A comprehensive histomolecular characterization of meningioangiomatosis: Further evidence for a precursor neoplastic lesion
Abstract
Meningioangiomatosis (MAM) remains a poorly understood lesion responsible for epileptic disease. In the past, MAM was primarily described in the context of neurofibromatosis type 2 before being mainly reported sporadically. Moreover, the malformative or tumoral nature is still debated. Because a subset of MAM are associated with meningiomas, some authors argue that MAM corresponds to an infiltration pattern of these tumors. For these reasons, MAM has not been added to the World Health Organization (WHO) Classification of Central Nervous System Tumors as a specific entity. In the present study, we characterized a series of pure MAM (n = 7) and MAM associated with meningiomas (n = 4) using histopathology, immunohistochemistry, genetic (fluorescent in situ and DNA sequencing analyses), and epigenetic (DNA-methylation profiling) data. We evidenced two distinct morphological patterns: MAM with a fibroblastic-like pattern having few lesional cells, and MAM with a more cellular pattern. A subset was associated with the genetic alterations previously reported in meningiomas (such as a KMT2C mutation and a hemizygous deletion of chromosome 22q including the NF2 gene). The DNA-methylation profile, using a t-distributed stochastic neighbor embedding analysis, evidenced that MAM (pure or associated with meningiomas) clustered in a separate group from pediatric meningiomas. The present results seem to suggest that MAM represents a neoplastic lesion and encourage the further study of similar additional series so that it may be included in a future WHO classification.
1 INTRODUCTION
Meningioangiomatosis (MAM) is a poorly studied, rare, benign, and epileptogenic brain meningovascular lesion. Only about 200 cases have been reported to date [1, 2]. The majority of MAM are sporadic, affecting predominantly male patients, younger than 20 years of age [3]. Epileptic seizures constitute the main symptom and represent a clinical concern with more than 80% of patients having uncontrolled seizures at the time of surgery [3]. Surgery is the first-line treatment. Histopathologically, MAM is characterized by an intracortical meningovascular proliferation with a perivascular spread of spindle-shaped cells along the Virchow-Robin spaces. It often encompasses psammoma bodies, fibrosis, and white matter infiltration [3]. Because the malformative or neoplastic origin of MAM is still not well understood, this lesion is not part of the meningioma chapter of the current World Health Organization (WHO) Classification of Central Nervous System (CNS) Tumors. Very few genetic data for MAM are available in the literature. MAM may be isolated (pure) or associated with meningiomas (MAM + M), supporting the idea that MAM represents a potential tumoral pattern of infiltration. Moreover, for the majority of patients being treated by a complete resection and having epileptic seizure control, no recurrence at the end of follow-up and no case of MAM transforming into meningioma has been reported. In this study, we performed histopathological and molecular analyses (including DNA-methylation profiling) for 11 cases of MAM (seven pure and four associated with a meningioma) in order to further suitably characterize these lesions.
2 MATERIALS AND METHODS
2.1 Study design, patients, data collection
This study included patients diagnosed with MAM, provided by the consultation archive database (1993–2022) from the department of neuropathology at GHU-Paris Psychiatry and Neurosciences, Sainte-Anne Hospital. Epidemiological data (sex and age at diagnosis) and lesion and treatment-related data (location of lesion, preoperative magnetic resonance imaging (MRI) features, duration of symptoms, extent of resection, relapses, and complementary treatments) were retrospectively analyzed. The extent of the initial resection was assessed by early (within 48 h) postoperative MRI. All patients' parents or legal guardians signed informed consent forms before treatment began. We obtained human subjects approval for genetic analyses.
2.2 Central histopathological review
The central pathology review was performed conjointly by two neuropathologists (ATE and PV). Samples were stained with hematoxylin-phloxin-saffron (HPS) according to standard protocol. For each case of MAM, the following pathological features were researched: cell density, pattern (nodules of lesional cells or only infiltration of Virchow Robin spaces), and psammoma bodies. Mitotic count was monitored using 5 high-power fields (HPF), which corresponded to 1.6 mm2 on our microscope, and was counted jointly by two neuropathologists in the hotspot area. Meningioma subclassification was performed in accordance with the current WHO 2021 classification. For each MAM + M case, each component was macrodissected and molecularly analyzed (fluorescence in situ hybridization—FISH, DNA sequencing, and DNA-methylation profiling).
2.3 Immunohistochemistry
Unstained 3-μm-thick slides of formalin-fixed, paraffin-embedded (FFPE) tissues were obtained and submitted for immunostaining with an automated stainer (Dako Omnis, Glostrup, Denmark). The following primary antibodies were used: a marker of meningioma SSTR2a (1:200, clone UMB1, Abcam, Cambridge, UK), GFAP (1:200, clone 6F2, Dako, Glostrup, Denmark), INI1 (1:50, clone 25/BAF47, BD-Biosciences, Erembodegem, Belgium), BAP1 (1:100, clone C4, Diagomics, Blagnac, France) which may be lost in a subset of meningiomas and meningiomatosis, and Ki-67 (1:200, clone MIB-1, Dako, Glostrup, Denmark). External positive and negative controls were used for all antibodies and staining.
2.4 FISH analyses
A FISH study was performed on interphase nuclei according to the standard procedures and the manufacturer's instructions. The SMARCB1 and NF2 gene copy numbers were assessed using the following centromeric and locus-specific probes: Z-2178-50 (Zytovision, Bremerhaven, Germany) and FG0003 (Abnova, Taipei, Taiwan). A deletion was defined if more than 30% of nuclei presented no signal for CDKN2A locus (at least 100 nuclei counted for each case). Results were recorded using a DM600 fluorescence imaging microscope (Leica Biosystems, Richmond, IL) fitted with appropriate filters, a charge-coupled device camera, and digital imaging software from Leica (Cytovision, v7.4).
2.5 DNA sequencing
The design of a custom next-generation sequencing (NGS) panel called DRAGON (for the Detection of Relevant Alterations in Genes involved in Oncogenetics by NGS) and marketed by Agilent under the name of SureSelect CD Curie CGP has been developed specifically for the molecular analysis of lesions. It is composed of 571 genes of interest in oncology from diagnostic, prognostic, and molecular therapy points of view, including, the full sequence of NF2, SMARCB1, AKT1 SMO, and PIK3CA, and the hotspot region of interest at the codon 409 of KLF4. The nucleotide sequence (variant calling is performed using Varscan2) as well as the number of copies (deletion and focal amplification) were explored. 50 ng of DNA input was extracted from FFPE lesions and used to prepare the library with the Agilent SureSelect XT-HS preparation kit in accordance with the manufacturer's protocol. The design uses 571 genes and an additional backbone of probes across the whole genome with an average resolution of one probe every 200 Kb. This allowed us to determine a ploidy and an estimated cellularity, together with a genomic profile spanning every chromosome. The copy number profile for each case was estimated using a combination of homemade R scripts and a facets package (v0.6.0) with a sex-specific unmatched-germline control, previously sequenced using the same panel for normalization. Thirty-two DNA were sequenced per 2 × 100 Sp flowcell of the NovaSeq Sequencer (Illumina) to reach an average depth of 1500X and a minimum depth of 100X on the region of interest.
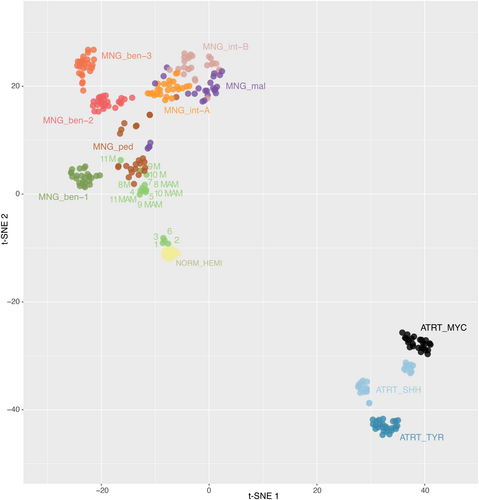
2.6 DNA methylation profiling
DNA was extracted from FFPE tissue samples (a macrodissection of FFPE blocks was performed in cases of low cellular density) using the Qiagen DNeasy Blood & Tissue Kit (Cat NO./ID 69504), according to the manufacturer's instructions. 500 ng of DNA was extracted from each tissue sample. The DNA was sent to the Genotyping facility of the German Cancer Research Center (Heidelberg, Germany). All patient samples were analyzed using either Illumina Infinium Methylation EPIC or HumanMethylation450 BeadChip arrays according to the manufacturer's instructions. Affiliation predictions were obtained from a DNA methylation-based classification web platform for CNS tumors (www.molecularneuropathology.org, version 11b4). Next, a t-distributed stochastic neighbor embedding (t-SNE) analysis was performed and compared with the genome-wide DNA methylation profiles from the brain tumor reference cohort [4], as well as with a series of 30 pediatric meningiomas from our center. Data was generated by the DKFZ Genomics and Proteomics Core Facility (Heidelberg, Germany) as previously described [4]. Dimensionality reduction was then performed using the uniform manifold approximation and projection method (uwot R package) with the following non-default parameters: n_neighbors = 10, spread = 2, min-dist = 0.2.
3 RESULTS
3.1 Clinical characteristics
The main results are summarized in Table 1. All patients included in this series were pediatric, except one (#2). The median age at diagnosis was 10.0 years (patients' ages ranged from 9 to 28 years) for patients with pure MAM and 9.0 years (ranging from 1 to 12 years) for patients with MAM + M. The male/female sex ratio was 1.3 (4 males and 3 females) and 3.0 (3 males and 1 female), respectively in pure MAM and MAM + M. Lesion locations varied, with the frontal lobes being the most common location (6/11 cases, 55%). All cases, except one (case #7), were revealed by seizures. The remaining patient was followed for a medulloblastoma, non-WNT/non-SHH, treated by surgery, chemotherapy, and craniospinal radiation therapy, and presented during the radiological follow-up (6 years after) an asymptomatic supratentorial MAM. There was no context of neurofibromatosis type 2 in the whole cohort. Another patient (#11) presented in the same surgical time, an atypical rhabdoid and teratoid tumor (AT/RT) component, SMARCB1-deficient, a meningioma, and a MAM. All three lesions were located in the left parietal lobe. There was no known SMARCB1 germline alteration. All patients, except one (case #3) underwent total resection. No patient received adjuvant treatment. Outcome data was available for all patients included in the cohort. Only one (9%) patient (case #3) had progression (after subtotal resection), with a mean progression-free survival of 124.1 months. All patients were alive at the end of follow-up (median overall survival: 193 months, ranging from 41 to 690 months).
| Case | Age | Sex | Histopathological diagnosis (grade) | Symptoms | Location | Medical history | OS (months) | DNA-methylation profiling v12.5 (calibrated score) | CNV/FISH analyses | DNA-sequencing analyses |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | M | MAM with a fibroblastic-like pattern | Epilepsy | Right temporal | 0 | 98.5 | Ganglioglioma (0.19) | Flat | KMT2C |
| 2 | 28 | F | MAM with a fibroblastic-like pattern | Epilepsy | Left temporal | 0 | 371 | Control hemispheric tissue (0.12) | Flat | WTa |
| 3 | 10 | M | MAM with a fibroblastic-like pattern | Epilepsy | Left frontal | 0 | 473.33 | Glioblastoma, IDH-wildtype (0.09) | Flat | WTa |
| 4 | 10 | M | MAM with cellular areas | Epilepsy | Parietal | 0 | 68.75 | Desmoplastic infantile ganglioglioma/astrocytoma (0.16) | Flat | WTa |
| 5 | 9 | M | MAM with cellular areas | Epilepsy | Left temporal | 0 | 301.83 | Desmoplastic infantile ganglioglioma/astrocytoma (0.14) | Hemizygous del. 22q, del. 13 | WTa |
| 6 | 9 | F | MAM with cellular areas | Epilepsy | Right frontal | 0 | 147.92 | Pilocytic astrocytoma, hemispheric (0.18) | Gain 1q | WTa |
| 7 | 15 | F | MAM with cellular areas | Fortuitous | Left frontal | Medulloblastoma | 41.33 | Meningioma, subtype benign, subclass 3 (0.30) | Hemizygous del. 22q, del. 1q | WTa |
| 8 | 11 | F | Meningothelial meningioma (1) | Epilepsy | Left frontal | 0 | 470.5 | Meningioma, subtype benign, subclass 3 (0.09) | Hemizygous del. 22q, gain 3p | WTa |
| 11 | F | MAM | Epilepsy | Left frontal | 0 | 193 | Desmoplastic infantile ganglioglioma/astrocytoma (0.21) | Hemizygous del. 22q, gain 3p | WTa | |
| 9 | 7 | M | Atypical meningioma (2) | Epilepsy | Right frontal | 0 | 690.33 | Meningioma, subtype benign, subclass 1 (0.60) | Hemizygous del. 22q, del. 1q, del. 17p | WTa |
| 7 | M | MAM | Epilepsy | Right frontal | 0 | 165.92 | Desmoplastic infantile ganglioglioma/astrocytoma (0.13) | Hemizygous del. 22q, del. 1q, del. 17p | WTa | |
| 10 | 12 | M | Atypical meningioma (2) | Epilepsy | Convexity | 0 | 98.5 | Meningioma, subtype benign, subclass 3 (0.30) | Hemizygous del. 22q, EGFR amplification, gain 5p | WTa |
| 12 | M | MAM | Epilepsy | Convexity | 0 | 371 | Supratentorial ependymoma, ZFTA fusion-positive (0.08) | Hemizygous del. 22q | WTa | |
| 11 | 1 | M | Atypical meningioma (2) | Epilepsy | Left parietal | AT/RT | 473.33 | Meningioma, subtype benign, subclass 3 (0.99) | Hemizygous del. 22q, del. 1p, del. 2p | WTa |
| 1 | M | MAM | Epilepsy | Left parietal | AT/RT | 68.75 | Teratoma (0.33) | Hemizygous del. 22q, del. 2p | WTa |
- Abbreviations: AT/RT, atypical teratoid and rhabdoid tumor; CNV, copy number variations; del., deletion; F, female; M, male; MAM, meningioangiomatosis; OS, overall survival; WT, wildtype.
- a Wildtype for all the 571 genes of the panel used.
3.2 Radiological characteristics
Preoperative imaging was available for 10/11 cases (see Supplementary Table 1 and Figure 1). All lesions affected the cortex and 8/10 cases (80%) were associated with subcortical white matter abnormalities. MRI showed thickening of the cortex, with a T2-weighted low signal in 7/10 cases (70%) and an intermediate signal in 1/10 cases. The T1-weighted signals were slightly higher than the normal cortex in 6/10 cases, and intermediate in 3/10 cases. An existence of cortical calcifications in all the cases with available CT (7/7) may explain this phenomenon. Only one case (#7) exhibited a high cortical signal on T2-weighted images and low signal on T1-weighted images. This case was detected during postoperative medulloblastoma follow-up, without any symptoms. The unique imaging characteristics may be attributed to the early detection of the radiological diagnosis before MAM calcification. Contrast enhancement varied from none to high (similar to choroid plexus). When available, diffusion was not restricted (high or intermediate apparent diffusion coefficient, 8/10 cases). Cerebral blood flow was low on arterial spin labeling perfusion MRI (7/10 cases).
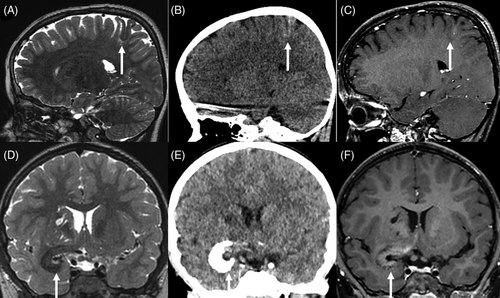
In 80% of cases, white matter involvement manifested as a subcortical T2-weighted high signal; however, in case #11, the T2-weighted low signal and calcifications extended into the white matter toward the lateral ventricles. Three cases presented with enlarged Virchow-Robin spaces. These spaces varied in size and location, with some being subcortical with minor extensions (#11), while others were very large extending from the frontal cortex to the lateral ventricle (#3) or found within the basal ganglia, associated with an infiltration around the proximal middle cerebral artery (#1).
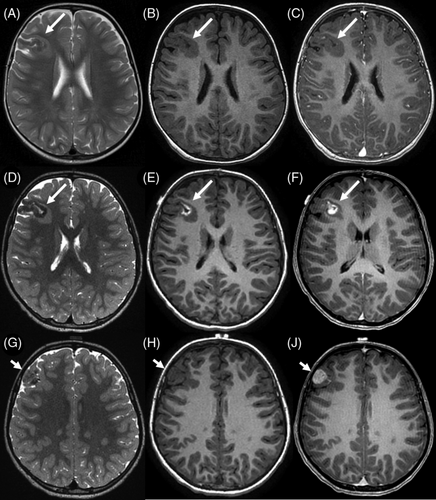
MRI was available for 3/4 MAM + M cases. The cortical MAM presented similarities to others but was connected to an extra-axial nodular mass in the immediate vicinity, without dural thickening. These masses showed imaging features typical of meningioma, with strong contrast enhancement. In one instance (#9, Figure 2), the meningioma was not detected during the radiological diagnosis, it appeared after a follow-up of 3 years with subsequent growth after 2 years, and a gradual radiological progression of the MAM.
3.3 Histopathological and Immunohistochemical characterization
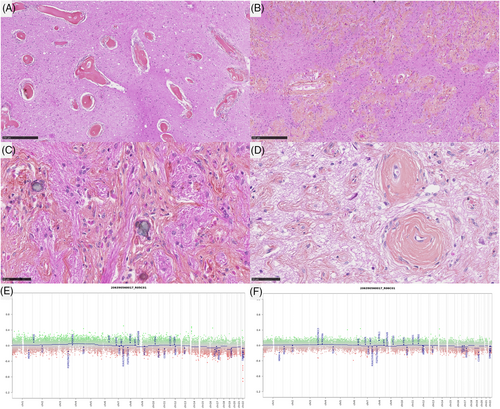
In the MAM cohort, two morphological patterns were observed. First, three lesions (cases #1–3) presented low cellular density, composed of a fibrous infiltration of the Virchow-Robin spaces (MAM with a fibroblastic-like pattern) (Figure 3A–D). The four other cases (cases #4–7) presented an association of fibrous patterns and small nodules of meningothelial cells (MAM with cellular areas) (Figure 4A–D). There were whorls and psammoma bodies, and the meningothelial cells did not present cytological atypia or mitoses. In these cases, we observed a transition between a fibrous component at the surface of the leptomeninges with a fibrous spread in the Virchow-Robin spaces and the formation of meningothelial nodules (Supplementary Figure 1). Necrosis was absent in all MAM cases. Using IHC, INI1, and BAP1 immunoexpressions were retained in all MAM cases. The MIB1 labeling index was weak (less than 1% in all cases) and all lesions expressed SSTR2a.
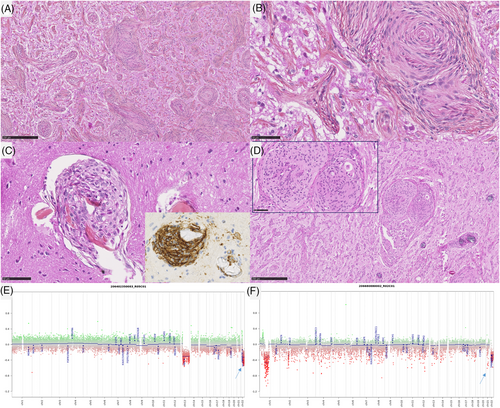
In the MAM + M cohort, all cases presented a morphological spectrum from a paucicellar fibrous infiltration of the Virchow-Robin spaces to the formation of a circumscribed meningothelial proliferation, corresponding to a meningioma. All meningiomas were of a meningothelial subtype. Three of them presented 4, 5, and 6 mitoses per 1.6 mm2 (grade 2), whereas the remaining case was grade 1. There was no brain parenchyma invasion (no irregular infiltration or protrusion of tumor cells into the underlying GFAP-positive parenchyma). Using IHC, INI1, and BAP1 immunoexpressions were retained in all MAM + M cases. The MIB1 labeling index was weak in the MAM component (less than 1% in all cases) whereas it varied from 1% to 8% in the meningioma component. In all lesions, both components (MAM and meningiomas) expressed SSTR2a.
3.4 Molecular results
DNA-sequencing analyses failed to reveal any mutation NF2, AKT1, KLF4, SMO, or PIK3CA genes in the whole cohort (pure MAM, and in both components of MAM + M). A hemizygous deletion of chromosome 22q was detected in 2/7 pure MAM (cases #5 and 7) and in all MAM + M (presence of the deletion in both components) (Figure 4E, F). An inactivating nonsense KMT2C mutation with an allele frequency of 4.38% was evidenced in one pure MAM (case #1). Concerning the case #11, the hemizygous deletion of chromosome 22q included SMARCB1 gene, and no alteration, no nucleotidic mutation or structural variant such as deletion, of SMARCB1 gene, was detected in the MAM and the meningioma (whereas the AT/RT component harbored a homozygous deletion of SMARCB1 gene). FISH analyses for NF2 and SMARCB1 failed to reveal any deletion in MAM cases which did not present a deletion of chromosome 22q on CNV (Figure 3E, F). MAM with a cellular pattern presented additional CNV alterations such as losses of chromosomal material on chromosome 1q (case #7) and chromosome 13q (case #5). MAM associated to meningiomas presented additional CNV alterations such as losses of chromosomal material on chromosome 1q (case #9) and chromosome 2 (case #11), or gain on chromosome 3p (case #8) and chromosome 5 (case #10). These alterations were also found in the meningioma component except for the gain of chromosome 5 of the case #10 but, interestingly, a focal amplification of EGFR appeared in the meningioma component in this case (confirmed by DNA-methylation profiling). The size of the loss of chromosome 22q differ from one case to another. For one case (case #7), the entire chromosome 22 was lost, for some cases, the chromosome 22q loss start from chromosome band 22q11.22 (case #9) or 22q11.23 (case #10) to the end of the chromosome 22q (hence encompassing both SMARCB1 and NF2), and finally for one interesting case (case #8), the deletion started from chromosome band 22q12.1 to the end of chromosome 22q hence encompassing NF2 but not SMARCB1. This last result hence defined a minimal deleted region that include NF2 gene that seems the most important for MAM development but not SMARCB1.
According to the DNA methylation-based classification and the DKFZ Classifier (version 12.5), none of the lesions were classifiable (calibrated scores for DNA methylation class <0.9), except one meningioma (case #11, which classified as a benign meningioma, with a calibrated score of 0.99). A t-SNE analysis was performed to compare the genome-wide DNA methylation profiles of pure MAM, MAM + M, pediatric meningiomas, and the different methylation classes (MC) of adult meningiomas. Most cases (three pure MAM and the four pairs of MAM + M) clustered within close proximity to the pediatric meningioma methylation class (Figure 5 and Supplementary Figure 2). Interestingly, meningiomas associated with MAM clustered within the MC of pediatric meningiomas. Moreover, MAM (pure or associated with meningiomas) formed a distinct cluster from other meningioma MC. The four remaining cases (#1, 2, 3, and 6) clustered in close vicinity with control hemispheric tissue.
4 DISCUSSION
The first description of MAM was in 1915 and was initially described as a lesion encountered in the context of neurofibromatosis type 2 (see review in [3]) [5, 6]. Since then, sporadic cases have been reported, and MAM associated with tumors (mainly meningiomas) have been reported in the literature [1, 7-10]. The neoplastic origin of MAM remains debated and the WHO classification does not include it in the meningioma chapter. The recent literature analysis and the current study bring several arguments in favor of a neoplastic origin for MAM. MAM are mainly isolated (without a meningioma), grow slowly in the brain parenchyma, and are responsible for epilepsy, which becomes refractory. Moreover, rare observations of progressions (but only after biopsy or partial resection) (cf., review in [4]) and multifocal forms have been reported [1, 11]. The current study evidenced that a subset of pure MAM (2/7, 29%), and all MAM + M harbored a hemizygous deletion of chromosome 22q (including the NF2 gene), which is the most frequent alteration in meningiomas (adult and pediatric) [2, 12-15]. Interestingly, this deletion was present in both components (MAM and meningioma) supporting the argument of a neoplastic origin for MAM. The current series showed that MAM cases did not present any mutation described for the NF2, AKT1, KLF4, SMO, or PIK3CA genes. The fact that these alterations have been reported in adults and are absent in pediatric meningiomas, may explain why they are not found in MAM, which mainly affect children [13]. Another potential argument for a neoplastic origin for MAM may be that one case of the current series harbored a pathogenic variant of the KMT2C gene, which was previously reported in a subset of chordoid meningiomas [11]. Based on epigenetic profiling, we showed that the main subgroup of lesions (three pure MAM and the four pairs of MAM + M) did, in fact, cluster together, and were in close vicinity to the pediatric meningioma MC. Because DNA methylation profiles are thought to represent a combination of both somatically acquired DNA methylation changes and a signature reflecting the cell of origin [16], it seems that MAM belong to the lesional spectrum of pediatric meningiomas, potentially as a neoplastic lesion. The absence of an alteration in a subset of pure MAM (3/7 cases) and the close vicinity of the DNA-methylation profile with control hemispheric tissue may be related to the low cellular density of these lesions that include normal brain parenchyma. Moreover, the current series showed that, even though some molecular alterations are present in both MAM and M components, the meningioma may present additional alterations. We can hypothesize that if the MAM component was a brain invasion or Virchow-Robin space involvement of a meningioma, there would be additional molecular alterations and histopathological features of aggressiveness in this component, which was not the case. In the cellular pattern of MAM and in the MAM component of mixed forms, 3/7 cases presented additional CNV alterations (losses of chromosomal material on chromosomes 1 and 5), that are also found in pediatric meningiomas [13] and in the meningioma component of mixed cases. Considering these results, we can hypothesize that there exists a histomolecular continuum between the pure MAM (primarily represented as a hypointense lesion on T1 weighted sequences with hyper signal in T2 weighted sequences, composed of a fibrous spread in the Virchow-Robin spaces, showing calcifications on MRI and becoming more cellular with the constitution of cellular nodules harboring a 22q hemizygous deletion) and the MAM associated with authentic meningiomas (Figure 6). Because those lesions are cortical, symptoms include epileptic seizures. Consequently, we can suggest that histopathological findings encountered in surgical samples may depend on the age of patients and the age of epilepsy onset, with the constitution of meningiomas over the long run, being associated with pharmaco-resistant seizures.
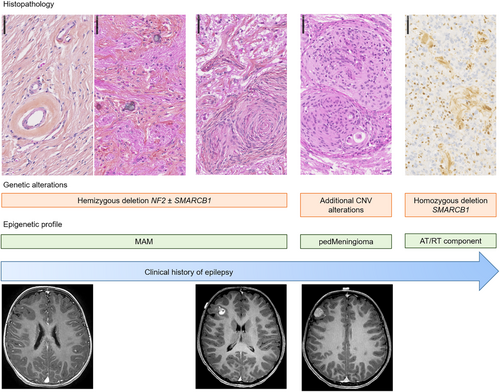
Previously, MAM have been described as being associated with AT/RT or tumors having CNS SMARCB1-alterations [17, 18]. However, molecular data (including 22q status in the two components, germline status of SMARCB1, and epigenetic analyses) are lacking for these cases. The current study showed that MAM and meningioma components harbored only a hemizygous deletion of chromosome 22q including the SMARCB1 gene, whereas a homozygous deletion was present in the AT/RT component. The DNA-methylation profiling of these three components are distinct. This may illustrate the fact that an additional genetic event may be responsible for a subset of patients with MAM where AT/RT later appears. Moreover, meningiomas belong to the tumor spectrum for cases of rhabdoid tumor predisposition syndrome [19, 20]. This hypothesis is reinforced by the fact that this AT/RT component was classified as an AT/RT subtype MYC, which is known to be similar to extracranial forms of rhabdoid tumors (non-neuronal), and in this case, potentially shares the same cellular origin as MAM and meningiomas.
To conclude, the current work brought additional findings arguing a potential neoplastic nature for MAM, which might be isolated or progress to form a meningioma. If further series confirm these data, MAM will need to be included in the meningioma chapter of a future version of the WHO Classification of CNS Tumors.
AUTHOR CONTRIBUTIONS
ATE, VDR, NB, OA, AR, JPTB, KB, FB, and SP compiled the MRI and clinical records; ATE, AM, FC, and PV conducted the neuropathological examinations; JMP, PS, and FS conducted the molecular studies; ATE, JMP, PS, LH, and PV drafted the manuscript; all authors reviewed the manuscript.
ACKNOWLEDGEMENTS
We would like to thank the laboratory technicians at GHU Paris Neuro Sainte-Anne for their assistance, as well as the Integragen platform for their technical assistance with DNA-methylation analyses and the RENOCLIP-LOC.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest directly related to the topic of this article.
ETHICS STATEMENT
This study was approved by the GHU Paris Psychiatry Neurosciences, Sainte-Anne Hospital's local ethics committee. All the patients' parents or legal guardians signed informed consent forms before treatment was started. We obtained human subjects approval from our institutional review board.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




