes it difficult to conclude whether enrichment strategies produce translocated organisms that are indistinguishable from wild conspecifics with respect to post-release fitness (Mathews et al., 2005).
Finally, the metric used to gauge relative performance can vary in its representation of fitness, thereby shaping study conclusions (Moyes et al., 2009; Wilson & Nussey, 2010; Hendry et al., 2018). The lifetime reproductive success of an individual is the ideal measure of fitness when focusing on population dynamics and phenotypic adaptation, but lifetime reproductive success and the component life-history traits that determine it (e.g. survival and fecundity) are difficult to measure in natural conditions (Coulson et al., 2006; Fairbairn & Reeve, 2001; Hendry et al., 2018; Kozłowski, 1993). Consequently, fitness is often approximated using traits that typically correlate with fitness (e.g. body size, body condition, movement). However, the extent to which correlates and components of fitness (hereafter collectively referred to as fitness surrogates) covary and ultimately relate to lifetime reproductive success remains uncertain (Moyes et al., 2009; Hendry et al., 2018). For example, Mulder et al. (2017) found that while translocated and wild-resident desert tortoises (Gopherus agassizii) had similar body condition and survivorship 4 years post-release, translocated males fathered none of 92 offspring for which paternity was determined. This suggests that survival is a potentially inappropriate component of fitness to gauge ACT success in adult organisms with high adult survivorship. Conservation translocations should consider multiple relevant fitness components during project assessment to identify potential life-history trade-offs and avoid misleading conclusions (Fincke & Hadrys, 2001; Wilson & Nussey, 2010; Pekkala, Kotiaho & Puurtinen, 2011; Hendry et al., 2018).
The translocation literature has grown to a point that quantitative reviews are necessary to summarize the high volume of papers, while also accounting for heterogeneity and bias (Bajomi et al., 2010; Stewart, 2010). Seminal reviews have evaluated ACT efficacy from the published literature and via mail-in questionnaires sent to conservation program managers (e.g. Griffith et al., 1989; Wolf et al., 1996; Fischer & Lindenmayer, 2000; Kraaijeveld-Smit et al., 2006; Seddon et al., 2007; Rummel et al., 2016; Brichieri-Colombi et al., 2019). In these approaches, the response variable (translocation success versus failure) reduces a highly nuanced outcome to a binary classification based in part on unstandardized criteria (e.g. creation of a self-sustaining population) and professional opinion. These syntheses are an invaluable resource for applied conservation and management (Nason et al., 2021), but their largely narrative format is not conducive to quantitative synthesis across broad and multifactorial data sets while accounting for bias and heterogeneity among studies.
Meta-analysis is a quantitative tool that integrates the findings of published papers to examine broader patterns and draw more powerful conclusions about a focal topic (Glass, 1976; Stewart, 2010; Gurevitch et al., 2018). Meta-analyses standardize and pool effect sizes from multiple studies while preserving the magnitude and variance of the original effects, thus enabling predictions about particular driving variables to be modelled as covariates that account for unexplained variation among studies (Glass, 1976; Bennett et al., 2017). A properly executed meta-analysis of the conservation literature will synthesize existing trends, assess knowledge gaps, and develop recommendations for future research and management actions.
Meta-analysis is well-equipped to gauge translocation efficacy for establishing viable populations based on the post-release performance of animal subjects (Ducatez & Shine, 2019; Jule, Leaver & Lea, 2008; Linklater et al., 2011; Skikne et al., 2020; Tetzlaff et al., 2019a; Zhang, Gao & Zhang, 2022; Zhu et al., 2022; also see Resende et al., 2021). Previous meta-analyses fail to include a wild-reference group (but see Stuparyk et al., 2018) and therefore cannot address the way by which translocated individuals may contribute to the population viability goals of ACTs. For instance, translocated animals that are on average less likely to survive, reproduce, or perform relative to wild conspecifics in the recipient population (i.e. a sympatric control group comparison) will have limited contributions to genetic or evolutionary mechanisms increasing population persistence (i.e. genetic and evolutionary rescue; Carlson, Cunningham & Westley, 2014; Reid et al., 2021). The performance of wild members of the recipient population should be considered by conservation managers as an adaptive baseline shaped by local environmental and social pressures that translocation programs should strive to emulate (Mathews et al., 2005). Even though translocated cohorts might exhibit lower survival or reproductive success compared to wild control groups, translocations may positively affect other demographic and ecological processes (e.g. demographic rescue and Allee effects) that fulfil primary goals of ACTs (IUCN/SSC, 2013). Distinguishing these beneficial effects on the wild resident recipient population requires comparison with wild allopatric control groups to separate the effects of translocation from the measurement of performance in both the translocated cohort as well as the wild sympatric recipient group. However, use of both wild allopatric and sympatric control groups has rarely been reported (e.g. Molony et al., 2006; Sah et al., 2016).
- (1)
Animals incorporated into conservation translocation programs represent a wide taxonomic breadth in which natural history, sensory capabilities, and native environments will vary substantially. These factors will also affect the capacities of conservation managers to recreate natural conditions in captivity and in turn influence organismal response. We predict that taxonomic groups will vary broadly in their relative post-release performance.
- (2)
Disparity in performance among translocated and wild-resident organisms will increase as a function of whether a trait is considered an indirect correlate (e.g. movement and body condition) or a direct component of an organism's fitness (e.g. growth, survival, reproductive success).
- (3)
Within species, sexes can vary in physical form and physiology, seasonal and annual energetic investments, optimal life-history strategy, spatial ecology, and behaviour (Trivers, 1972; Parker, 1979; Ruckstuhl & Neuhaus, 2005; Bonduriansky & Chenoweth, 2009; Fairbairn, 2013). We predict significant intraspecific variation in post-release relative performance among male and female cohorts.
- (4)
We predict varying amounts of captive ancestry will explain variation in relative post-release performance of translocated cohorts. We predict that ESRs lasting >1 generation will show greater disparity in relative performance than ESRs lasting <1 generation (e.g. head-starting programs). We also predict that ISRs involving zero captive generations will have more similar post-release performances to wild-reference groups compared to ESRs.
- (5)
Translocated organisms exposed to one or more forms of enrichment will perform better post-release relative to wild conspecifics compared to unenriched translocated conspecifics.
II. METHODS
(1) Literature search and inclusion criteria
We conducted our literature review, screening, and analysis according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig. 1; Moher et al., 2009) and following a PRISMA EcoEvo checklist (see online Supporting Information, Appendix S1; O'Dea et al., 2021). We searched the literature using Web of Science, including all databases (01/01/1900–08/23/2019), for peer-reviewed studies concerning animal conservation translocations with some reference to a wild-resident group used as a control. Our query was: TS = [(conservation OR reintroduc* OR repatriat* OR releas* OR supplementation OR transloc*) AND (‘captive born’ OR ‘captive breed*’ OR ‘captive bred’ OR ‘captive rais*’ OR ‘captive rear*’ OR fisher* OR hatcher* OR ‘headstart*’ OR ‘head start*’ OR relocat*) AND (native OR resident OR wild) AND (animal OR invertebrate OR fish* OR amphib* OR reptil* OR mammal* OR bird* OR avian*)].
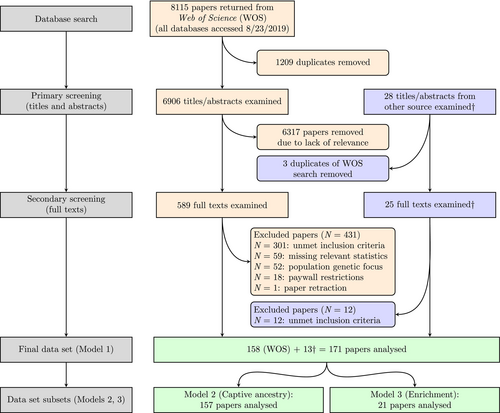
We also screened studies reviewed in Stuparyk et al. (2018) for inclusion because our primary Web of Science query did not encompass the terminology used in several ISR studies concerning ISRs intended to mitigate human–animal conflicts. After screening (Fig. 1), the resulting 589 full-text papers – along with 25 additional papers synthesized by Stuparyk et al. (2018) (Table S1 in Appendix S2) – were assessed for our required inclusion criterion: the paper must include at least one direct, quantitative comparison of a fitness component (growth, survival, reproduction) or fitness-correlated trait (movement, body condition) between one translocated (either ESR or ISR) and one wild-resident animal group. The comparison(s) must have been made in a natural setting following the release of the translocated cohort into an area occupied by wild-resident conspecifics of comparable age and size distributions. We excluded papers making comparisons based on ambiguous traits without the author(s) clearly defining a relationship to fitness. These ambiguous traits include, but are not limited to, stable isotope or itemized diet analysis (e.g. Bourass & Hingrat, 2015; Quinn, Seamons & Johnson, 2012), habitat use (e.g. Himes et al., 2006; Mann et al., 2011), behavioural ethograms (e.g. Rantanen et al., 2010), or varying morphotypes (Larsen et al., 2013). Papers were also excluded if they did not contain the necessary descriptive statistics to calculate effect sizes (see Section II.2) and corresponding authors did not respond to email requests for supplementary statistics. Finally, we excluded papers assessing the influence of translocations on population genetic indices, as these data sets do not involve distinct animal groups and sample sizes that are necessary to calculate effect sizes.
(2) Effect size extraction and calculation
An effect size is a statistic used to estimate the magnitude of a relationship between two experimental groups or variables (Nakagawa & Cuthill, 2007). We quantified the disparity in performance between wild-resident and translocated cohorts using two effect size metrics. In studies comparing animal groups based on mean estimates of continuous traits (e.g. growth rate, survivorship, clutch size), we calculated standardized mean differences (SMDs; Rosenthal & Rubin, 1982). For comparisons based on counts of dichotomous outcomes (e.g. full versus empty stomachs, proportion recaptured, successful versus unsuccessful nests), we calculated ln odds ratios (lnORs; Haddock, Rindskopf & Shadish, 1998). Effect sizes were calculated using the function escalc in the R package metafor (Viechtbauer, 2010). From each paper, we extracted descriptive statistics necessary to calculate effect sizes for all relevant fitness-related comparisons from the published and supplemental texts and data tables, from additional materials requested from corresponding authors, and from relevant figures and plots using the R package digitize (Poisot, 2011).
We converted all SMD estimates to lnORs for more intuitive interpretation of effect size magnitudes (Chinn, 2000; Rosenberg, Rothstein & Gurevitch, 2013). LnORs are centred at zero and were calculated such that positive values indicate translocated individuals exhibited better relative performance than wild-resident individuals, and negative values indicate translocated individuals under-performed relative to wild individuals. For traits where lower values indicate better performance (e.g. mortality rate, parasite load), raw trait values were multiplied by −1 for consistent interpretation.
(3) Moderators
We extracted several study-level and estimate-level attributes to evaluate our predictions in the final statistical model. Comparisons were assigned a study ID based on the paper from which they were extracted and a group ID to distinguish unique groups of organisms within each study. Identical group ID values were assigned to comparisons involving measures of different traits in the same organisms (Noble et al., 2017). We also retrieved taxonomic class, order, family, genus, and species names of all assessed organisms from the Integrative Taxonomic Information System database (ITIS, 2021). We identified the fitness-related trait under consideration in each comparison and reclassified each into one of five broad fitness surrogate categories: movement, body condition, growth, survival, and reproduction. We also identified the predominant sex (i.e. female-only, male-only, or mixed-sex) of organisms in each comparison. We characterized the amount of captive ancestry of translocated individuals represented in each comparison using two separate variables. First, we distinguished comparisons according to whether the translocation program involved a captive phase or not (i.e. ISR versus ESR strategy). In those studies that described the amount of captive ancestry among translocated cohorts, we further classified captive ancestry using an ordered set of categories: (i) ISR organisms without any captive ancestry (i.e. 0 generations in captivity); (ii) ESR organisms with wild-origin parents not raised in captivity (<1 generation in captivity); and (iii) ESR organisms with one or more direct ancestral generation/s raised in captivity (>1 generation in captivity). Finally, we classified the absence/presence (0/1) of enrichment techniques for select comparisons according to the circumstances of each paper. Due to the arbitrary nature of enrichment treatments across our data set, we only assessed enrichment efficacy within studies that included post-release performance comparisons with wild-resident conspecifics for both enriched and unenriched translocated cohorts.
(4) Statistical analysis
(a) Model structure
We first fitted a meta-analytical taxonomic mixed model (Clutton-Brock & Harvey, 1977; Hadfield & Nakagawa, 2010) to quantify the overall effect size – relative post-release performance among translocated and wild-resident cohorts – across the entire data set, and also assessed how individual-level (i.e. sex, taxonomic group) and study-level (i.e. fitness surrogate, ESR versus ISR strategy) factors influence this outcome (model 1; Table S2 in Appendix S2). We modelled the lnOR responses as a function of categorical fixed effects for sex, taxonomic class, fitness surrogate, and study strategy. We examined two-way interactions between sex and fitness surrogate, and between sex and strategy to determine whether these individual- and study-level factors had sex-specific relationships with relative post-release performance. We also included a two-way interaction between fitness surrogate and strategy to examine whether post-release performance in various fitness-related traits is influenced by translocation strategy.
(b) Quantification of study heterogeneity
We included several random effects terms to account for different sources of non-independence among effect sizes. To account for shared taxonomy (Clutton-Brock & Harvey, 1977; Hadfield & Nakagawa, 2010), we included all Linnaean taxonomic ranks below the level of class as separate random effects terms in each model. Taxonomic class was maintained as a fixed effect to quantify average differences in post-release performance across broad, recognizable animal groups of potential relevance to translocation managers. We chose the nested taxonomic random effects approach over the incorporation of a phylogenetic covariance matrix because the taxonomic breadth of our data set precluded access to a comprehensive phylogeny with empirically derived branch lengths (Hadfield & Nakagawa, 2010; Lajeunesse, Rosenberg & Jennions, 2013) and the goals of our analysis are not concerned with estimating the extent of correlated evolution. We included study and group identities as two additional random effects terms to account for non-independence of effect sizes within studies and among repeated measures of unique organisms (Noble et al., 2017). We also included a term to model the variance in sampling errors for each effect size, which incorporates study-level precision into the model (see Section II.4.c; Hadfield, 2010; Mengersen et al., 2013). To assess the overall dispersion of our effect sizes and thus the generalizability of the findings of our models, we calculated total model heterogeneity (Higgins & Thompson, 2002; Table S3 in Appendix S2). Since measures of heterogeneity tend to be high in ecological studies assessing diverse taxa (Senior et al., 2016), we further calculated the heterogeneity apportioned across the aforementioned taxonomic and study-specific random effects terms to investigate in more detail patterns of dispersion in effect sizes (Nakagawa & Santos, 2012).
(c) Meta-analysis model specifications
The model was fitted in a Bayesian framework using a Markov chain Monte Carlo (MCMC) algorithm as implemented in the R package MCMCglmm (Hadfield, 2010). We specified diffuse normal prior distributions for all fixed effects (mean = 0, variance = 1010) and parameter expanded priors on all variance components to yield scaled non-central F-distributions with numerator and denominator degrees of freedom equal to 1 and a scale parameter of 1000 (Gelman, 2006; Hadfield, 2010). The model was constructed as a random effects meta-analysis by including a term that contained the estimated sampling variance for each effect size and fixing the variance associated with this term to 1 (in the prior). The MCMC chain for each model was run for an initial burn-in of 3000 iterations with samples from the chain being saved every 150th iteration until 3000 samples were obtained. All models were checked for autocorrelation values < |0.1|, and model convergence was confirmed using a Heidelberg stationarity test and through visual assessment of posterior distributions.
(d) Influence of captive ancestry and enrichment
To assess the potential influence of domestication selection on the relative post-release performance of translocated cohorts, we used a second, similar model to analyse a subset of studies from the model 1 data set that specified the number of captive generations experienced by translocated cohorts (model 2; Table S4 in Appendix S2). We fitted model 2 by substituting the fixed effect of ESR versus ISR strategy from model 1 with captive ancestry, a categorical fixed effect with three levels classifying the focal ACT cohort as either part of an ISR (without any captive ancestry, i.e. 0 generations in captivity), a head-starting program (ESR with <1 generation in captivity), or a captive-breeding program (ESR with >1 direct ancestral generations in captivity). We were unable to analyse captive ancestry as a continuous variable due to a lack of reporting of exact ancestral generation times, and due to cohorts of potentially mixed ancestry. Model 2 also included categorical fixed effects for sex, taxonomic class, and fitness surrogate, and two-way interactions between sex and fitness surrogate, sex and captive ancestry, and between fitness surrogate and captive ancestry.
Finally, we developed model 3 to test the influence of enrichment strategies on relative post-release performance among wild and translocated cohorts by analysing a subset of studies from the model 1 data set that gathered relative post-release performance data with a wild-control group from both enriched and unenriched cohorts within the same experimental design (Table S5 in Appendix S2). In model 3, we included categorical fixed effects for sex, taxonomic class, fitness surrogate, ESR versus ISR strategy, and enrichment. We also included two-way interactions between enrichment and sex, enrichment and fitness surrogate, and enrichment and strategy.
(e) Results reporting
We addressed whether conservation programs produce organisms that perform similarly to or better than wild-resident conspecifics after release by calculating the total probability that the lnOR for a particular comparison is greater than or equal to zero. We also reported the marginal posterior mode and 95% highest posterior density credible interval (CrI) of the OR by exponentiating posterior distributions of lnORs. We further interpreted each effect size according to established threshold values delimiting small, medium, and large magnitudes of effect (see Table 1; Cohen, 1992; Olivier & Bell, 2013). While these values provide potential useful benchmarks for the assessment of biological importance in effect sizes (Nakagawa & Cuthill, 2007), we focused our interpretations on the true effect size values and associated credible intervals. We used the R package emmeans to report the marginal values of individual parameters and overall model effects to account for the influence of other model parameters (Lenth, 2021). All derived parameters are calculated across the entire posterior distribution to carry through all uncertainty.
| Magnitude, direction of effect | Ln odds ratio (lnOR) | Odds ratio (OR) |
|---|---|---|
| Large, negative | −1.10 | 0.33 |
| Medium, negative | −0.62 | 0.54 |
| Small, negative | −0.20 | 0.82 |
| No effect | 0 | 1.00 |
| Small, positive | 0.20 | 1.22 |
| Medium, positive | 0.62 | 1.86 |
| Large, positive | 1.10 | 3.00 |
(5) Meta-analysis validation
(a) Outlier identification
We performed several meta-analysis validation tests to assess our data set and findings for influential outliers and publication bias (Koricheva, Jennions & Lau, 2013). We identified outliers by calculating studentized deleted residuals for each comparison (Viechtbauer & Cheung, 2010). Comparisons with estimates over 1.96 standard deviations from the overall mean effect were considered outliers. We re-ran all models with outliers excluded to examine whether our findings were significantly affected.
(b) Publication bias
To test for temporal patterns in effect sizes and potential time-lag bias (Jennions & Møller, 2002; Koricheva et al., 2013), we fitted a linear model with our data set's calculated effect sizes as the response variable and year of publication as the predictor. We included study ID, group ID, and all Linnaean taxonomic ranks below the level of phylum as random effects. To assess the data set for evidence of publication bias, we used Egger's regression to model the relationship of the meta-analytic residuals of each effect size against their precision (Egger et al., 1997; Nakagawa & Santos, 2012). Funnel plots were also created to visualize this relationship and identify any systematic absence of values, which could also indicate publication bias. To test directly the influence of publication bias on our overall effect, we conducted a trim-and-fill analysis using the trimfill function in the R package metafor, which quantifies funnel plot asymmetry and estimates the number of missing studies on either side of the overall mean effect (Duval & Tweedie, 2000; Viechtbauer, 2010).
III. RESULTS
(1) Description of data
Our initial Web of Science query returned 6906 unique articles. After filtering based on the inclusion criteria, our data set comprised 821 unique effect sizes (Table 2) for relative performance of translocated organisms and their wild-resident conspecifics collected from 171 peer-reviewed studies (all studies included in the meta-analysis are identified using asterisks in the reference list). The studies investigated 101 unique species in either ISRs (599 effect sizes) or ESRs (222 effect sizes). We calculated standardized effect sizes as lnORs of performance metrics for translocated versus wild-resident cohorts such that positive values indicate translocated individuals exhibited higher relative performance than wild-resident conspecifics.
| Movement | Condition | Growth | Survival | Reproduction | Total | |
|---|---|---|---|---|---|---|
| Bivalvia | 0 | 0 | 0 | 6 | 0 | 6 |
| Gastropoda | 0 | 3 | 5 | 9 | 6 | 23 |
| Malacostraca | 0 | 4 | 0 | 14 | 5 | 23 |
| Echinoidea | 1 | 1 | 0 | 0 | 0 | 2 |
| Actinopterygii | 38 | 43 | 26 | 158 | 77 | 342 |
| Amphibia | 4 | 2 | 0 | 3 | 0 | 9 |
| Reptilia | 101 | 12 | 26 | 54 | 9 | 202 |
| Aves | 14 | 12 | 0 | 59 | 38 | 123 |
| Mammalia | 18 | 13 | 2 | 46 | 12 | 91 |
| Total | 176 | 90 | 59 | 349 | 147 | 821 |
(2) Meta-analysis
We analysed the lnORs and subsets of these data using three linear mixed effects meta-analytical models with fixed effect moderator variables to determine the impact of organismal and methodological factors, and random effects to attribute sources of variation among effect sizes.
(a) Model 1: overall model
The primary model's overall lnOR (posterior mode: –1.02; 95% CrI: −1.59 to −0.32; Fig. 2; Table S2 in Appendix S2) was converted to an OR by exponentiating across the posterior distribution, indicating that translocated organisms have 63.9% decreased odds of out-performing their wild-resident counterparts (OR posterior mode: 0.36; 95% CrI: 0.16 to 0.66). These effect size values correspond to a large magnitude of effect (Table 1; Cohen, 1992; Olivier & Bell, 2013). By quantifying the proportion of posterior samples with lnOR estimates greater than or equal to zero, we infer that translocated organisms had an overall 0.001 probability of out-performing wild-resident conspecifics following release (Fig. 2). Disparity in post-release performance did not vary widely across taxonomic classes since marginal posterior distributions of all classes generally exhibited negative lnOR effect sizes of moderate-to-large magnitude (Fig. 2). This suggests poor performance of translocated organisms relative to wild-resident conspecifics across taxa (Table S2 in Appendix S2). Similarly, relative post-release performance was largely unaffected by ESR versus ISR translocation strategy.
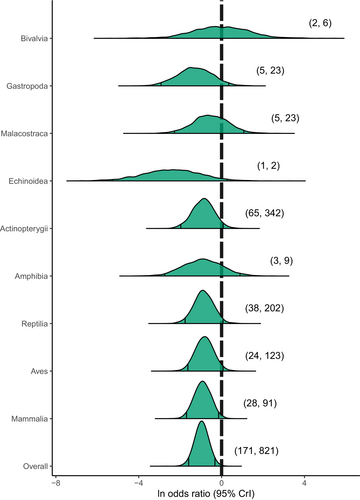
Model results suggest that in studies involving mixed-sex cohorts, comparisons based on fitness components (growth, survival, reproduction) tend to have similar lnOR marginal posterior mode estimates to comparisons based on fitness correlates (movement, condition), which indicates a consensus in the interpretation of relative under-performance of translocated cohorts across a variety of performance metrics (Table S2 in Appendix S2). The more variable effect size outcomes observed in sex-specific comparisons relative to mixed-sex comparisons might suggest the presence of unequal life-history consequences of translocation among sexes. In movement-based comparisons, for example, both male-only and female-only comparisons exhibited lower lnOR values than mixed-sex comparisons. However, low sample sizes in condition-, growth-, and survival-based estimates, and uneven taxonomic representation in movement-based estimates (limited to N = 5 reptilian studies, N = 1 avian study, and N = 3 mammalian studies) preclude conclusive interpretations for sex-specific results.
(b) Data set heterogeneity
Table S3 in Appendix S2 provides heterogeneity summary statistics for all three models. For model 1, representing the full data set, heterogeneity values from individual taxonomic levels were relatively small and consistent in magnitude, but their combined taxonomic signal accounted for 60.6% (95% CrI: 47.0% to 74.7%) of total heterogeneity. Study- and group-level effects accounted for 23.9% (95% CrI: 12.4% to 34.0%) and 13.3% (95% CrI: 8.67% to 19.7%) of total heterogeneity, respectively.
(c) Subset analyses
Due to limited reporting of key moderator variables across studies, we analysed subsets of the data in two additional models to assess whether the amount of captive ancestry or the presence of enrichment, respectively, influenced relative post-release performance of translocated cohorts. In general, lnOR marginal posterior mode estimates were similar for the overall model effects and for parameters that also appeared in the primary model 1 (Tables S4 and S5 in Appendix S2). Therefore, we limit the remainder of our reporting to model-specific parameters.
(i) Model 2: influence of captive ancestry
Post-release performance was largely unaffected by the length of captive ancestry, as indicated by the high overlap in the credible intervals of relocated (i.e. 0 captive generations), head-started (i.e. <1 captive generation), and captive-bred (i.e. >1 captive generation) parameter estimates (Table S4). However, in condition-based comparisons we did observe that relocated organisms without any captive ancestry had a lower probability (0.00; OR mode: 0.07; OR 95% CrI: 0.03 to 0.21) of out-performing wild-resident conspecifics relative to head-started (0.15; OR mode: 0.55; OR 95% CrI: 0.22 to 1.28) and captive-bred cohorts (0.25; OR mode: 0.23; OR 95% CrI: 0.02 to 2.52 Table S4 in Appendix S2). Comparisons with growth-based estimates exhibited a similar pattern, but these growth-based comparisons for relocated, head-started, and captive-bred cohorts were based on reduced data sets of only two, eight, and one studies, respectively (Table S4 in Appendix S2).
(ii) Model 3: influence of pre-release enrichment
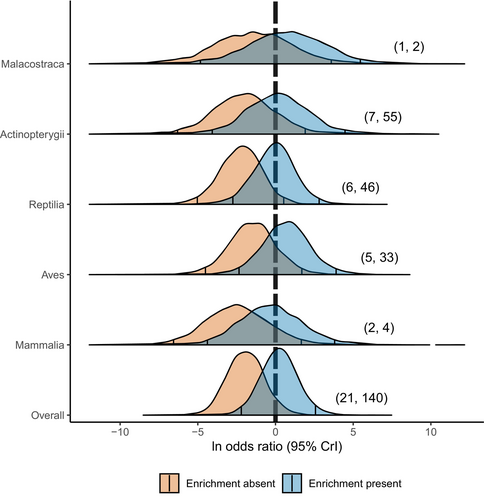
To test the influence of enrichment strategies on relative post-release performance among translocated and wild cohorts, we analysed a subset of studies that gathered data from both enriched and unenriched cohorts within the same experimental design and compared their post-release performances relative to a common wild-control group. Unenriched translocated organisms had a 0.06 probability of out-performing their wild-resident conspecifics (OR mode: 0.06; 95% CrI: 0.002 to 1.06), whereas enriched translocated organisms had a 0.58 probability of out-performing their wild counterparts (OR mode: 0.75; 95% CrI: 0.008 to 9.36; Fig. 3; Table S5 in Appendix S2).
(d) Validation
(i) Outlier identification
We identified 56 outlying effect sizes based on studentised deleted residuals greater in magnitude than 1.96. Removal of outliers generally reduced effect sizes across all parameters and caused the 95% credible intervals for the overall effects of model 1 and model 2 to overlap with zero (Tables S6–S8 in Appendix S2). However, outlier exclusion did not qualitatively affect our overall findings nor the indices of publication bias. Moreover, the examination of all outlying effect sizes revealed these effect sizes were calculated correctly and reflect legitimate patterns in relative post-release performance (Aguinis, Gottfredson & Joo, 2013). Therefore, we completed the final analysis with outliers included.
(ii) Publication bias
Visual examination of the marginal posterior distributions across years shows consistent effect sizes, indicating no time-lag bias in our data set (Fig. 4). Removing outliers did not affect patterns of effect size magnitude across years (Fig. S1 in Appendix S3).
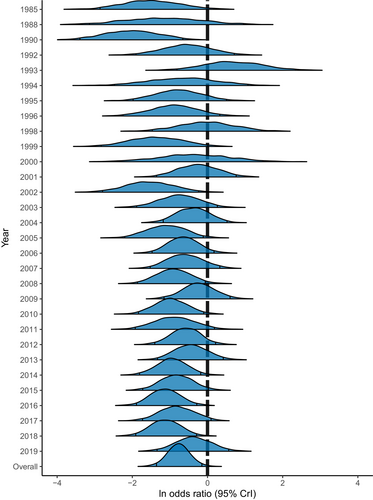
The Egger's regression slopes were significantly different from zero for both the data set including (slope = 0.18; SE: 0.04; P < 0.001) and excluding (slope = 0.19; SE: 0.03; P < 0.001) outliers, suggesting the presence of publication bias. The resulting funnel plots revealed the presence of some effect size asymmetry in the data set including outliers (Fig. 5), and to a lesser degree in the data set excluding outliers (Fig. S2 in Appendix S3). However, the significant Egger's regression slopes for both data sets could be an artifact of the high model heterogeneity and not publication bias (Higgins & Thompson, 2002).

The trim-and-fill analysis estimated that the overall model including outliers was missing zero estimates to the left side of the mean and three estimates from the right side (SE: 2.83; P = 0.06), whereas the model excluding outliers had zero missing estimates to either side of the mean (SE: 1.41; P = 0.5).
In conclusion, our validation tests indicate negligible amounts of publication bias. Moreover, we consider our estimates to be highly conservative given the fact that conservation translocations describing unsuccessful or uncertain outcomes are less likely to be published than those with successful outcomes (Fischer & Lindenmayer, 2000; Miller, Bell & Germano, 2014; Reading, Clark & Griffith, 1997).
IV. DISCUSSION
Our meta-analysis gauged the efficacy of conservation translocation programs for benefitting population and species persistence based on direct, in situ comparisons of animal performance between translocated organisms and wild-resident conspecifics. We synthesized estimates of relative performance based on five fitness-surrogate categories across 101 unique species spanning nine taxonomic classes. Our findings indicate that translocated organisms tend to under-perform relative to their wild counterparts, and that this pattern is largely independent of taxonomic class or the type of fitness surrogate measured. This supports previous claims for the existence of general issues across ACTs and suggests that translocated organisms are often at a major disadvantage following release (see Section I; e.g. Snyder et al., 1996; Fischer & Lindenmayer, 2000; Pérez et al., 2012; Germano et al., 2015; Sullivan et al., 2015). Under-performance of translocated cohorts was also consistent regardless of translocation strategy or the amount of captive ancestry, with some caveats. Finally, post-release performance was markedly improved in translocated cohorts exposed to pre-release enrichment relative to unenriched cohorts. Here we explore the implications of our findings and make recommendations for future translocation program assessment and monitoring procedures.
(1) Overall model
The unexpected lack of variation in effect sizes across taxonomic classes (Fig. 2) hints at a general influence of ACTs on animals, which is in direct contrast with the large proportion of study heterogeneity explained by taxonomic rank (Table S3 in Appendix S2). The high taxonomic signal (60.6%) indicates that taxonomic position explains a majority of variation in effect sizes, which would lead us to expect large differences in relative performance values among higher taxonomic groups. For example, Dochtermann et al. (2019) observed a consistent pattern in a phylogenetic meta-analysis quantifying behavioural heritability: little difference in effect size among species and a low phylogenetic signal. However, we found the proportion of heterogeneity explained by individual taxonomic tiers to be consistently low, which could explain the minimal differentiation in effect sizes at the level of taxonomic class. Equal variances estimated at each taxonomic level is consistent with an explanation of constant phylogenetic inertia across the phylogeny, in which equal branch lengths of the phylogeny coincide with equal taxonomic branch lengths. Alternatively, if phylogenetic and taxonomic branching patterns do not coincide, then the observed results would imply variation in taxonomic inertia across evolutionary time (Hadfield & Nakagawa, 2010). To explore these alternative explanations further, and to investigate patterns of correlated evolution with respect to performance of translocated animals, comparative studies focusing on biological patterns at the broadest taxonomic scales could adopt a mixed phylogenetic and taxonomic approach until an appropriate phylogeny with empirically derived branch lengths can be resolved for all species in the data (Lynch, 1991; Hadfield & Nakagawa, 2010; Johnson et al., 2018).
Our meta-analysis highlights a clear bias in taxonomic representation among ACTs (Table S9 in Appendix S2), with most studies involving charismatic species or those with economically important fisheries. Most notably, there is a significant lack of ACTs involving amphibians that meet our inclusion criteria. Amphibian populations are experiencing global declines and will likely require human interventions in the form of ACTs to avoid extirpation (Wake & Vredenburg, 2008; Germano & Bishop, 2009; Scheele et al., 2021). Recent quantitative reviews describe a positive trend in the success of amphibian translocations relative to earlier benchmarks (Dodd & Seigel, 1992; Germano & Bishop, 2009). However, these efforts fail to include comparisons among translocated and wild-resident conspecifics as a potential index for project feasibility. Recent amphibian studies using this comparative approach have provided novel insights regarding the multi-generational influence of ACTs as well as potential pitfalls of cryopreservation techniques on ACT feasibility (Cayuela et al., 2019; Poo et al., 2022). Future assessments of translocation success should include simultaneous monitoring of a wild-control group from the donor and/or recipient populations as an additional criterion for determining project feasibility and success.
Our primary model found no differences in effect sizes across fitness surrogates, indicating that phenotypic traits considered as correlates of overall fitness have the potential to characterize performance differences among translocated and wild-resident cohorts. We initially predicted that fitness correlates would exhibit less-consistent disparities across animal cohorts since fitness correlates are less-reliable proxies of fitness than fitness components (Hunt & Hodgson, 2010). One explanation for the observed pattern is that fitness surrogates can vary in how well they reflect overall individual fitness based on the relative length and positioning of the measurement interval within the organism's lifespan (Pekkala et al., 2011; Hendry et al., 2018). Additionally, the lack of differences in fitness surrogate effects may be due to unaccounted-for differences in life-history strategies within and among study systems. Life-history theory suggests that the uneven allocation of finite resources results in life-history trade-offs among fitness components such that maximizing one fitness component can decrease overall lifetime reproductive success [e.g. increased annual fecundity decreases annual survival (Haave-Audet et al., 2022; Moyes et al., 2009; Roff, 2002; van Noordwijk & de Jong, 1986)]. Future ACT studies and meta-analyses should incorporate multiple fitness components at different life stages to assess relative quality of released organisms, such that the covariance among fitness components and thus the presence of fitness trade-offs or differing life-history strategies can be tested at the within-study level (Fincke & Hadrys, 2001; Hendry et al., 2018).
We were not able to evaluate sex-by-fitness surrogate interactions across all taxonomic classes because so few studies reported sex-specific effects. However, data from avian, mammalian, and reptile studies suggest that estimates of relative performance based on movement were substantially lower for both male- and female-only cohorts relative to estimates from mixed-sex cohorts, indicating greater disparity in post-release performance relative to wild conspecifics when males and females were analysed separately. Thus, only when considering cohort performances of a single sex do our findings align with the established relationship between maladaptive post-release movement patterns and reduced survival in the vertebrate literature (Griffith et al., 1989; Bonnet, Naulleau & Shine, 1999; Germano & Bishop, 2009). The influence of sex on post-release performance is poorly represented in the primary literature and rarely synthesized in subsequent reviews (Teixeira et al., 2007). To our knowledge, no other quantitative literature review or meta-analysis examining translocation efficacy has included sex as a moderating factor, despite well-documented and systematic differences in life history and ecology among the sexes (Immonen et al., 2018; Tarka et al., 2018).
(2) Influence of captive ancestry
We observed similar levels of under-performance among translocated cohorts relative to wild conspecifics, regardless of whether translocated organisms were ISR (0 captive generations), head-started (ESR with <1 captive generation), or captive-bred (ESR with >1 captive generation). This contrasts with existing quantitative reviews describing a negative relationship between captive ancestry and fitness, with ISRs performing relatively better than ESRs (Fischer & Lindenmayer, 2000; Rummel et al., 2016). One explanation for this discrepancy is the poor reporting in the literature of ESR captive histories and animal origins which precluded us from conducting a more powerful assessment of the effect of captive ancestry. As such, our head-starting versus captive-rearing levels possibly misclassified cohorts with ambiguous or mixed captive histories, which could explain the lack of significant performance deficits commonly reported in long-term captive-breeding populations (Araki et al., 2007). It should also be noted that the ACT strategies specific to certain taxonomic groups might be more prone to reporting ambiguous captive ancestry information (Table S9). For example, reptilian, mammalian, and avian studies regularly describe captive ancestries of largely closed systems in great detail (Jones et al., 2002), whereas tracking individual ancestries in fisheries systems may require additional consideration since captive-reared broodstocks of potentially non-local origin can often interact with wild conspecifics in natural systems during migratory and spawning life phases (O'Sullivan et al., 2020; Lutz et al., 2021). This can be especially problematic if there is unreported captive ancestry in the purported wild-resident group, which would deflate expected fitness differences between translocated and wild-resident conspecifics (Araki et al., 2007). Our findings suggest that while ISRs avoid the maladaptive selective pressures associated with extended captivity, managers should still be wary of individual- and population-level stressors characteristic of ISRs that can lead to disparities in post-release performance like those observed in ESRs. This underscores the necessity of ACT programs to report the capture histories of their organisms, and to conduct multi-generational monitoring to ensure accurate measures of meaningful life-history traits.
(3) Influence of pre-release enrichment
Disparity in post-release performance among translocated and wild individuals was markedly reduced when translocated organisms were exposed to some form of pre-release enrichment. This finding highlights the growing relevance of the behavioural ramifications of ISR and ESR, where patterns of allelic heterozygosity and population growth rate have historically been the primary factors used to assess translocation efficacy (Frankham, 2008; Greggor et al., 2016; Mathews et al., 2005; Morris et al., 2021). Our findings agree with those from other meta-analyses showing that enriched translocated organisms generally had higher performance than non-enriched translocated conspecifics in a variety of taxa (Tetzlaff et al., 2019a; Zhang et al., 2022). Since few studies incorporating pre-release enrichment also fulfilled our inclusion criteria by directly comparing translocated cohorts to wild-resident conspecifics, we were not able to refine our categorization of different enrichment strategies. Despite that, our study provides the broadest evidence yet, and the first synthesized evidence to incorporate wild-control groups, that pre-release enrichment strategies have the potential to reduce post-release performance disparities between translocated and wild-resident organisms.
(4) Future directions
Despite notable positive outcomes, ACTs have experienced historically low success rates without significant improvement observed over time (Berger-Tal, Blumstein & Swaisgood, 2020). Ensuring future success of ACTs requires improved alignment between the specific conservation benefits sought in the translocation (e.g. as defined in Annex 2 of IUCN/SSC, 2013) coupled with objective evaluation of success achieving the desired benefits. Conservation biology is in the midst of a revolutionary push for more evidence-based decision-making to replace historically anecdotal- or experience-driven strategies (Pullin & Knight, 2001; Sutherland et al., 2004). Formal meta-analysis is an important tool for synthesizing existing literature and directing new veins of inquiry, particularly in the field of conservation where translocation studies are numerous but statistical power is limited by low sample sizes (Moseby, Hill & Lavery, 2014). Here we discuss several aspects of ACT practices and conservation goals that are relevant to increasing insight from future meta-analyses.
The conservation benefits of many ACTs (sensu IUCN/SSN, 2013) target population persistence and preventing outright species extinction. Short-term strategies for achieving these goals can be generalized according to three different ways to ‘rescue’ populations (Carlson et al., 2014): increase population density and buffer against ecological effects and demographic stochasticity (i.e. demographic rescue); increase genetic heterozygosity and reduce the accumulation of deleterious recessive alleles (i.e. genetic rescue); or increase adaptive potential (i.e. evolutionary rescue). Ultimately, to achieve these goals, translocation activities must contribute to populations in ways that enable recipient populations to adapt to their environment, increase average individual fitness, and thereby bolster population growth rate (Lande, 1998; Carlson et al., 2014; Hendry et al., 2018; Shaw, 2019). To evaluate the density-dependent ecological dynamics associated with demographic rescue, the recipient population cannot serve as the control group and so an allopatric population should be used instead. In our systematic review, we found only one study that included both sympatric and allopatric control groups (Molony et al., 2006). Thus, our results can only be interpreted in light of how successfully ACTs contribute to increasing mean fitness of the recipient population.
Our findings show that ISRs and ESRs produce organisms that under-perform and should therefore be implemented only after careful consideration of the recipient population's trajectory and the existing environmental threats at the recipient site (IUCN/SSC, 2013). For example, if a wild population is declining due to extrinsic threats, then the release of under-performing translocated conspecifics is unlikely to reverse declines (Heppell et al., 1996). Alternatively, if environmental threats have been removed, then the allelic diversity of a remnant wild population could benefit from the influx of translocated cohorts, despite certain performance deficits (Jensen et al., 2018; Cayuela et al., 2019). Pilot studies employing the post-release comparative techniques analysed in this study could therefore use relative post-release performance, population demographics, and environmental forecasts to predict the efficacy of ACT programs.
The initial prevention and reversal of invasive species spread is a major aspect of global habitat restoration which shares a conceptual basis with conservation translocations (Bright & Smithson, 2001; Armstrong & Seddon, 2008) and thus stands to benefit from in situ comparisons with wild-control groups. The potential for non-invasive species establishment and persistence can be assessed through sympatric comparisons with non-conspecifics of overlapping ecological niche, or through allopatric comparisons with conspecifics from the original donor population. If an invasion is ongoing, direct comparisons between recent invaders and the offspring of previous-generation invaders could also promote the investigation of the genetic basis of invasive adaptations. Similar investigations could act as the prelude to assisted colonization or rewilding efforts to ensure that species introduced to areas outside of their historic range are capable of colonizing successfully without risk of disrupting existing ecosystems (Corlett, 2016; Hunter-Ayad et al., 2021).
The often-wide credible intervals in our findings suggest there are opportunities in which ACTs can produce viable individuals and contribute to recipient populations. We recommend that ACT managers include sympatric and allopatric wild-reference groups in their programs properly to evaluate post-release performance of translocated organisms, and assess any negative density-dependent impacts of ACTs on resident conspecifics (Mathews et al., 2005; Molony et al., 2006). Managers should also take advantage of next-generation sequencing technologies and parentage assignment software to promote long-term, multi-generational monitoring of relative reproductive success and other fitness surrogates (Araki et al., 2007).
V. CONCLUSIONS
- (1)
Translocated organisms generally exhibited poorer performance following release compared to wild-resident conspecifics. The translocated organisms showed an overall 64% decreased odds of out-performing their wild-resident counterparts (OR posterior mode: 0.36; 95% CrI: 0.16 to 0.66) and this degree of disparity was consistent across taxonomic classes as well as whether an ISR versus ESR strategy had been implemented.
- (2)
The overall disparity between translocated and wild-resident cohorts was consistent across studies regardless of the amount of captive ancestry in any particular implementation.
- (3)
Post-release performance of translocated organisms generally benefitted as a result of some level of enrichment activity incorporated into the translocation program.
- (4)
The data set exhibited a high overall taxonomic signal but low heterogeneity at higher individual taxonomic levels. Variation across taxonomic levels suggests a potential influence of shared evolutionary history on relative post-release performance, but more comprehensive taxonomic sampling coupled with comparative analyses in a phylogenetic framework is necessary to draw accurate conclusions.
- (5)
Overall, the disparity in post-release performance between translocated organisms and their wild-resident counterparts was not affected by sex, taxonomic group, type of fitness surrogate measured, or ESR versus ISR translocation strategy.
- (6)
Only 2% of animal conservation translocation studies returned during the initial literature search of our systematic review report or design translocations that use wild-resident control groups. We suggest use of both sympatric and allopatric wild-reference groups will enable post-release performance of translocated organisms to be evaluated further for any negative density-dependent impacts of ACTs on resident conspecifics.
- (7)
Conservation managers should standardize the use of wild-resident controls and multiple performance metrics that capture several different aspects of the life history in their assessments of potential conservation translocation strategies to ensure population or species persistence.
ACKNOWLEDGEMENTS
We would like to thank members of the D. A. Warner and M. E. Wolak labs, and students from the Auburn University meta-analysis course (taught by A. E. W.) for providing comments on earlier drafts.
AUTHOR CONTRIBUTIONS
Conceptualization: I. P. G. and M. E. W.; data curation: I. P. G. and M. E. W.; formal analysis: I. P. G., with input from A. E. W. and M. E. W.; investigation: I. P. G.; project administration: M. E. W.; visualization: I. P. G. and M. E. W.; writing original manuscript: I. P. G.; writing – review and editing: A. E. W. and M. E. W.
Open Research
DATA AVAILABILITY STATEMENT
All data and R code are available from the Zenodo Digital Repository (https://doi.org/10.5281/zenodo.7535746).




