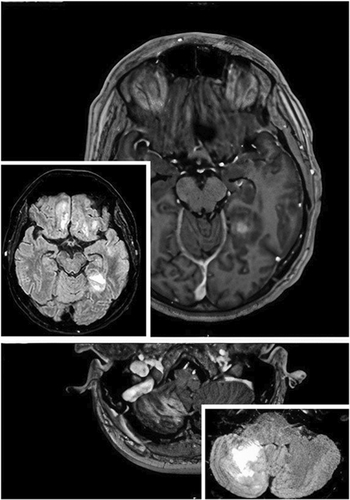
Multiple intra-axial lesions in a 57-year-old male with a history of B-cell chronic lymphocytic leukemia
BOX 1. Virtual glass slide
Access at https://isn-slidearchive.org/?col=ISN&fol=Archive&file=21B088241-1F%20.svs
1 CLINICAL HISTORY
A 57-year-old male with a recent history of B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (BCLL/SLL), developed a persistent left-frontal headache. One month after the onset of headache and 2 days after the second dose of anti-SARS-CoV2 mRNA vaccine, he developed a persistent high fever. He was monitored for the following days and 20 days later, he experienced tonic–clonic seizures with post-critical coma (Day 1). CT and MRI scans showed multiple sub and supra-tentorial intra-axial edematous lesions with contrast enhancement and without mass effect, as shown in Figure 1. He started dexamethasone and levetiracetam with subsequent total recovery of vigilance on Day 3 (Box 1).
On Day 6, a stereotactic biopsy of the major frontal lesion showed the presence of necrotic tissue. Following clinical improvement, on Day 9 he was discharged, with dexamethasone taping from Day 20. On Day 40 a control RMI showed edema reduction, but new nodules appeared. He was admitted again and underwent CSF analysis, lymphocyte characterization, blood culture, and total body 18FDG-PET-CT, all non-contributive.
Still, under steroid tapering, the patient became unresponsive and febrile again, so corticosteroid dosing was increased. After initial improvement he went into hyponatremic coma, consistent with inappropriate ADH secretion syndrome. A new MRI showed a further increase in the lesions and expanded edema. On Day 67 a new open biopsy of the left frontal lesion was performed. After histologic diagnosis, the patient was referred to the Onco-Hematology department where a bone marrow biopsy confirmed a modest infiltration by BCLL/SLL (10% of cellularity). Chemotherapy with MATRix regimen was started with only mild improvement. He ultimately succumbed to the disease on Day 98.
2 FINDINGS
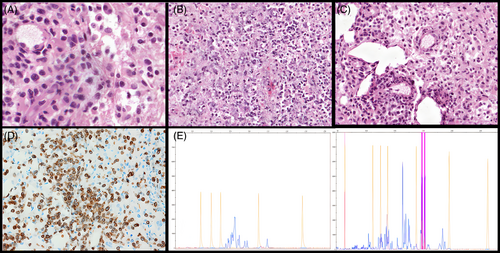
On hematoxylin–eosin the lesion showed extensive necrosis with predominantly medium-sized, atypical lymphoid cells with irregular nuclei, moderate amount of pale cytoplasm with vascular proliferation and histiocytic elements (Figure 2A–C). The neoplastic cells displayed angiocentric growth, mitoses, and apoptotic bodies. CD3 (Figure 2D) and CD2 were positive in neoplastic cells (Figure 2E) as well as Beta-F1 for the αβ T-cell receptor (TCR) dimer; CD20, PAX5, CD5, CD7, CD4, CD8, TdT, CD56, Tia-1, and TCR-γ dimer were negative. Epstein–Barr virus-encoded small RNAs were not detected at in situ hybridization. TCR Gamma rearrangement (TRG) was analyzed by GeneScanning and two distinct reproducible peaks were detected (Figure 2E) interpreted as either a biallelic or biclonal rearrangement.
3 DIAGNOSIS
Peripheral T cell lymphoma, Not Otherwise Specified (NOS) consistent with Primary CNS T cell lymphoma.
4 DISCUSSION
Peripheral T-cell lymphoma (PTCLs) accounts for 2%–4% of primary CNS lymphomas in Europe. Immunodeficiency might play a role in the etiology of the disease in a subset of patients. Involved sites include the frontal and temporal lobes, cerebellum, pituitary, and (rarely) leptomeninges. Involvement can be single or multifocal.
Clonal proliferation, extensive necrosis, prominent angiocentric growth, histological and genomic heterogeneity, phenotypic aberrancy, and cytotoxic phenotype are typical hallmarks of biopsy-proven PTCLs, while symptoms and neuroradiological examinations are often nonspecific. Therefore the awareness of this entity is important for distinction with other intra-axial tumors.
The main differential diagnosis is with other primary CNS lymphomas with medium-to-large cell composition, frequently of B-cell derivation, including primary CNS diffuse large B-cell lymphoma (CNS-DLBLC), Burkitt lymphoma, and EBV+ large B-cell lymphomas. Other nodal or extranodal B-cell and T-cell lymphomas can involve the CNS, such as anaplastic T-cell lymphoma, most commonly in refractory/advanced-stage patients. Immunophenotype is crucial, with minimal contribution from morphology or clonality analysis (TCR-γδ is detected in only 50% of primary CNS T-cell lymphomas).
- CD4/CD8 negative phenotype is very unusual in peripheral T-cell lymphoma-NOS and raises the differential diagnosis with other double-negative T-cell lymphomas (i.e., γδ T-cell (γδTCL) or NK-cell lymphomas (NKCL), anecdotally described in the CNS) [1].
- γδTCL and NKCL however, commonly express cytotoxic markers (CD8, TIA1), that rarely occur in NOS subtype; NKCL is usually associated with massive necrosis and angioinvasion, CD3, CD5, and EBV positivity and germline TCR configuration, whereas the γδTCL expresses the γδ dimer.
- EBV negativity excludes an EBV-positive peripheral T-cell lymphoma [2, 3].
- Precursor/lymphoblastic T-cell lymphomas may involve the CNS and can be CD4/CD8 negative, but the cytological features are not so pleomorphic, and the cells are typically positive for TdT.
- Advanced stages of adult T-cell lymphoma/leukemia can also show CNS involvement but arise in specific epidemiologic settings, are HTLV-1 associated, and typically CD4+.
AUTHOR CONTRIBUTIONS
Matteo Martinoni: acquisition of data. Elena Sabattini, Rocco Liguori, and Caterina Tonon: revising the manuscript. Sofia Asioli, Luisa Di Sciascio, and Lina Cardisciani: study design, drafting design, interpretation of pathological results, revising the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.




