Conformity in mate choice, the overlooked social component of animal and human culture
ABSTRACT
Although conformity as a major driver for human cultural evolution is a well-accepted and intensely studied phenomenon, its importance for non-human animal culture has been largely overlooked until recently. This limited for decades the possibility of studying the roots of human culture. Here, we provide a historical review of the study of conformity in both humans and non-human animals. We identify gaps in knowledge and propose an evolutionary route towards the sophisticated cultural processes that characterize humanity. A landmark in the study of conformity is Solomon Asch's famous experiment on humans in 1955. By contrast, interest in conformity among evolutionary biologists has only become salient since the turn of the new millennium. A striking result of our review is that, although studies of conformity have examined many biological contexts, only one looked at mate choice. This is surprising because mate choice is probably the only context in which conformity has self-reinforcing advantages across generations. Within a metapopulation, i.e. a group of subpopulations connected by dispersing individuals, dispersers able to conform to the local preference for a given type of mate have a strong and multigenerational fitness advantage. This is because once females within one subpopulation locally show a bias for one type of males, immigrant females who do not conform to the local trend have sons, grandsons, etc. of the non-preferred phenotype, which negatively and cumulatively affects fitness over generations in a process reminiscent of the Fisher runaway process. This led us to suggest a sex-driven origin of conformity, indicating a possible evolutionary route towards animal and human culture that is rooted in the basic, and thus ancient, social constraints acting on mating preferences within a metapopulation. In a generic model, we show that dispersal among subpopulations within a metapopulation can effectively maintain independent Fisher runaway processes within subpopulations, while favouring the evolution of social learning and conformity at the metapopulation scale; both being essential for the evolution of long-lasting local traditions. The proposed evolutionary route to social learning and conformity casts surprising light on one of the major processes that much later participated in making us human. We further highlight several research avenues to define the spectrum of conformity better, and to account for its complexity. Future studies of conformity should incorporate experimental manipulation of group majority. We also encourage the study of potential links between conformity and mate copying, animal aggregations, and collective actions. Moreover, validation of the sex-driven origin of conformity will rest on the capacity of human and evolutionary sciences to investigate jointly the origin of social learning and conformity. This constitutes a stimulating common agenda and militates for a rapprochement between these two currently largely independent research areas.
I. INTRODUCTION
Social learning is a form of learning that specifically uses information obtained from conspecifics or other animals or the products of their activities to gain information about the environment (adapted from Heyes, 1994). It has been recognized as a crucial learning process for the transmission and spread of adaptive behaviours within and among populations (Laland, 2004; Rendell et al., 2011; Aplin et al., 2015b). As such, social learning is the cornerstone of cultural traditions, since culture relies by definition on socially transmitted information (Boyd & Richerson, 2005).
A major form of social learning that is essential for the establishment of cultural traditions and social norms in humans is conformity (Boyd & Richerson, 1985; Henrich & Boyd, 1998). Several definitions of conformity (or conformist bias) have been used in the literature, depending on the field. They range from ‘copy the majority’, which is a simple positive frequency-dependent bias in learning (e.g. Laland, 2004) to ‘Aschian conformity’, where personal knowledge or preference is overridden by countervailing options performed by others (Asch, 1955). Perhaps the most popular and widespread definition of conformist bias or conformist transmission is defined as the disproportionately likely adoption of the most common variant within the local population (Boyd & Richerson, 1985). Conformity allows individuals easily to grasp local behaviours and norms. In turn, it can generate socially learned traditions that are both resistant to erosion and robust to invasion of alternative variants, potentially persisting over generations. Thus, conformity can produce a combination of within-group homogeneity and between-group heterogeneity (see discussion in Section III).
Because of its central role for the emergence and maintenance of culture, conformity has long been a major topic of research in human sciences (Boyd & Richerson, 1985; Banerjee, 1992; Bernheim, 1994; Aoki, Lehmann & Feldman, 2011; Merrell, 2011; Claidière & Whiten, 2012; Efferson et al., 2016; Denton et al., 2020). By contrast, the study of conformity in non-human animals became a major topic in evolutionary sciences only recently (e.g. Whiten, Horner & de Waal, 2005; Aplin et al., 2015b; van Leeuwen et al., 2015; Danchin et al., 2018), and a synthesis between these two scientific domains is still lacking. Here, we review the literature on conformity in both humans and non-human animals, thus drawing a link between these two sides of the literature. This review allows us to identify gaps in knowledge and suggest a general model for the evolution of social learning and conformity. Our review further suggests that conformity in mate choice deserves closer attention as sexual selection is a strong evolutionary force that has been largely overlooked up to now in this context. We aim to inspire new research agendas in both human and non-human animals to determine in which context, e.g. mate choice or foraging, the cognitive processes that underpin the evolution of animal and human culture could have evolved and how they interact.
II. EARLY DEFINITIONS: CONFORMITY OR POSITIVE FREQUENCY-DEPENDENT LEARNING?
In this section, we propose a brief history of the term ‘conformity’ pre-dating Boyd & Richerson's (1985) definition of conformist transmission bias. Most uses of the term ‘conformity’ over this period would now be more accurately described as positive frequency-dependent social learning [see Efferson et al. (2008) for a discussion around existing definitions of conformity].
The influence of groups on individual behaviour first attracted attention in the early twentieth century (Jenness, 1932; Sherif, 1935), but it was Asch (1955) who popularized the term conformity itself. Using a simple ‘visual judgement’ task, Asch documented that people were willing to abandon their own personal preferences (or convictions) when confronted with a disagreeing majority opinion. He ascribed this behaviour to social pressure and called this innate tendency to self-align on group opinion ‘conformity’. Asch's findings became momentous in developing the field of social psychology. They were replicated many times across different age classes and cultures, even when factors such as group size, motivation, task difficulty or relevance were varied (Baron, Vandello & Brunsman, 1996; Bond, 2005; Bond & Smith, 1996; Griskevicius et al., 2006). Later, Latané's (1981) work on ‘social impact theory’ provided a tentative conceptual framework for these findings. Drawing a parallel with physics, Latané pictured social influence as a force emanating from the group, and acting on individuals (e.g. peer pressure). The intensity of this force could depend on the source group's size, its behavioural composition (the proportion of individuals performing the various behaviours), its status (such as prestige), or its proximity in time and space to the target individual (see also Latané & Wolf, 1981). Overall, social psychology had suggested a proximate reason for ‘conforming’, namely that people inherently dislike going against the majority.
Note that these early studies equated conformity with a linear increase in the likelihood of adopting a behaviour as that behaviour becomes more common in the population – what today is called positive frequency-dependent social learning. Conformist transmission bias as defined by Boyd & Richerson (1985) is a particular case of this class of learning rules (adoption of the most common variant needs to be disproportionately likely). Still, debates from this early period may provide useful insights regarding positive frequency-dependent social learning, and therefore, regarding conformist transmission bias in the Boyd & Richerson sense.
One such example is the question of whether positive frequency-dependent social learning arises because of normative or informational reasons. In Asch's studies, following the majority was a response to peer pressure, but this does not have to be the case. Early on, Deutsch & Gerard (1955) remarked that social psychology had focused mainly on a single side of ‘conformity’ (here again understood as positive frequency-dependent social learning), namely, the normative side. In what they called normative social influence (later also referred to as social conformity; e.g. Coleman, 2004), the commonest behaviour defines a social norm. If the norm is explicitly enforced, for instance by rewarding compliers and punishing non-compliers, then individuals might adopt the normative behaviour through ordinary cognitive mechanisms for avoiding social and environmental risks; in this case, conforming simply means avoiding punishment. But individuals might also adopt the norm not because they consciously try to avoid social risks, but because of specific preferences for conforming, which might have proved adaptive over long timescales. In other words, the drive to conform could be an individually optimized response to social incentives, but it could also be the result of evolved, wired-in preferences. For now, the literature remains unclear on whether normative conformity includes both these mechanisms, or just the latter (conforming because of an evolved, intrinsic drive to do so, e.g. self-esteem concerns).
But Deutsch & Gerard (1955) described another driver of majority-following: informational social influence (sometimes also called instrumental conformity; cf. Burdett et al., 2016). In this case, the behaviour of the majority does not define a norm, but instead reveals useful information about the environment. Take the example of durian (Durio zibethinus), a tropical fruit with a distinctly unpleasant smell which is widely popular in South-east Asia. The naïve tourist might be willing to try it despite the stench, not for fear of social sanctions if he or she does not, but rather because he or she can infer from observing the majority behaviour that the fruit is actually palatable. As pointed out by Cialdini & Goldstein (2004) however, informational and normative conformity are actually interrelated – for instance, from these definitions it is unclear whether an individual using social information to navigate a social environment (e.g. to avoid punishment) is an example of normative or informational conformity.
Economists summarized the mechanisms and limitations of the informational channel in a famous decision problem. Suppose that, on a night out and looking for a good dinner, you stumble upon two adjacent restaurants. One is crowded, the other almost empty. Which restaurant will you choose? First, you should realize that the behaviour of others probably conveys information about quality. The safest choice is therefore to follow the ‘wisdom of the crowd’ and go for the crowded restaurant (i.e. conform). However, sometimes the crowd is wrong, as Banerjee (1992) and Bikhchandani, Hirshleifer & Welch (1992) pointed out. Indeed, in this problem, the first few customers are pivotal in determining group behaviour: if they choose the lower-quality restaurant, all subsequent customers will copy their mistake. Such informational cascades were later observed in laboratory experiments with human subjects (Anderson & Holt, 1997). What these studies emphasized is the fragility of mass behaviour when it is driven by conformity, in the sense that it is susceptible to shifts when accurate information is publicly released. Back to the restaurant example, it means that if customers had had access to the restaurants' ratings on Yelp or Tripadvisor, then they could have ignored the mistake of the first few customers and chosen the best restaurant instead despite it being empty.
Economists also studied the normative channel. For instance, Bernheim (1994) considered a population in which individuals must balance their self-interest with social esteem. Social esteem is obtained by adhering to a group norm, but individuals vary in how their self-interest aligns with this norm. Bernheim (1994) showed that individuals whose self-interest closely aligns with the norm will conform to it in order to reap the social esteem benefits. On the other hand, individuals whose self-interest departs a lot from the norm will ignore that norm, but by doing so their behaviour flags them to the rest of society as undeserving of social esteem. This model illustrates how reward and punishment can enforce conformity around a social norm, even in the presence of individual heterogeneity. In a similar spirit, Kuran & Sandholm (2008) studied how individuals with heterogeneous preferences behave in the presence of coordination incentives; they also found that individuals tend to align their behaviour with the social norm.
This informational versus normative dichotomy, together with the above studies by economists, provides a convenient analytical structure for evolutionary biologists. They suggest that any tendency towards positive frequency-dependent social learning should be rooted in one of these two channels, i.e. that it should have normative or informational benefits. However, informational and normative conformity are interrelated (David & Turner, 1996), and as such they are difficult to disentangle theoretically and empirically. This is reflected in the research on individual differences in conformity. For example, DeYoung, Peterson & Higgins (2002) looked at two personality types, namely Stability (emotional stability, agreeableness and conscientiousness) and Plasticity (extraversion, openness), and found that Stability is a positive predictor of conformity while Plasticity was negatively correlated with conformity. Thus, strong conformists tend to be very stable but also less able to adjust to novelty or change. This also suggests that positive frequency-dependent social learning is not always the right answer to an environmental challenge, as we will see more in detail below. Finally, as a particular case of positive frequency-dependent social learning, conformist transmission in the Boyd & Richerson sense should also obey these insights.
III. CONFORMITY EVOLVES: A BENEFICIAL BIAS?
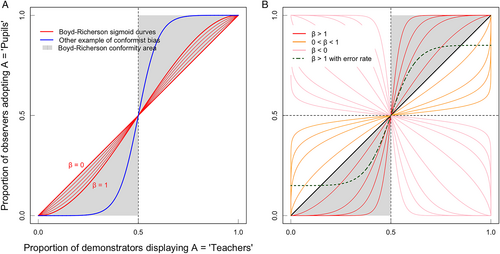
A few decades after Asch's experiments, biologists incorporated conformity into the study of cultural evolution, i.e. the change of culture over time (Claidière & Whiten, 2012; Haun, van Leeuwen & Edelson, 2013; van Leeuwen & Haun, 2013; Whiten, 2019, 2021) where culture is the phenotypic variation that is inherited through social learning (Danchin et al., 2018). Integrating socially transmitted information into a population genetics framework raised the question of how individuals use social information to maximize fitness (Cavalli-Sforza & Feldman, 1981; Boyd & Richerson, 1985). This predicts that such socially aware individuals would be equipped with a wide range of social transmission biases that dictate whom they copy and when (Feldman, Aoki & Kumm, 1996; Henrich & Boyd, 1998; Henrich & McElreath, 2003; Laland, 2004; Enquist, Eriksson & Ghirlanda, 2007; Wakano & Aoki, 2007; Kendal, Giraldeau & Laland, 2009). For instance, individuals could preferentially copy prestigious models (prestige bias), or models that look like themselves (similarity bias), or even – our focus here – models that display the majority behaviour (conformist bias, or conformity for short). A rigorous definition of conformity was established early on by Boyd & Richerson (1985): an individual is conformist if it is more likely to adopt the majority behaviour than if it copied a demonstrator picked at random (Morgan & Laland, 2012; Muthukrishna, Morgan & Henrich, 2016; see Fig. 1). Given the right conditions, this ‘over-copy-the-majority’ rule could be highly profitable.
Subsequent evolutionary models examined conditions favouring different learning biases. Conformity's ‘sweet spot’ turned out to be a spatially variable environment with migration, since conforming helps migrants to adopt the locally adaptive behaviour quickly (Boyd & Richerson, 1985; Henrich & Boyd, 1998; reviewed in Aoki & Feldman, 2014). More generally, conformists perform better when environmental change is slow, social learning is easy (relative to individual learning), transmitted traits have high adaptive value, and fitness payoffs vary greatly with space (Nakahashi, 2007; Kendal et al., 2009).
Conformity adds robustness to the transmission process overall (Dindo, Whiten & de Waal, 2009; Danchin et al., 2018; Lachlan, Ratmann & Nowicki, 2018). A conformist learner is unlikely to pick up a one-off behavioural mistake, since its decision is based on many demonstrators. Yet this redundancy also dampens innovation (Sterelny, 2006), and in rapidly changing environments it may cause a carryover of old behaviours that are no longer adaptive if the individuals are not flexible enough to adapt at the same speed as the environment changes (Aplin, Sheldon & McElreath, 2017). In the context of cultural evolution, this can in turn slow down the response to environmental change and thus promote the maintenance of outdated maladaptive behaviours (Whitehead & Richerson, 2009). In practice however, a mix of conformity and individual learning may allow populations to switch to newly adaptive behaviours. This was shown by Aplin et al. (2017) in an experimental study on great tits (Parus major): by manipulating behaviour rewards, they documented that both individuals and populations were able to move away from established, now-suboptimal behavioural traditions. The takeaway message is that conformity can catalyse a behavioural change that is initially driven by individual learning. A related point was made by Perreault, Moya & Boyd (2012), in providing theoretical evidence that conformity is expected to be a universal feature of social learning when individual learning is also in play.
Given the right conditions, conformity can stabilize any trait – adaptive or not (Laland, 1994). For example, in some models conformity can foster cooperation if interactions and learning are spatially constrained in the population, thus giving rise to ‘local clusters’ of cooperators (Peña et al., 2009; Mengel, 2009; Molleman, Pen & Weissing, 2013a). Instead, in large homogeneous populations, cooperation is diluted, and conformity can block its evolution (Lehmann & Feldman, 2008; Molleman, Quiñones & Weissing, 2013b). Similarly, conformity can coevolve with other cultural or genetic traits such as altruism (Lehmann & Feldman, 2008) or the ability to copy (Wakano & Aoki, 2007). Empirical studies to verify these predictions remain needed, for instance to study how conformity interacts with group size to affect traditions of cooperation.
Crucially, conformity can generate a stable combination of within-group homogeneity and between-group heterogeneity. Indeed, conformity fixes a given group on a single socially learned tradition that remains stable over generations, making the tradition resistant to erosion and robust to invasion of alternative variants. Importantly, different groups can fix on different traits depending on which trait is locally optimal, cultural drift or some specific initial conditions. This is illustrated by patterns of tool use among chimpanzees (Pan troglodytes) from the Taï National Forest (Ivory Coast). These chimpanzees crack Coula edulis nuts with stone and/or wooden hammers and use roots as anvils. Which tools they use depends on the community they live in, and is culturally transmitted. Despite high levels of female intergroup migration, differences between neighbouring groups persist over time with incoming females conforming to their new group-specific tradition (Luncz & Boesch, 2014).
(1) Looking for normative conformity in non-human animals
All the evolutionary models mentioned above relate to informational benefits of conformity. They depict cases where the majority behaviour is a local fitness-maximizing social cue. Hence, predicting the evolutionary success or failure of conformity rests on the statistical concept of Bayesian inference, as made explicit by Perreault et al. (2012). All models thus follow more or less the same reasoning, at least implicitly. They (i) take a naïve individual seeking the fitness-maximizing alternative amongst several behaviours; (ii) assume that the best behaviour is selected for so that it increases in frequency; (iii) hence, conclude that the most-displayed behaviour should be the best, provided that the demonstrator population had enough time to undergo selection. Although this reasoning holds under many circumstances, it may fail when the environment is too unstable for the demonstrator population to have reached the best strategy.
In the normative case, benefits instead occur when the population itself rewards a given behaviour (the norm), or punishes non-compliers. Administering these rewards or punishments is often costly, which impedes the evolution of such social machinery. However, conformity can overcome this evolutionary obstacle so that hardwired conformity allows costly social norms to evolve, implying that conformity might be responsible for the emergence of a reward-and-punishment culture (Henrich & Boyd, 2001). Once established, costly social norms can even coevolve with conformity (Gúzman, Rodríguez-Sickert & Rowthorn, 2007) although such associations are harder to obtain than previously thought (van Cleve, 2016). Since conformity also accounts for the stable combination of intergroup heterogeneity and intragroup homogeneity, it can in turn trigger cultural group selection (Boyd & Richerson, 1985).
Until now, informational benefits have been the dominant explanation for conformist behaviour in evolutionary biology (for an extended discussion see Claidière & Whiten, 2012). Nonetheless, in a non-conformity context rewards or punishments for complying (or not) with group-specific rules or norms have been documented in animals (Singh & Boomsma, 2015; reviewed by Raihani, Thornton & Bshary, 2012) particularly in insects and non-human societies where it seems to maintain intense levels of cooperation, suggesting the existence of normative benefits in non-human animals. We thus suggest that the normative channel deserves more attention.
(2) Viability of conformity in strategic settings
Is it always beneficial to copy the majority? As a preliminary answer to this question, consider how you, as a conformist individual, might fare in the following three scenarios.
(1) Choosing between two plentiful food sources, each potentially healthy or poisonous.
(2) Choosing between two identical but limited-supply food sources.
(3) Choosing between two paths to avoid a predator that preys preferentially on smaller groups.
Scenario 1 is a straightforward win for informationally driven conformity: if a majority of people feed from a given food source, it probably means that it is healthy, so conforming is a good bet. That changes, however, in scenario 2: since the food sources are in limited supply, the more individuals feed from one, the less food remains for you there. Here, conforming will not work well: the situation calls for not copying the majority, and you would be better off by taking the less-popular option. In scenario 3, conformity makes a comeback: the more individuals take a given path, the less likely you are to be attacked by also taking that path (Cresswell & Quinn, 2011). To improve your chances of survival you should learn which is the safe way according to the number of individuals using it, i.e. copy the majority.
The crucial difference between these scenarios is how the behaviour of others affects your fitness. In scenarios 2 and 3 the viability of each option depends on what others do – neither option is intrinsically better independently of the actions of others. Economists call such interactions strategic. In scenario 2, those you copy affect your fitness negatively. Such behaviours are called strategic substitutes, which means that the attractiveness of an option decreases as more individuals choose it (negative frequency dependence). In scenario 3, those you copy affect your fitness positively – such behaviours are called strategic complements, which means that the attractiveness of an option increases as more individuals choose it (positive frequency dependence) (Bulow, Geanakoplos & Klemperer, 1985). Scenario 1 is non-strategic as there is an intrinsically better option (the healthy one) and the behaviour of others affects your fitness only insofar as it provides social information on what that better option is.
These scenarios sketch a qualitative result: conformity seems to thrive only when behaviours are strategic complements, or non-strategic. While a rigorous theoretical exploration may be needed to confirm this simple intuition, it suggests that strategic complements or substitutes may provide a useful lens to understand why conformity evolves for some traits but not others. Further research is needed, both on theoretical and empirical fronts, to investigate this potential link between conformity and strategic settings.
(3) Information acquisition, the value of observations, and memory
As a matter of fact, the classical formalisms used to model information acquisition largely are idealized. Models commonly assume a simple group structure where individuals observe simultaneously everyone else in their group, and then infer the majority behaviour from this far-reaching, sweeping look. More realistically, observations in fact are gathered sequentially from a subset of the whole population (Morgan, Acerbi & van Leeuwen, 2019), as in a recent study on chimpanzees (Watson et al., 2018). Organisms must deduce the majority behaviour from such imperfect samples, probably by using individual learning and memory, which none of the models of conformity to date has implemented. Different environments could promote different ways to deal with information. In unstable environments for instance, older observations may be discounted in favour of recent ones, since reliance on past observations could cause deleterious behavioural inertia. Regarding the size of the sample of demonstrators, Denton et al. (2020) showed that evolutionary dynamics can be more complex than previously thought when there are more than three role models, as in Boyd & Richerson's (1985) classical treatment.
Similar arguments hold for how to integrate space, prestige or other characteristics into the evaluation of observation. Should an immigrant completely ignore information from its home environment in favour of local practices? Should it give more weight to demonstrators like itself? Is a prestigious demonstrator worth two, 10, or a 1000 ordinary ones? Mesoudi (2018) addressed similar questions by modelling immigrant conformist acculturation. Basically, this comes down to studying the interaction between conformity and other biases, such as prestige, success, locality, ethnicity, age, sex, etc. The core problem is to determine the value of adding a single demonstrator into the decision process, based on that demonstrator's personal characteristics relative to the observer. This is both a theoretical and an empirical question that needs to be explored.
Finally, while most current studies concern binary choices, natural situations usually involve many more options. Expanding the binary approaches would raise empirical challenges (what would occur when the majority is still an absolute minority, as in a 40–30–30 split?) and theoretical ones. A first unanswered problem is to devise a suitable learning rule (an S-shaped probability, symmetric in all behaviours) when there are more than two behaviours to choose from, or even when traits are continuous. These issues remain under-explored for the moment, with a few exceptions (e.g. Nakahashi, Wakano & Henrich, 2012; Mesoudi, 2018). In the next section we outline a possible step in this direction.
(4) Conformity with more than two choices: a functional form and a statistical test
β > 1 corresponds to conformist transmission in Boyd & Richerson's (1985) sense;
0 < β < 1 corresponds to weak conformity;
β < 0 corresponds to anti-conformity.
IV. CONFORMITY IN NON-HUMAN ANIMALS
(1) Behavioural ecology
Boyd & Richerson's (1985) conformist transmission model has recently attracted a lot of attention in behavioural ecology (e.g. Brown & Laland, 2002; Pike & Laland, 2010; Nelson & Poesel, 2014; Aplin et al., 2015b, 2017; Battesti et al., 2015; Danchin et al., 2018; Lachlan et al., 2018; Ayoub, Armstrong & Miller, 2019), the domain of evolutionary sciences that studies the evolution of behaviour (Davies & Krebs, 1984; Danchin, Giraldeau & Cézilly, 2008). However, although conformity in humans rapidly attracted attention, it remained largely overlooked in non-human animals for a long time, with the first studies of animal conformity being published in the early 2000s, more than 50 years after Asch's studies. Although non-human animal conformity is now becoming a hot topic, the junction with the domain of human conformity remains to be made to allow the full study of the evolutionary origins of human conformity.
In the non-human animal literature, conformity is often defined as ‘behaving like, or copying, the majority’ which is in many cases a simplification of Boyd & Richerson's (1985) ‘disproportionate likelihood of adopting the majority strategy’ definition. At the very least, this broader definition raises several important issues that are also subject to recent debate. First, the definition ‘copy the majority’ does not specify whether the majority concerns the larger number of individuals performing a behaviour or whether it concerns the behaviour that is most frequently displayed (van Leeuwen et al., 2015). Indeed, it is interesting to notice that this is not specified in Boyd & Richerson's (1985) model either. From an evolutionary standpoint, the majority should refer to the number of individuals performing a behaviour. This allows an outsider to grasp easily what is the appropriate behaviour under given circumstances. Otherwise, there might be only a few individuals over-displaying a given behaviour, making it the most frequent, but not the one adopted by the majority of individuals in the population (van Leeuwen et al., 2015; Acerbi et al., 2016). However, in some cases, it can also be beneficial if the behaviour with the highest frequency is adopted; namely when particularly highly skilled or successful individuals perform one behaviour especially frequently. For example, when great tits trained to open a puzzle box to get a food reward were released into the wild, these few skilled individuals frequently opened the slider of the puzzle box in a specific way and this technique was adopted by other individuals in the population. Thus, conforming with respect to the frequency of options performed may be as adaptive as conforming with respect to the majority of individuals (Aplin et al., 2015a). Similarly, models showed that both the number of individuals and the frequency of the behaviour can lead to the same sigmoidal conformity curve (Smaldino, Aplin & Farine, 2018). It is likely that if at the beginning the most frequent behaviour is copied, soon a majority of individuals in the population will also exhibit this behaviour. Second, this simplified definition of ‘copy the majority’ is sometimes used only for cases where an individual performing behaviour A changes to behaviour B to follow the majority (e.g. Cialdini & Goldstein, 2004; Cherng et al., 2014; Haun, Rekers & Tomasello, 2014). For example, wild male vervet monkeys (Chlorocebus pygerythrus) that migrate to a new group will abandon personal foraging preferences in favour of the new group norms (van de Waal, Borgeaud & Whiten, 2013). This restriction that behaviour A must first be learned before switching to B seems unnecessarily restrictive as even naïve individuals can show conformist behaviour after observing others. Last but not least, it is important to focus on the fact that frequency-dependent copying without disproportionately likely copying of the most common variant cannot foster stable local traditions in which all group members adopt the same behaviour. This is illustrated in Fig. 1A where Boyd & Richerson's (1985) definition of conformity corresponds to the grey areas, while the common broader definition of conformity would also consider strategies in the white areas as conformist, despite the fact that such strategies would invariably rapidly drive populations towards a stable equilibrium with no majority (i.e. at 0.5). Thus, the ‘disproportionate likelihood of adopting the majority strategy’ is crucial to study and compare conformity both in non-human animals and humans.
Although research on non-human animal conformity is relatively recent, there is some evidence for conformity in several taxa from insects to great apes and in various contexts including foraging, song learning, problem-solving tasks, tool use and mate choice (Table 1). Unfortunately, these studies use very different kinds of definition of conformity ranging from a ‘copy the majority’ rule, over Aschian conformity where a personal preference/behaviour is replaced by the majority preference/behaviour to Boyd & Richerson's (1985) definition of conformist transmission. Thus, the field would benefit from clearer definition and consistent use of terminology to allow for comparisons across species and studies. More generally, Table 1 does not include the well-studied phenomena of herding or shoaling and other processes of collective behaviour [for instance, coloniality in birds (e.g. Siegel-Causey & Kharitonov, 1990; Buckley, 1997; Danchin & Wagner, 1997; Danchin, Boulinier & Massot, 1998; Dukas & Edelstein-Keshet, 1998; Rolland, Danchin & de Fraipont, 1998; Doligez et al., 1999, 2003; Barta & Giraldeau, 2001; Brown & Bomberger Brown, 2001; Serrano et al., 2001; Boulinier et al., 2002; Doligez, Danchin & Clobert, 2002; Eberhard, 2002; Parejo et al., 2007; Varela, Danchin & Wagner, 2007)] that are not usually called conformity, despite the fact that such processes can be envisioned as forms of conformity in space as long as they involve social learning, which was clearly suggested by several authors from both observational and experimental data (Danchin et al., 1998; Boulinier et al., 2002; Doligez et al., 2002, 2003; Parejo et al., 2007).
| Context | Species | Working definition used | References |
|---|---|---|---|
| Cooperation | Ant (Paratrechina longicornis) | “Conformist group members align their actions with those of their neighbours” (p. 2) | Gelblum et al. (2015) |
| Oviposition site | Fruit fly (Drosophila melanogaster) | “The tendency to disproportionately adopt the most commonly encountered social information” (p. 84) | Battesti et al. (2015) |
| Mate choice | Fruit fly (Drosophila melanogaster) | “An exaggerated tendency to copy the majority” (p. 362) | Danchin et al. (2018) |
| Shoaling | Mosquitofish (Gambusia holbrooki) | No clear definition | Herbert-Read et al. (2013) |
| Guppy (Poecilia reticulata) | “Positive frequency-dependent social learning” (p. 917) | Day et al. (2001); | |
| “Many animals are disproportionately likely to adopt via social learning the behaviour of the majority” (p. 41) | Brown & Laland (2002); | ||
| “Strong compulsion for individuals within social groups to remain in close contact and look and behave similarly” (p. 95) | Brown & Irving (2014) | ||
| Rummy-nose tetra (Hemigrammus rhodostomus) | “Tendency to follow the majority of their neighbours nonlinearly” (p. 1) | Lecheval et al. (2018) | |
| Personality | Solitary crab (Carcinus maenas) | “Animals compromise their own behaviour to the level of a certain behaviour displayed by another individual or a group” (p. 131) | Fürstbauer & Fry (2018) |
| Eurasian perch (Perca fluviatilis) | “Behaving uniformly” (p. 501) | Hellström et al. (2011); | |
| No clear definition | Magnhagen (2012) | ||
| Gouldian finch (Erythrura gouldiae) | “Individuals will tend to synchronize their behaviour in time and space, altering their behaviour in line with their group mates, and potentially suffering consensus costs” (p. 26) | King et al. (2015) | |
| Marmoset (Callithrix jacchus) | No clear definition | Koski & Burkart (2015) | |
| Song | White-crowned sparrow (Zonotrichia leucophrys) | “When a young pupil models its song(s) on those of one or more tutors” (p. 433) | Nelson & Poesel (2009); |
| “Disproportionate tendency to copy the most common behavioural variant” (p. 1742) | Nelson & Poesel (2014) | ||
| Swamp sparrow (Melospiza georgiana) | “Disproportionate tendency to copy the majority” (p. 2) | Lachlan et al. (2018) | |
| Indo-Pacific bottlenose dolphin (Tursiops aduncus) | “Being increasingly likely to adopt the most frequent behaviour” (p. 6) | Cantor & Whitehead (2013) | |
| Humpback whale (Megaptera novaeangliae) | “Being increasingly likely to adopt the most frequent behaviour” (p. 6) | Cantor & Whitehead (2013) | |
| Sperm whale (Physeter macrocephalus) | “Learn preferentially the most common codas” (p. 1) | Cantor et al. (2015) | |
| Tool use | Japanese macaque (Macaca fuscata) | “Immature individuals should adopt the same type of stone-directed activities as most of the older group members” (p. 124) | Leca et al. (2010) |
| Chimpanzee (Pan troglodytes) | “A powerful tendency to discount personal experience in favour of adopting perceived community norms” (p. 738) | Whiten et al. (2005); | |
| No clear definition | Whiten et al. (2007); | ||
| “Personal knowledge was dropped in order to adopt the behaviour of the group” (p. 650) | Luncz & Boesch (2014) | ||
| Foraging | Nine-spined stickleback (Pungitius pungitius) | “Positive frequency dependent social learning where the probability of acquiring a trait increases disproportionately with the proportion of other individuals performing it” (p. 466) | Pike & Laland (2010) |
| Threespined stickleback (Gasterosteus aculeatus) | No clear definition | Webster & Hart (2006); | |
| No clear definition | McDonald et al. (2016) | ||
| Zebrafish (Danio rerio) | “Copy the majority strategy” (p. 1519) | Zala et al. (2012); | |
| “Individuals will appear to disproportionately copy the most common behavioural choice demonstrated by their group” (p. 164) | Ayoub et al. (2019) | ||
| Great tit (Parus major) | “Tendency for naïve individuals to disproportionately adopt the most common behaviour (‘conformist transmission’) and by a tendency for individuals with experience of both techniques to change their behaviour to match the common variant (‘conformity’)” (p. e5) | Aplin et al. (2015a); | |
| “Disproportionate tendency to copy the most common behavioural variant” (p. 7830) | Aplin et al. (2017) | ||
| Norway rat (Rattus norvegicus) | “Changing one's behaviour to match that of others” (p. 769) | Jolles et al. (2011) | |
| Indo-Pacific bottlenose dolphin (Tursiops aduncus) | “Being increasingly likely to adopt the most frequent behaviour” (p. 6) | Cantor & Whitehead (2013) | |
| Humpback whale (Megaptera novaeangliae) | “Being increasingly likely to adopt the most frequent behaviour” (p. 6) | Cantor & Whitehead (2013) | |
| Redfronted lemur (Eulemur rufifrons) | “Adoption of the group's norm, despite being in principle able to behave differently, or overriding of individually learned by socially acquired information” (p. 506) | Schnoell & Fichtel (2012) | |
| Capuchin monkey (Cebus apella) | “Conform to the foraging preferences of their closest social partners, despite having the knowledge of alternative techniques” (p. 4) | Dindo et al. (2009); | |
| No clear definition | Crast et al. (2010); | ||
| No clear definition | Franz & Matthews (2010) | ||
| White-faced capuchin monkey (Cebus capucinus) | “The tendency for individuals to preferentially exhibit behavioural alternatives that they witness most frequently in their peers, or to exhibit the behaviours that are performed by peers who are considered most prestigious or successful, or those peers with whom they have the highest quality social relationships” (p. 706) | Perry (2009) | |
| Vervet monkey (Chlorocebus pygerythrus) | “Conformity to local behavioural norms” (p. 483) | van de Waal et al. (2013) | |
| Chimpanzee (Pan troglodytes) | “Follow-the-majority (= the number of animals in a group performing a specific behaviour increases, so does the likelihood of a naïve individual adopting that same behaviour, thus driving the preservation)” (p. 1195) | Hopper et al. (2011); | |
| “The increased likelihood for learners to end up not with the most frequent behavior but rather with the behavior demonstrated by most individuals” (p. 727) | Haun et al. (2012); | ||
| “Foregoing a pre-existing behaviour in favour of adopting one demonstrated by a majority of conspecifics” (p. 407) | Watson et al. (2018) |
All these considerations suggest that there is probably more evidence for the existence of informational conformity in animals than usually thought. This vast breadth of taxonomies and contexts (Table 1) raises the question of the evolutionary origin of conformity. In itself, this breadth suggests convergent selection for conformity rather than a homologous capability (Laland, Atton & Webster, 2011). However, we are still lacking a general framework by which conformity may have emerged in the first place during the course of evolution.
(2) Conformity in mate choice and the Fisher runaway process
Our review of the literature on non-human animal conformity has revealed a quasi-absence of evidence for conformity in the context of mate choice (Table 1, with the nuance we introduce in Section V). The only clear example of conformity in mate choice in any animal including humans is a study in fruit flies (Drosophila melanogaster) where females develop mating preferences for a certain male phenotype in a conformist manner (Danchin et al., 2018). In that study, as long as there was a majority of demonstrator females copulating with males of a given phenotype, observer females copied this choice and developed a similarly significant bias for males of that specific phenotype whatever the level of majority in the population. As a result, the response function of observer females followed a step function (as the green dotted curve of Fig. 1B), with females learning equally well to prefer the most commonly chosen male colour whatever the level of majority, which in that study varied experimentally from 100% to only 60%. However, a certain number of individuals did not copy the majority for whatever reason (e.g. being anti-conformists or individual learners). A simple model also suggested that this strong conformity in mate choice might generate surprisingly long-lasting traditions of preferring one male phenotype at the local scale (Danchin et al., 2018).
The Drosophila result on conformity calls for an evolutionary explanation. Interestingly, conformity in the context of mate choice is reminiscent of the well-known Fisher runaway process (Fisher, 1930). According to this process, both male traits and female preferences are supposed to be under genetic control. Under such conditions, as soon as females develop some preference, females tend to mate with their preferred male phenotype, which automatically generates a correlation between the male trait and the female preference. This generates a linkage disequilibrium of purely statistical nature (Bailey & Moore, 2012). This statistical linkage participates in what we now call ‘inclusive heritability’, i.e. parent–offspring resemblance due to genetic or non-genetic information being transferred from parents to offspring (Danchin & Wagner, 2010; Danchin et al., 2011). Such resemblance can involve DNA sequence variation (which is probably partly valid for the male trait) but could also result from the cultural transmission of female preference. What matters is that females transmit their preference to their daughters, and fathers their trait to their sons, so that the information responsible for the two traits (e.g. male trait A, and the preference for A) are transmitted jointly. Hence, the female trait becomes statistically associated with the male trait, producing a self-reinforcing process called the Fisher runaway process (Fisher, 1930). Based on this understanding of the Fisherian process, we now propose and provide a generic model for a possible two-step evolutionary scenario rooted in this process, which unfolds at two different spatial scales.
Let us first imagine an ancestral metapopulation with two heritable male phenotypes A and B with equal fitness (as is the case, for instance, in Danchin et al., 2018). There is no initial female preference for one of these male phenotypes, but some inclusively heritable variation in females' tendency to copy others (due to the inheritance of genetic or non-genetic information). In a given subpopulation, chance may sometimes lead to more females mating with, for instance, A males. As soon as such a majority becomes detectable, copier females tend to mate more often with A males. As a result, they will tend to have A sons and daughters that tend to copy the choices of their elders, implying that they reject B males (left panel, Fig. 2). At the scale of the subpopulation, because of the Fisher runaway process, this reinforces both the proportion of A males and the tendency socially to learn to prefer A males. On the other hand, females of the initial subpopulation choosing B males (i.e. mostly non-copiers) would have B sons and mostly non-copier daughters that mate randomly with A and B males. So, at the beginning, while copier females tend to amplify the proportion of A males and the tendency to copy, non-copier females produce sons in proportion to the frequency of each male type in the subpopulation, while producing non-copier daughters. Thus, non-copier females do not affect the proportion of male types.
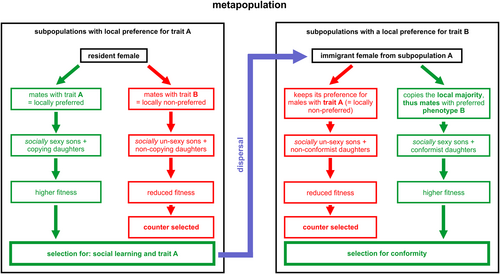
Thus, at the scale of the subpopulation, locally both the proportion of A males and of copier females slowly increase at a rate that accelerates with the proportions of copier females in a kind of snowball effect. As a result, non-copier females mating with B males will have B sons that will be more and more socially unsexy over the course of generations. On the other hand, copier females mating with A males are more and more favoured (Fig. 2, left panel). In summary, at the local scale, this process transitorily selects for A males, and for social learning in mate choice. This process, however, is transitory because social learning becomes neutral after the local disappearance of the B male phenotype (see simulations in Section VI). Note that even if the two male phenotypes initially did not differ in terms of fitness, the trait soon quits neutrality because of the social transmission of mating preferences in females. This is the runaway process.
Now, this logic unfolding within a single subpopulation should simply transitorily select for females preferring A males. Nonetheless, that transitory phase probably generated a correlation between male trait and female preference for it. As this correlation is likely to persist for some time after the disappearance of B males, this implies that if dispersing B males enter that local population the Fisher runaway process will immediately resume. In fact, at the scale of a metapopulation, which consists of subpopulations connected by dispersing individuals, in some subpopulations selection would favour females mating with A by chance (Fig. 2, left panel), and in other subpopulations females mating with B males (Fig. 2, right panel). In such a system, dispersal hampers the local evolution of a heritable preference for A, and favours conformity, as immigrants detecting the local preference and conforming to it have higher fitness (Fig. 2, right panel and Section VI). Hence, it is the spatial structure of the metapopulation with individuals dispersing among subpopulations that generates selection for the more integrated rule of ‘mate with males of the locally preferred phenotype’, i.e. for conformity.
Note that this process is superficially similar to a process by which conformity could arise for foraging preferences, but it is different at a deeper level as in the latter case conformity evolves due to natural and not sexual selection as proposed above. Suppose that in the first subpopulation fruit C is nutritious and fruit D is poisonous, while in the second subpopulation there is fruit E, resembling C, that is poisonous and fruit F, resembling D, that is nutritious. A preference for C and a preference for copying the majority would be equally adaptive in the first subpopulation, just as a preference for F and a preference for copying the majority would be equally adaptive in the second subpopulation. However, for dispersers between the two, a preference for copying the majority would continue to be adaptive, while a preference for C, translated to the second environment, might be fatal, as would a preference for F, translated to the first environment. So, conformity would be more adaptive on average than either direct nutritious preference.
Unlike the Fisher process, though, this process would not be self-reinforcing – conformity would not become more adaptive as it became more common in the population. In addition, its adaptive value would depend on the absence of consistency in the nutritious value of foraging preferences across environments. This may be true in some ecologies but lacks the general autocatalytic runaway character of the Fisher process at the metapopulation scale, which is independent of other characteristics of the environment.
In view of the generality of the Fisher runaway process in sexually reproducing organisms, and in view of its autocatalytic properties, we should expect conformity in mate choice to be far more common than usually understood. In other words, it should have evolved very early in evolution, soon after the moment when sexually reproducing organisms developed enough cognitive capacities to detect the local majority. This raises the question of the links between mate copying and conformity.
Mate copying is a special case of social learning in the context of mate choice in which females build their own preference from observations of another female choosing between male phenotypes. Mate copying can be performed in a conformist manner (Danchin et al., 2018), i.e. over-copying what is preferred by the majority on one hand, and on the other hand conformity in mate choice can be the result of positive frequency-based mate copying. Unfortunately, very few studies of mate copying have tested whether it is performed also in a conformist fashion, precluding us from studying the links between these two processes. In fact, most studies on mate copying use only a single demonstration for practical reasons (reviewed in Varela, Matos & Schlupp, 2018), thus preventing us from talking about the majority. We suggest that mate-copying experiments may in fact constitute a specific test of conformity in which the experimental design limits itself to a sample size of demonstrators of 1. As animals have been selected to conform in mate choice (as we developed above), as soon as they see one demonstration, they may interpret it as a majority preference in the population, because chance makes it more likely that this single observation represents the local majority. But the smaller the majority the higher the risk of opting for the locally non-preferred male phenotype (which corresponds to the black random copying line in Fig. 1B). In nature, however, by observing multiple matings, observer females can considerably increase the quality of their assessment of the local majority and thus can considerably increase their chance of building a preference for the majority male phenotype.
This reasoning would suggest that the rather large literature on mate-copying experiments may reveal the existence of conformity in mate choice in the many concerned species. Hence, we can predict that a quick way to increase the evidence for conformity in non-human animals would be to test for conformity in species known to preform mate copying by simply adapting the experimental design in order to be able to show multiple demonstrations in order to manipulate the majority as in the testing of Danchin et al. (2018)'s criterion 5. If, as we predict, many such studies provide evidence for conformity in sex, this would support the idea that mate copying and conformity are tightly linked, and that mate copying and conformity in mate choice are two faces of the same coin that jointly accelerate and maintain the evolution of local traditions for preferring a specific male phenotype over generations. Thus, future studies should systematically test for conformity in mate copying. It is thus of prominent importance to design experiments that manipulate the level of majority in order to study the response function of conformity in mate choice in a wide range of species.
V. FROM FISHER TO HUMAN CULTURE
Beyond the fact that the existence of conformity in numerous animal taxa generates a continuum from non-human animals to humans, it is necessary to integrate some of the major consequences of the evolutionary scenario presented in the previous section in terms of its potential to foster cultural transmission in general and in humans in particular.
As discussed above, the first step in this evolutionary pathway unfolds locally and temporarily favours social learning (Step 1 of Fig. 3). Similarly, the second component results from individuals dispersing among subpopulations within the metapopulation, which stabilizes social learning and leads to the evolution of conformity (Step 2 of Fig. 3). Thus, in highly mobile species, conformist mate choice might have fostered the development of a kind of ‘conformist module’ for detecting the majority and following it in order to mate preferentially with the locally preferred male phenotype. These two first steps are detailed in the previous section and should foster the emergence of local traditions in mating preferences (Step 3 of Fig. 3). In view of the generality of the Fisher runaway process and of the scenario proposed in Section IV.2, these capacities probably evolved as soon as sexually reproducing ancestral species acquired the capacity to detect the majority, so that they should be present in a vast array of species.
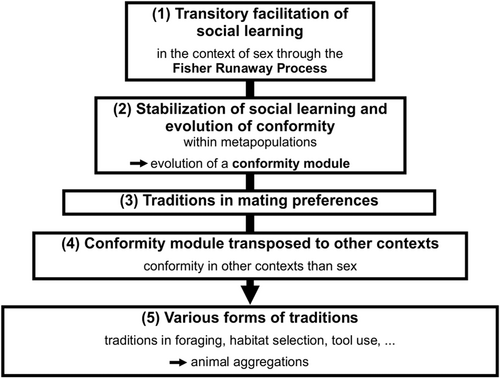
However, the story does not end there. We have detailed above that there is some theoretical and empirical evidence that conformity is potent in fostering a cultural process (Fisher, 1930; Sterelny, 2006; Efferson et al., 2008; Laland et al., 2011; Danchin et al., 2018; Lachlan et al., 2018). Like norms or punishment, conformity can play the role of a ‘repair mechanism’ to maintain an existing preference/behaviour. Thus, once acquired and potent, the conformity module may have percolated to other contexts such as foraging, problem solving, tool use, etc. (Step 4 of Fig. 3). Alternatively, cognitive processes involved in copying foraging behaviour may have been subsequently co-opted for use in mate choice. Some mechanisms, however, do not seem easily co-opted from foraging to mate choice – for instance, the pheromone trails that yield conformity in ant foraging (Sumpter & Beekman, 2003). Another alternative is that conformity emerged independently in foraging and mate choice within lineages. Further studies are needed to rule out the different possibilities. Nonetheless, as in this scenario initial fitness benefits accrue from reproduction, it predicts that social learning should remain efficient during the whole reproductive period, which appears consistent with some non-human animal and human studies.
The evolution of a conformity module that expanded to other contexts then may have fostered the emergence of local traditions in many domains of behaviour (Step 5 of Fig. 3). This pathway would thus imply that all the reported cultural processes, whatever the domain involved, would find their origin in mate choice and sexual reproduction in the first ancestors that acquired the capacity to detect and comply with the majority, which must have produced the initial trigger of the Fisher runaway process.
VI. A GENERIC MODEL OF THE EVOLUTION OF CONFORMITY
We investigated the verbal model that we developed above using simulations of population dynamics, in order to explore whether conformity can indeed evolve via such a mechanism (Figs. 4 and 5). Consider a group of individuals, with the same number of males and females. There are two diallelic genes (or two non-genetic inclusively heritable variants). The first one, with variants A and B, is expressed in males only and is neutral for fitness. The second one, with variants C and c, is expressed in females only: females c mate randomly, while females C use the logistic conformist learning rule described in Section III.4. without associated cost. The strength of this conformist rule is expressed by the parameter β. Initially, the two inclusively heritable types of variants are neither physically nor statistically linked so that they vary independently from each other. Each female has two offspring, one male and one female that inherit the traits from their parents.
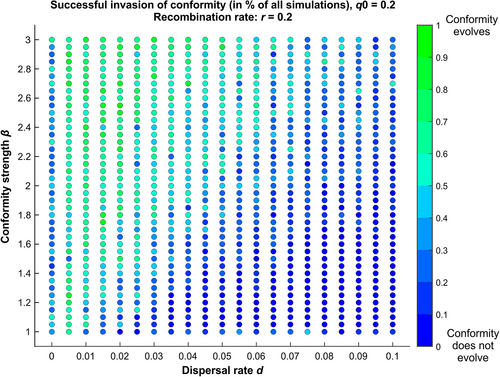
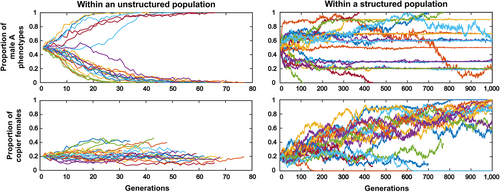
The metapopulation is composed of several such groups. In each new generation, a random fraction d of the offspring migrates to another randomly chosen subpopulation on one of the spots left vacant by other migrants (males take male spots and females take female spots to prevent sex bias in groups). Initially, alleles A and B are randomly allocated in the meta-population in 50:50 proportions. Alleles C and c are also randomly allocated, with an initial proportion q0 of allele C. We explored the effect of the dispersal rate d and of the conformity strength parameter β on the proportion of A and B males and of C and c females (Fig. 4), taking the initial proportion of q0 of conformists and the recombination rate r fixed (q0 = r = 0.2).
Simulations show that conformity can indeed evolve under the sole effect of the Fisher runaway process unfolding independently in every subpopulation (Fig. 4). This happens in a particular region of the parameter space, namely, when the dispersal rate d is not too high compared to the conformity strength parameter β. A likely explanation is that when the dispersal rate is too high, the spatial structure of the population becomes less relevant. However, more theoretical work is needed to understand the precise role of each parameter in the evolution of conformity via the Fisher runaway process.
Our simulation results mirror those of Somveille et al. (2018), who study the effect of similar parameters in a computational model of the emergence of local traditions. Similarly, they find that local traditions emerge when the dispersal rate is not too high compared to the strength of conformity. The difference with our model is that they consider conformity as a common feature of all individuals and study the consequences on behaviour adoption. By contrast, we study whether conformity can evolve in the first place through the Fisher runaway process. In our case, local traditions emerge because conformity has been able to evolve. Our goal here is not to illustrate all the properties of this simple model but rather to show that under some parameter values, conformity does evolve under the sole effect of the Fisher runaway process unfolding independently in every subpopulation.
As an illustration, we also show how the population dynamics unfold for a set of parameters for which conformity evolves (Fig. 5) and compare this to the case wherein the population is spatially unstructured. For these parameter values, conformity evolves readily in metapopulations, and leads to among-group diversity on the male phenotype. When the population is unstructured however (the same number of individuals but no subgroups and therefore no migration), male diversity is quickly lost, and conformity becomes neutral.
VII. CONCLUSIONS
(1) The strength of our proposed pathway rooted in mate choice and runaway sexual selection is that it explains the evolution of social learning and conformity, as well as culture.
(2) One of the major challenges therefore for empirical studies of social learning is to find out whether the detection of majority behaviour in mate copying preceded (in evolutionary time) the detection of majority behaviour in other contexts such as foraging and to what extent it evolved analogously or homologously. For that goal we will need to determine in a large range of species the shape of the response function of conformity in mate choice, and other contexts with experiments manipulating the level of majority. Altogether, this provides a rich agenda for future research.
(3) In the expectation of such information, the tentative model we propose and simulate here for the evolution of conformity, and all its cultural evolution consequences, casts surprising light on one of the major processes that has participated in making us humans. Sex might play a bigger role than previously thought in the long-run development of cultural traditions.
ACKNOWLEDGEMENTS
We thank Sergey Gavrilets for his help in the building of the simulation model, as well as Suzanne Alonzo, Alexis Chaine, Alex Mesoudi and Jorge Peña for insightful comments on previous versions of this review. This work was supported by the CNRS in the framework of its mission for transverse and interdisciplinary initiatives (MITI) project CNRS Prime|80 2019. A.J., P.S., and S.N. acknowledge IAST funding from the French National Research Agency (ANR) under the Investments for the Future (Investissements d'Avenir) program, grant ANR-17-EUR-0010. E.D. and G.I. were supported by the ‘Laboratoires d'Excellence (LABEX)’ TULIP (ANR-10-LABX-41), the Toulouse Initiative of Excellence ‘IDEX UNITI’ (ANR-11-IDEX-0002-02) transversality grant, and MoleCulture (ANR-18-CE37-0015). G.I. benefitted from a CNRS Excellence Chair.




