Astrocytes for brain repair: More than just a neuron’s sidekick
Abstract
Transplantation of glial enriched progenitors provides therapeutic effects on axonal damage, cognitive and motor function following white matter stroke.
White matter (WM) strokes account for up to 30% of all stroke subtypes leading to permanent and progressive cognitive and motor impairments (1). Unlike large artery strokes, these lesions encompass small infarcts in deep penetrating blood vessels in the subcortical brain. The main components of the WM are long extensions of myelinated axons that are organized into tracts and surrounded by oligodendrocytes and astrocytes. These cells are in state of close metabolomic co-dependence; damage to these structures leads to local activation of astrocytes and microglia, loss of oligodendrocytes, and axonal demyelination (2). Myelin is fundamental for metabolic support, structural integrity, and neural function; its absence increases the axons’ vulnerability to degeneration and progressive WM lesions. As a result, WM lesions may cause gait abnormalities and cognitive decline over time that present as vascular dementia. To date, there is no therapy that enhances the brain's own ability to regenerate damaged brain circuits. Cell-based therapies are emerging experimental strategies to restore brain function after stroke. Despite great promise, experimental efforts almost exclusively focused on replacing neural cells to enhance brain regeneration and neglected the therapeutic potential of glial cells (3, 4). However, there is increasing evidence that accelerating remyelination supports axonal integrity and prevents neural loss in experimental models of central nervous system (CNS) injury (5). A promising strategy to promote remyelination is the transplantation of immature glia. Astrocytes are known to promote oligodendrocyte progenitor cell production and maturation in brain development and following CNS lesions (6). Furthermore, ablation of astrocytes has been reported to impair neurovascular remodeling in stroke (7). Glial progenitors have been recently transplanted in for example spinal cord injury models and multiple sclerosis with promising results. Writing in Science Translational Medicine, Llorente et al. test the hypothesis that induced pluripotent stem cell (iPSC)-derived glial progenitor cells promote functional repair in a mouse model of subcortical WM stroke (Figure 1) (8).
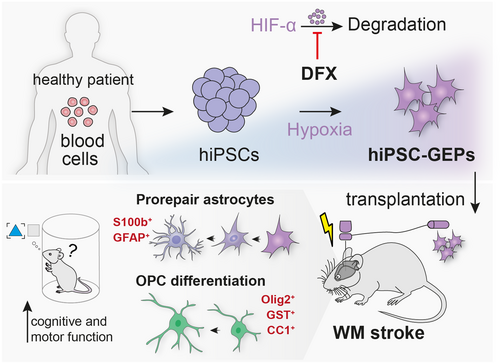
The work has two important implications. First, the study describes a rapid and efficient protocol to differentiate iPSC-derived neural progenitor cells (NPCs) toward glial-enriched progenitors (GEP). Building on previous work (9), Llorente et al. expose the cells to HIF activation using deferoxamine (DFX) to permanently direct the differentiation toward glia. Transcriptional analysis confirmed a successful induction of GEP upon HIF activation by upregulation of glial-specific genes (e.g., CD44, S100A10, NFIX). Differentiation of GEP under this protocol resulted in a considerable increase for markers of the astrocyte-lineage (60% GFAP, 85% S100b, 0% DCX, 0% NeuN), whereas control NPCs without astrocytic differentiation bias, preferentially expressed neural markers (45% DCX, 25% NeuN, 0% GFAP, 0% S100b), 4 months following cell transplantation.
Second, the study describes the regenerative potential of GEPs following transplantation in a mouse model of WM stroke. The expansion of WM strokes typically results in permanent astrocyte and oligodendrocyte damage and is associated with the degeneration of demyelinated axons. Deficits in motor and memory function could be observed up to 4 months after injury. To test the beneficial effects of GEPs, these cells were injected into the brains of mice at a subacute time period after stroke induction to recapitulate human disease presentation. Months after transplantation, GEPs migrated to the contralateral WM, striatum, and cortex, and additionally displayed a higher proliferation rate compared with control NPCs, which were not found to leave the injury site.
After 2 and 4 months >50% of GEPs differentiated into adult astrocytes (GFAP+, S100b+), whereas most NPCs remained in an immature state (DCX+ NeuN–). Both NPCs and GEPs foster axonal projections in the injured brain regions. In addition, transplanted GEPs restored lost myelin around the infarct site, associated with the increase of mature oligodendrocytes. To determine functional recovery, motor and cognitive functions were tested at regular intervals using standard behavioral tests (grid walk, cylinder test, novel object recognition). Motor control was improved more substantially following GEP transplantation compared with mice receiving NPCs. Similar effects were also observed in the cognitive tests; only GEP-treated mice revealed cognitive improvements 4 months after injury.
To understand the casual relationship between the transplanted cells and improved functional recovery, Llorente et al. chemogenetically ablated the grafted NPCs and GEPs. Interestingly, NPC ablation partially worsened motor recovery, whereas GEP ablation did not alter functional recovery. These findings suggest that unlike NPCs, GEPs initiate local repair responses and are not required to maintain the recovered function.
To understand these mechanisms of WM repair, gene expression profiles of NPCs and GEPs were compared in an in vitro system. Transcriptional profiling of GEP revealed key molecular and cellular pathways enriched for a prorepair astrocytic phenotype for up to 4 months. Among others, genes driving myelination (e.g., LGALS2, SPHK1, ERG2) and neuroprotection (S100A10, GDF15) were enriched in hiPSC-GEPs. Most relevant released growth factors that may be involved during WM repair were FGF2, CXCL1, and VEGF.
There are two major limitations to this study. First, the use of immunocompromised mice may likely have had an impact on the endogenous response to WM stroke. While necessary for the context in this study, the authors suggest that future studies should opt for the use of engineered GEP lines that are able to avoid rejection and be used as an allogenic cell-based therapy for stroke. Second, and more importantly, the effect of hiPSC-GEP transplantation in aged animals, at distinct time points other than this study, variable sites of injection, and lastly different doses must still be determined. Carrying out further experiments to address this knowledge gap will prove to be vital in the quest for the clinical translation of such a novel stem cell therapy.
Overall, Llorente et al. reported that a one-time injection of GEPs limits the progression of WM stroke and improves motor and cognitive function in mice, thereby providing a renewable and scalable cell source for therapeutics and research (Figure 1). Despite their immense therapeutic potential, first generation of cell therapies has previously revealed major drawbacks with variable clinical data and some safety concerns. Results contributed by high-quality basic research – like the study from Llorente et al. – provide supportive evidence that cell-based approaches can be developed into next-generation therapies for brain injury. However, major hurdles (e.g., immune incompatibilities, scaling to clinical manufacturing of the cells) have to be overcome and given the heterogeneity of stroke pathology, different cell sources have to be tested. Only then will it be possible to offer clinically useful strategies that provide stroke patients with substantial improvements of their quality of life.
CONFLICT OF INTEREST
The author declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTIONS
Rebecca Z. Weber, Patrick Perron, and Ruslan Rust wrote the manuscript. Rebecca Z. Weber and Ruslan Rust prepared the illustration.




