Decreased microvascular claudin-5 levels in cerebral amyloid angiopathy associated with intracerebral haemorrhage
Graphical Abstract
1 INTRODUCTION
Cerebral amyloid angiopathy (CAA) comprises the accumulation of the amyloid-β protein (Aβ) in the cerebral vasculature. Moderate-to-severe CAA has a prevalence of 23% in the elderly population (>55 years) [1]. Consequences of CAA may include cognitive impairment and intracerebral haemorrhages (ICH). It remains unclear what molecular mechanisms cause vessels to be more susceptible to rupture in some patients compared to others. Blood–brain barrier (BBB) dysfunction has been associated with CAA and CAA-associated ICH [2]. BBB dysfunction has also been shown to contribute to the pathogenesis of Alzheimer's disease (AD) [3]. Claudin-5 is a tight junction protein that plays a critical role in functioning of the BBB as it regulates its permeability to solutes and ions. Therefore, we hypothesised that decreased expression of claudin-5 in the cerebrovasculature, which may be linked to loss-of-function of the BBB, may play a role in CAA-associated ICH. We performed a comparative immunohistochemical study of claudin-5 in the occipital and temporal lobe microvasculature of CAA cases who developed ICH in lobar locations (CAA-ICH; n = 20, supplementary table S2), non-haemorrhagic CAA cases (CAA-NH; n = 40), and controls (n = 42; Table 1). Using Fiji Software, the microvascular claudin-5 immunostained area was determined and compared between CAA-ICH, CAA-NH, and control cases (see Supplementary Materials S1).
| Control | CAA-NH | CAA-ICH | p-Value | ||
|---|---|---|---|---|---|
| Na | 42 | 40 | 20 | ||
| Age (mean ± sd) | 78.4 (±8.4) | 77.4 (±11.7) | 78.3 (±7.3) | 0.88g | |
| Sex (% female) | 52.4 | 50.0 | 55.0 | 0.93h | |
| CAA grade (mean ± sd) | Occipital | N.A. | 3.3 (0.9) | 3.6 (0.6) | 0.47i |
| Temporal | N.A. | 2.9 (1.1) | 3.2 (1.2) | 0.18i | |
| Brain bank origin | 43% RUMC 17% IBB 40% NBB |
45% RUMC 15% IBB 40% NBB |
25% RUMC 30% IBB 30% NBB 15% UMCU |
0.51h,j | |
| AD pathology reported (%)b | N.A. | 75% | 68%e | 0.76h | |
| Large vessel artherosclerosis reported (%)c | Not assessed | 39%d | 39%d | 1.00h | |
| Capillary CAA reported (%)c | N.A. | 44%e | 29%f | 0.36h |
- Abbreviations: AD, Alzheimer's disease; capCAA, capillary CAA; CAA-NH, CAA non-haemorrhagic; CAA-ICH, CAA-associated intracerebral haemorrhage; IBB, Institute Born-Bunge; N.A., not applicable; RUMC, Radboud University Medical Center; NBB, Netherlands Brain bank; sd, standard deviation; UMCU, University Medical Center Utrecht.
- a For analysis of occipital lobe tissue, data were not available for 2 CAA-ICH cases (UMCU) and one control case (RUMC). Due to unavailability of temporal/occipital tissue, temporal tissue was replaced by frontal tissue for two CAA-ICH cases, and occipital tissue was replaced by frontal tissue for one CAA-ICH case.
- b The presence of AD pathology was defined as the presence of AD pathological hallmarks to a degree that they match a clinical diagnosis of AD according to board-certified neuropathologists judgements (generally at least Braak 4B).
- c Reported in neuropathological assessments in autopsy reports.
- d Information unavailable for two cases.
- e Information unavailable for one case.
- f Information unavailable for six cases.
- g Tested with One-Way ANOVA.
- h Tested with Pearson Chi-Square.
- i Tested with Mann Whitney.
- j UMCU and NBB cases were pooled for statistical analyses.
2 RESULTS
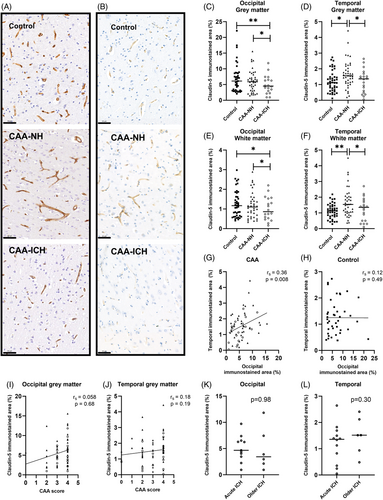
We demonstrate a decreased degree of claudin-5 staining in the occipital grey matter in CAA-ICH (median 4.5%, interquartile range (IQR) 2.8–6.7) compared to CAA-NH (median 5.9%, IQR 3.1–7.9; p = 0.027) and controls (median 6.1%, IQR 3.1–8.6; p = 0.003, Figure 1A,C). Similarly, the degree of claudin-5 staining in the occipital white matter area was lower in CAA-ICH (median 0.9%, IQR 0.5–1.6) compared to CAA-NH (median 1.1%, IQR 0.6–1.5; p = 0.021) and controls (median 1.2%, IQR 0.6–1.7; p = 0.018, Figure 1E). The degree of claudin-5 staining in the temporal grey matter was lower in CAA-ICH (median 1.4%, IQR 0.5–1.6) compared to CAA-NH (median 1.6%, IQR 1.1–2.2; p = 0.035), but not controls (median 1.1%, IQR 0.7–1.5; Figure 1B,D). In addition, CAA-NH had a higher degree of claudin-5 staining compared to controls (p = 0.011). The degree of claudin-5 staining in the temporal white matter was lower in CAA-ICH (median 1.3%, IQR 0.5–1.7) compared to CAA-NH (median 1.5%, IQR 1.0–2.0; p = 0.015), but not controls (median 1.1%, IQR 0.8–1.4; Figure 1F), whereas CAA-NH had a higher degree of claudin-5 staining compared to controls (p = 0.001).
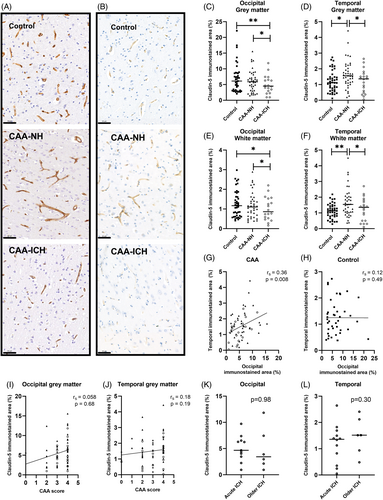
The degree of claudin-5 staining in the grey matter of the two investigated brain regions correlated with each other in CAA (rs = 0.36, p = 0.008; Figure 1G), but not in controls (rs = 0.12, p = 0.49; Figure 1H). We neither found associations between CAA grade and grey matter claudin-5 immunoreactivity in the complete CAA cohort, nor in the separate groups of CAA-NH and CAA-ICH in these two brain regions (Figure 1I,J). The degree of claudin-5 staining in the occipital and temporal grey matter did not differ between acute ICH cases (passed away <1 week after last ICH) and older ICH cases (occipital p = 0.98, temporal p = 0.37, Figure 1K,L). Sensitivity analyses with other cut-off points (3 days, 1 month) yielded similar results. We found no differences in claudin-5 expression between patients with and without capillary CAA (Supplementary Materials S1).
3 DISCUSSION
We demonstrate that (micro)vascular claudin-5 expression is decreased in patients with CAA-ICH compared to CAA-NH, independent of CAA severity. This is in line with a previous neuropathological study of 469 patients with AD in which temporal lobe claudin-5 levels did not correlate with CAA grade [4]. In our patients with CAA-ICH, haemorrhagic events had occurred at different lobar locations, whereas claudin-5 expression was consistently decreased in both temporal and occipital brain regions. This suggests that the claudin-5 reduction was not a local effect of the ICH. In addition, the expression of claudin-5 did not differ between CAA-ICH patients with older ICH and patients with semi-recent (fatal) ICH, suggesting that the claudin-5 reduction is not an acute response to ICH. We therefore speculate that decreased expression of claudin-5 may be mechanistically linked to an increased risk of vessels to rupture in patients with CAA. In addition to making vessels more prone to rupture, other—yet unidentified—factors may determine the exact site of ICH. Recently, BBB leakage in CAA has been suggested as a potential trigger for perivascular inflammation and vascular remodelling leading to haemorrhage [5].
We did not observe reduced claudin-5 expression in CAA-NH cases compared to controls. This is in contrast to previous research showing that in human brain tissue, Aβ-affected capillaries had decreased expression of claudin-5 compared to unaffected vessels [6], although it was not reported whether the studied tissue also included CAA-ICH cases. Another study demonstrated reduced claudin-5 levels in Aβ40-treated isolated rat microvessels and in microvessels of 9-months-old Tg2576 mice, a widely used mouse model of AD [7]. On the other hand, no differences in claudin-5 expression were observed between wildtype and Tg-SwDI mice (a CAA mouse model) [8], and another study did not detect differences in claudin-5 expression between controls and AD patients with CAA [9]. Results are likely influenced by the analytic method as well as the level of observation (studying only Aβ-affected (micro)vessels versus the whole (micro)vasculature). Remarkably, in our study claudin-5 expression patterns in controls differed across the two brain regions. In contrast to patients with CAA, the expression levels of claudin-5 in the two studied brain regions did not correlate in controls. This suggests that different mechanistic pathways regulate claudin-5 expression levels in patients with CAA and controls. Claudin-5 expression is highly regulated by endothelium-specific transcriptional regulators [10], and other—yet unknown—mechanisms may be at play here.
Strengths of our study include our unique cohort, which allowed for the comparison of CAA-ICH cases with CAA-NH cases. Since we studied cases with ICH in different lobar locations (frequently distant from at least one of the studied brain regions), our data suggests that the decreased claudin-5 levels observed in CAA-ICH reflect generally altered protein expression levels in the microvasculature in CAA-associated ICH. We cannot rule out the possibility that claudin-5 expression levels may be altered as a consequence of the haemorrhage, or that the varying time intervals between ICH and death may have affected claudin-5 expression. However, we did not find such a relation when comparing acute ICH to older ICH. Limitations of our study include the heterogeneity of our patient group regarding the presence of AD pathology, atherosclerosis and capillary CAA, which may affect claudin-5 expression, although the proportions of these pathologies were similar between CAA-NH and CAA-ICH groups, and we found no differences in claudin-5 expression between patients with and without capillary CAA. Furthermore, differences in vessel density may have affected results although CAA is not expected to result in a structural decrease of vessel density [9].
Another limitation of our study is the use of tissue from different brain banks, although brain bank was included as covariate in our analyses to account for potential tissue source differences. Differences in post-mortem interval may also have affected results. Finally, we only studied one tight junction marker, for future studies it would be informative to include other tight junction proteins such as occludin or zonula occludens 1.
Decreased levels of claudin-5 are associated with CAA-associated ICH. Future studies in larger cohorts with more biomarkers of BBB function are needed to substantiate these findings. Also, mechanistic studies (e.g., longitudinal studies in animal models of CAA-associated ICH) aimed at unravelling the molecular mechanisms leading to decreased claudin-5 expression and to the subsequent ICH are warranted.
AUTHOR CONTRIBUTIONS
LJ and MMV conceptualised and designed the study. WMTJ, YV and BK provided and characterised brain tissue, and contributed clinicopathological information. KKWJC and LJ performed experiments and data-analysis and AMK and HBK contributed to interpretation of data. HBK, FHBMS, CJMK and MMV revised the manuscript. All authors read and approved the manuscript.
ACKNOWLEDGEMENTS
We thank Tuur Smolders and Carla Hernández Utrilla for their assistance in assessing CAA burden.
FUNDING INFORMATION
Lieke Jäkel is supported by a grant from Alzheimer Nederland (WE.03-2022-17). This study was financially supported by the CAFÉ project (the National Institutes of Health, USA, grant number 5R01NS104147-02) and the BIONIC project (no. 733050822, which has been made possible by ZonMW as part of ‘Memorabel’, the research and innovation programme for dementia, as part of the Dutch national ‘Deltaplan for Dementia’: zonmw.nl/dementiaresearch). The BIONIC project is a consortium of Radboudumc, LUMC, ADX Neurosciences, and Rhode Island University. Marcel M. Verbeek is also supported by the SCALA project, funded by ‘The Galen and Hilary Weston Foundation’ (NR170024), Stichting Alkemade-Keuls, Maag-Lever-Darm-stichting (WOO 2105), Parkinson NL (P2-21-18) and ZonMW—Dementia program (10510032120006 and 10510032120003). Floris H.B.M. Schreuder is supported by a senior clinical scientist grant from the Dutch Heart Foundation (grant 2019T060). Catharina J.M. Klijn receives funding for research outside the submitted work of the Netherlands Cardiovascular Research Initiative, which is supported by the Dutch Heart Foundation, CVON2015-01: CONTRAST, and the support of the Brain Foundation Netherlands (HA2015.01.06). CONTRAST is additionally financed by the Ministry of Economic Affairs by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships (LSHM17016) and was funded in part through unrestricted funding by Stryker, Medtronic and Cerenovus. Radboudumc and Erasmus MC received additional unrestricted funding on behalf of CONTRAST, for the execution of the Dutch ICH Surgery Trial pilot study and for the Dutch ICH Surgery Trial from Penumbra Inc.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Brain samples obtained from the NBB, Netherlands lnstitute for Neuroscience, Amsterdam (NBB; Ref. No. 2009/148, open access: www.brainbank.nl), had been collected from donors that had provided written informed consent for the use of autopsy material and clinical information for research purposes. The study was performed in accordance with local regulations and approved by the medical research ethics committee of the UMCU (reference number 17–092). The use of autopsy materials from the Radboudumc was approved by the local ethics committee (reference number 2015–2215). The IBB-Neurobiobank of the Institute Born-Bunge (IBB) with FAMHP registration ID Institute with ID: BB190113 is subject to biannual evaluation by the local Ethics Committee of University hospital Antwerp (UZA)/University of Antwerp (UAntwerp), Belgium, approval reference 19/13/166. Samples were used anonymously in accordance with the Code of Conduct of the Federation of Medical Scientific Societies in The Netherlands.
Open Research
DATA AVAILABILITY STATEMENT
Anonymised datasets generated during this study are available from the corresponding author on reasonable request.