Mechanotransduction properties of the cytoplasmic tail of PECAM-1
Abstract
Background Information
Vascular endothelial cells (ECs) are a well-known cell system used in the study of mechanobiology. Using cultured ECs, we found that platelet EC adhesion molecule 1 (PECAM-1, CD31), a cell adhesion protein localised to regions of EC–EC contact, was rapidly tyrosine phosphorylated in ECs exposed to shear or cyclic stretch. Src-homology 2 domain-containing protein tyrosine phosphatase 2 (SHP2) binds phosphorylated PECAM-1 and activates the extracellular signal-regulated kinase1/2 (ERK1/2) signalling cascade, a known flow-activated signalling pathway.
Results
Although PECAM-1 tyrosine phosphorylation is characterised in ECs exposed to fluid shear stress, it is less well demonstrated in the cells stretched cyclically. Thus, we first show that PECAM-1 is tyrosine-phosphorylated in ECs cyclically stretched. We hypothesise that when an external force is applied to a monolayer of ECs, the force is directly transmitted to PECAM-1 which is then stretched and phosphorylation sites in its cytoplasmic domain are exposed and phosphorylated. This hypothesis requires the presence of any stretchable structure within the PECAM-1 cytoplasmic domain. Force spectroscopy measurements were performed with a construct containing cytoplasmic PECAM-1 domains inserted between I27 motifs, a recombinant string of the structural elements from titin. This strategy allowed us to identify the events in which a single molecule is being pulled and to detect the unravelling of the cytoplasmic domain of PECAM-1 by force. The response by PECAM-1 to mechanical loading was heterogeneous but with magnitudes as high as or higher than the naturally force bearing I27 domains.
Conclusions
The PECAM-1 cytoplasmic domain has a structure that can be unfolded by externally applied force and this unfolding of PECAM-1 may be necessary for its phosphorylation, the first step of PECAM-1 mechanosignalling.
Significance
When EC monolayers are mechanically stimulated, the PECAM-1 found at EC contacts is phosphorylated. We have proposed that under these conditions, the cytoplasmic domain of PECAM-1 is unfolded, which then exposes a phosphorylation site, allowing it to be accessed. The stretch induced unfolding is essential to this model of PECAM-1 mechanosignalling. In this study, we investigate whether the cytoplasmic domain of PECAM-1 has a stretchable structure, and the results are in line with our hypothesis.
Abbreviations
-
- AFM
-
- atomic force microscopy
-
- BAEC
-
- bovine aortic endothelial cell
-
- DPC
-
- dodecylphosphocholine
-
- ECs
-
- endothelial cells
-
- HPA
-
- PECAM-1 cytoplasmic domain flanked by an N-terminal 6xHis and a C-terminal AviTag
-
- PECAM-1
-
- platelet endothelial cell adhesion molecule 1
-
- RT
-
- room temperature
-
- SEM
-
- standard error of the mean
-
- SHP2
-
- Src-homology 2 domain-containing protein tyrosine phosphatase 2
Introduction
Biological mechanotransduction is an interesting but poorly understood biological process. Cells sense and respond to forces in their environment through mechanotransduction, a process that translates mechanical forces into biochemical signals (Orr et al., 2006; Vogel, 2006; Chatterjee et al., 2015). Mechanotransduction is crucial for cell and organ functions including growth and development, acquisition of hearing and of touch sensory signals and the mechanical regulation of organs like the lungs and heart that are cyclically stretched and compressed. Endothelial cells (ECs), which line the interior of blood vessels, respond to the shear stress arising from flowing blood. ECs are elongated in the direction of flow in the vessel area exposed to laminar flow (White et al., 1983; Davies, 1995). This process can be observed both in vivo and in vitro and cells that are exposed to low shear stress or disturbed flow will not adopt this morphological appearance. Instead the EC monolayer has a cobblestone-like pattern (Davies, 1995). Cells that do not adopt the aligned and elongated morphology are found in regions prone to atherosclerosis (Davies, 1995; Malek et al., 1999; Hahn and Schwartz, 2009), although it is not clear if this divergent morphology is a promoter of plaque development.
Platelet EC adhesion molecule 1 (PECAM-1) has been previously identified as a mechanotransduction receptor on the surface of ECs (reviewed in Fujiwara, 2006). PECAM-1 forms homophilic bonds and is a component found in regions of intercellular contact between ECs (Newman, 1997). When confluent monolayers of ECs are exposed to steady laminar fluid shear stress above a threshold of 5 dynes/cm2 (Harada et al., 1995), PECAM-1 has been shown to be rapidly tyrosine phosphorylated (Osawa et al., 1997). Other mechanical forces applied to ECs, including the tensile forces produced by cyclic cell stretching and cell shrinkage through hyperosmotic shock, have been shown to similarly activate PECAM-1 presumably through the same mechanism (Chiu et al., 2008). Pulling on ECs with PECAM-1-targeted magnetic beads has also been shown to activate the molecule (Osawa et al., 2002). In cells grown sparsely in vitro to eliminate the presence of cell–cell contacts and thus homophilic PECAM-1 bonds, PECAM-1 does not become phosphorylated nor induce downstream signalling in response to fluid flow or other mechanical perturbations of the cell (Osawa et al., 2002; Chiu et al., 2008). Therefore, the PECAM-1–PECAM-1 bond between neighbouring ECs is a necessary component in PECAM-1's response to mechanical force.
PECAM-1 has an extracellular domain composed of six Ig-like repeats of which the terminal two form intercellular bonds with those of the PECAM-1 molecules on adjacent cells (Sun et al., 1996; Jackson, 2003). The molecule has one transmembrane domain, and a cytoplasmic domain anchored to the cytoskeleton through β- and γ-catenins (Ilan et al., 2000). Forces exerted on the extracellular domain result in the phosphorylation of two tyrosine residues in the cytoplasmic tail (Osawa et al., 2002; Woodfin et al., 2007). The phosphorylation is mediated by the protein tyrosine kinase Fyn (Chiu et al., 2008), which has been shown to be attached to the membrane (van't Hof and Resh, 1997). We hypothesise that forces on the extracellular domain of PECAM-1 result in stretching of the cytoplasmic domain and exposure of tyrosines that may have been hidden from Fyn by secondary structure such as alpha helix (Paddock et al., 2011). Once phosphorylated, an adaptor protein with protein tyrosine phosphatase activity, Src-homology 2 domain-containing protein tyrosine phosphatase 2 (SHP2), recognises and binds to the phosphotyrosines within two immunoreceptor tyrosine-based inhibition motif (ITIM) domains and activates an endothelial mechanosignalling pathway (Osawa et al., 2002; Kandadi et al., 2010; Furcht et al., 2014).
Here, we show that upon cyclic stretch, ECs in a monolayer elongate and align perpendicular to the direction of stretch and that this cell shape response is a dynamic process. We also show that PECAM-1 is rapidly phosphorylated when EC monolayers are cyclically stretched. We hypothesise that when ECs are stretched, this force is transmitted to PECAM-1 which is anchored externally to another PECAM-1 from a neighbouring cell and intracellularly to the cytoskeleton. We further propose that the force transmitted to PECAM-1 will stretch (i.e. open up the secondary structure of) the molecule such that the cryptic tyrosine residues become exposed. Once exposed, these tyrosine residues within the two ITIM domains will be phosphorylated. To test our hypothesis, we performed further studies to determine whether or not the cytoplasmic domain of PECAM-1 does indeed have some structure that can be unravelled by pulling on this part of the molecule. To investigate how mechanical forces act on the PECAM-1 cytoplasmic tail, we use force spectroscopy analysis using an atomic force microscope. The response of the PECAM-1 cytoplasmic domain to these mechanical forces and additional observations regarding its interaction with lipids should allow us to develop a deeper understanding of mechanotransduction by PECAM-1.
Results
When cultured ECs are cyclically stretched, the long axis of these cells is known to align perpendicular to the direction of stretch (Naruse et al., 1998; Wang et al., 2001). Figure 1 shows that this alignment response is a dynamic process. Using our two-dimensional cyclic stretch apparatus, confluent bovine aortic ECs (BAECs) (upper left, before stretch) were stretched by 15% for 15 h first in one (Y) direction (upper right), then in another (X) direction (lower left) and finally in both (X and Y) directions simultaneously (lower right). To achieve the same 15% increase in the chamber area for the bi-directional stretch, we stretched 10.6% simultaneously in the X and Y directions (see Materials and Methods for detail). These low magnification (10× phase contrast objective) images were taken from the centre spot of the same chamber at the end of each stretch regimen. Note that ECs under the static condition exhibit a cobblestone shape and that the same field of cells reorient perpendicular to each new stretch direction (upper right and lower left). However, when they are stretched simultaneously in both the X and Y directions, they become cobblestone-shaped again (lower right). To quantify cell alignment, we measured the smaller angle (i.e. ≤90°) between each cell's long axis and the horizontal direction in the micrographs. The orientation of cells was scored at 5° increments and the total number of cells within each increment is shown as a bar in Figure 1b. The cumulative percent increase in cell number at each 5° increment, starting from 0° (orange line; scale on the right side of graphs) is also shown. It is interesting to note that while the orientation angle of unstretched cells is distributed more or less equally from 0° to 90°, the biaxially stretched cells show a strong preference to orient at 45°, even though the ratio between the long and the short axes is small like the unstretched cells. These experiments demonstrate the dynamic response of ECs to stretch and imply that ECs have some mechanotransduction mechanism to continuously monitor their mechanical environment and respond to it.
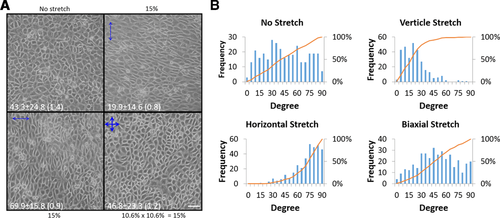
Reversible response of endothelial cells to cyclic stretch
(A) Confluent BAECs (upper left) were cyclically and uniaxially stretched by 15% at 1 Hz for three consecutive periods of 15 h each, the first period in the ‘north-south’ (upper right) and the second then ‘east-west’ (lower left) directions. The cells elongated and aligned perpendicular to the direction of stretch. When cells were stretched biaxially (lower right) for the last period, cell elongation and alignment were lost. These phase contrast images were taken from the centre area of the same two-dimensional stretch chamber at the end of each stretch regiment. Scale: 50 μm. (B) The long axis of each cell was determined by eye and the angle it makes with the horizontal direction was measured. If this angle (X) was larger than 90°, we assigned (180 – X) degree to it. The data are recorded as 5° increment from 0 to 90 (i.e. 19 steps). The graphs show the number of cells oriented at each 5° angle step (blue bars) and also percent increases in the cumulative event total (orange line, scale on the right side). The average orientation angle of the long cell axis is shown in each image of control and stretched cells. The angle is presented with ±SD (SEM) where SD is standard deviation and SEM is standard error of the mean. The number of cells analysed for each condition was as follows: 329 cells for the no stretch, 301 for ‘north-south’ stretch, 335 for ‘east-west’ stretch and 365 for the biaxial stretch. Vertical and horizontal refer to the direction of stretch indicated in (a), not the actual direction used in the experiments. The data are a representative set from five independent experiments.
PECAM-1 is an EC adhesion protein that participates in the formation of EC–EC adhesion and as such, is localised at interendothelial cell contact sites. Since protein phosphorylation is involved in many types of signalling events including mechanosignalling (Osawa et al., 1997, 2002), we used anti-protein phosphotyrosine as a marker of signalling events occurring when ECs are stretched for a short period of time (Figure 2). PECAM-1 is localised mainly in the cell–cell contact region outlining each EC. In unstretched cells, only faint anti-phosphotyrosine staining is seen at the cell border. When ECs were uniaxially stretched by 15% at 1 Hz for 10 min, there was an increased level of anti-phosphotyrosine staining at the cell border. To avoid anti-phosphotyrosine staining signals from focal adhesions, especially near the cell–cell border, we recorded these images away from the collagen-coated flexible membrane surface. These data indicate that interendothelial contacts play an important role in stretch-induced mechanosignalling. Similar observations were made in our earlier in vivo study in which we found increased protein tyrosine phosphorylation at the interendothelial cell contacts in high shear stress areas (Kano et al., 2000). However, in these experiments, the specific molecules that are tyrosine phosphorylated in an external force-dependent manner at cell contacts are unknown although PECAM-1 should be one of them. We arbitrarily chose 10 regions containing roughly 20 μm long cell–cell borders identified by anti-PECAM-1 staining from both stretched and unstretched cultures and using the Olympus Fluoview 1200MPE software, calculated the Pearson's coefficients between anti-PECAM-1 and anti-protein phosphotyrosine staining. We found that Pearson's correlation coefficients were 0.55 ± 0.02 (average ± SEM) for the stretched culture and −0.02 ± 0.02 (average ± SEM) for the unstretched monolayer, indicating that there is a good correlation between the two staining patterns in the stretched culture. The Olympus Fluoview 1200MPE software also allowed us to compare the cell border staining intensity of the anti-protein phosphotyrosine of the stretched with that of the unstretched cultures. Average pixel intensities in an arbitrary unit were 147 ± 11.7 (average ± SEM) and 50.3 ± 2.75 (average ± SEM) for stretched and unstretched cultures, respectively. Thus, the level of protein phosphotyrosine at the cell–cell contact increases when EC monolayers are passively stretched. To confirm that PECAM-1 is phosphorylated by stretch, we performed a Western blot analysis of ECs stretched in the same manner as those shown in Figure 2. Cells were stretched uniaxially for 0, 5, 10 and 15 min and lysed as described in Chiu et al. (2008). PECAM-1 was immunoprecipitated and probed simultaneously by Western blotting with rabbit anti-PECAM-1 (pseudo coloured ‘red channel’) and mouse anti-phosphotyrosine (pseudo coloured ‘green channel’) (Figure 3). These images were obtained using the Li-COR two colour system. Note that the intensity of the green colour increases within 5 min of stretch and that in the merged image, the yellow colour also increases with time. The graph shows the average increase in PECAM-1 phosphorylation in ECs stretched uniaxially for 0, 5, 10 and 15 min. The kinetics and the level of phosphorylation are comparable to those observed in flow-induced PECAM-1 phosphorylation (Harada et al., 1995).
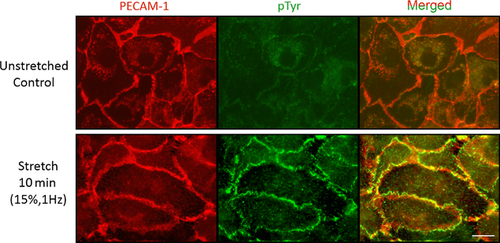
Increased tyrosine phosphorylation at interendothelial contact sites after cyclic stretch
Confluent monolayers of BAECs with or without exposing them to cyclical stretch at 1 Hz, 15% for 10 min were immunofluorescently double-stained with anti-PECAM-1 (red) and anti-protein phospho-tyrosine (green). Although the cell contacts show weak anti-phospho-tyrosine staining without stretching (upper panel), increased green fluorescence intensity is seen at the cell border after the stretching (lower panel). The merged images show increased yellow colour at the cell border after stretch. In order to avoid detecting focal adhesion-associated phosphorylation, the images were taken from above the chamber surface. Representative images from three independent experiments. Scale: 10 μm.
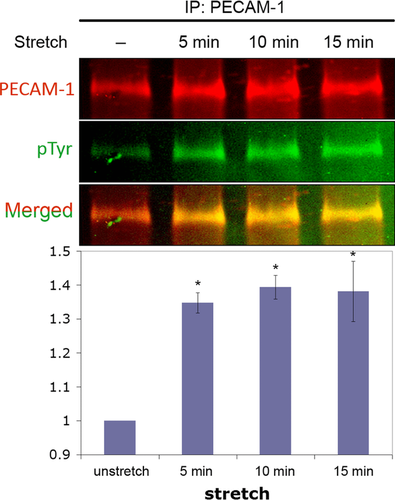
PECAM-1 phosphorylation in stretched endothelial cells
A confluent monolayer of BAECs was uniaxially stretched by 15% at 1 Hz for 5–15 min. At the end of each stretch experiment, cells were lysed and PECAM-1 was immunoprecipitated from the lysates and analysed by Western blotting using the two colour Odyssey detection system. The emission of IRDye 680 (PECAM-1 signal) was pseudo-coloured red (top panel) and IRDye 800 (phospho-tyrosine signal) was pseudo coloured green (middle panel). The third panel shows the merged image. When the level of PECAM-1 phosphorylation is low, the merged image appears red, but as the phosphorylation level increases, the yellowness of the band increases. The graph shows the time course of the average increase (±SEM) in PECAM-1 phosphorylation determined by the ratio of pTyr/PECAM-1 band intensity in three independent experiments. The level of phosphorylation (Y-axis) is expressed relative to unstretched cells. Asterisks indicate P < 0.05.
A protein construct containing a PECAM-1 flanked by an N-terminal 6xHis tag and a C-terminal AviTag tag (HPA; for detail, see Materials and Methods) was synthesised by and purified from AVB101 cells (Figure 4a). The presence and purity of the resulting ∼25 kDa protein was determined by SDS-PAGE and Coomassie brilliant blue staining (Figures 4b and 4c). Functional studies of the protein indicated that phosphorylation of the cytoplasmic tail of PECAM-1 occurred readily in the presence of Fyn and ATP, albeit rather slowly (Figure 5). A recent study by Paddock et al. (2011) has suggested that a portion of the cytoplasmic tail of PECAM-1 does not become structured unless it is in the presence of lipid. For this reason, dodecylphosphocholine (DPC) was titrated and it was found that 3 μM of DPC or greater prevented immediate phosphorylation from occurring (Figure 5). Therefore, the presence of a lipid may mask the phosphorylation sites as suggested by Paddock et al (2011). However, this inhibition may be due to other causes such as a possible inhibition of Fyn's activity by DPC. These and other mechanistic considerations are presently under investigation.
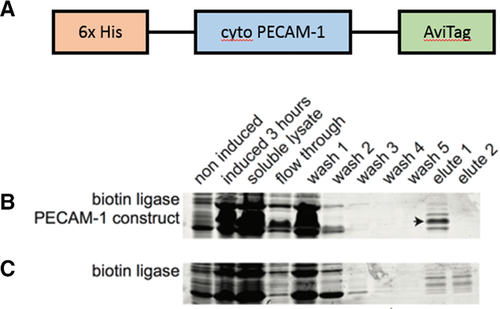
Purification of the HPA protein construct
(A) A schematic representation of HPA. (B) Coomassie brilliant blue stained SDS gels of proteins purified using nickel chelation columns from cells expressing the HPA protein construct (arrow). (C) Coomassie blue stained SDS gel of lysates from control non-expressing cells.
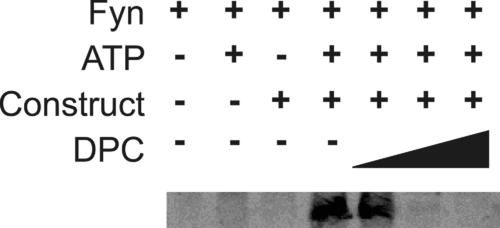
Phosphorylation of PECAM-1 cytoplasmic tail constructs by protein kinase Fyn
Increasing amounts of DPC (0.3, 3.0 and 30 μM) prevented phosphorylation by Fyn. Each trial contained where indicated 5 μg protein, 100 ng Fyn and 1.25 nM ATP and were incubated 60 min at RT.
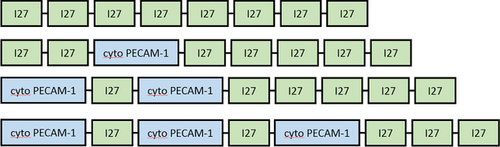
Force spectroscopy experiments were performed using a strategy developed for the study of molecules that do not contain the repeated force bearing motifs common to the mechanical proteins such as those found in muscle (Best et al., 2001). We used a recombinant construct that contains eight repeats of the I27 domain of titin. A variable number of PECAM-1 cytoplasmic domain repeats were spliced into the construct (Figure 6). The mechanical property of the construct consisting of eight I27 repeats has been well characterised (Best et al., 2001): when atomic force microscopy (AFM) was used to unravel the construct, eight sawtooth force curves with ∼200 pN peaks were recorded (Figure 7, black). For our PECAM-1 unravelling analysis, we used only force-extension curves having the expected number (5–8) of ∼200 pN sawtooth peaks, and we superimposed seven force-extension curves for each of the four I27 constructs prepared (Figure 7). When a single molecule is being pulled, the number of characteristic sawtooth force curves predicted by the construct was observed. Concentrations applied to the substrate surface were kept low so that only ∼10 out of 100 test points gave a force curve indicating the presence of a single molecule. In all samples, the signal from PECAM-1 unfolding events occurred prior to the opening of the I27 structures, and we observed heterogeneous peaks of varying forces within the 50–400 pN range (Figure 7). Constructs with more PECAM-1 domains inserted in the place of I27 motifs showed a greater number of these randomised peaks with forces as large as or greater than those of I27 rupture. Because of the random nature of the PECAM-1 unfolding events, a strong structural motif (i.e. beta sheet or alpha helix) does not likely exist. However, given the strength of the unfolding forces, strong adhesions between residues within the molecule appear to exist. The cytoplasmic tail of PECAM-1 is phosphorylated in solution, but it is not likely in a fully unfolded conformation.
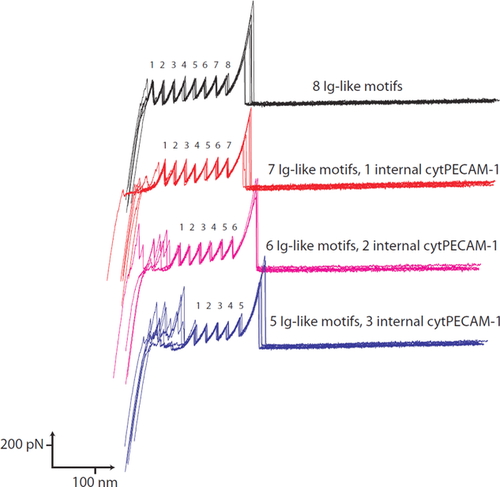
Force extension curves of the I27-PECAM-1 constructs
For each construct, seven individual curves were overlayed. The I27 motifs displayed reproducible curves with rupture forces of ∼200 pN. Single molecule pulling events were determined by the number of expected I27 curves. For constructs with increasing numbers of PECAM-1 repeats, a higher degree of random forces occurs before I27 repeats are unfolded. These forces were in some cases larger than those displayed by the I27-like motifs.
Discussion
Based on our earlier studies, we have hypothesised that the cytoplasmic tail of PECAM-1 is involved in the transduction of forces applied to the exterior of ECs into biochemical signals within the cells (Osawa et al., 2002; Fujiwara, 2006; Chiu et al., 2008). The conversion of a mechanical signal to a biochemical signal through PECAM-1 is hypothesised to be due to structural changes within the cytoplasmic tail of the molecule. The main goal of this study was to determine whether the PECAM-1 cytoplasmic region has a mechanically stretchable structure.
In this study, we used AFM to perform force spectroscopy measurements. This technique allows for the application of high forces on single molecules and is used to evaluate the response of mechanically loaded molecules (Neuman and Nagy, 2008). By using a procedure developed for the analysis of non-mechanical protein (Best et al, 2001), we were able to isolate the signal from the cytoplasmic portion of PECAM-1 and compare it to the known mechanically activated domains from titin. We have observed that PECAM-1 exhibits a heterogeneous array of structural responses to force. This may be due to the lack of a single strong structural element within the protein and may be caused by the random unravelling of adhesive units within the molecule. However, we note that the magnitude of the rupture forces is as large as or larger than the I27 domains from titin. This observation may indicate that the cytoplasmic domain of PECAM-1 folds in a manner such that high forces are required to fully open the molecule. Although there are unresolved issues regarding PECAM-1 deformation by force, our study suggests that the molecule can be unravelled by force. In our earlier study in which PECAM-1 on the cell surface was pulled using magnetic beads (Osawa et al., 2002), we estimated the magnitude of pulling force to be about 20 pN/bead based on the calculation method proposed by Glogauer et al. (1997). It is not known how many PECAM-1 molecules are captured by the beads, but it is likely that the number is more than 1. This suggests that PECAM-1 is activated by a force of less than 20 pN, which is less than the force level we detected in this study needed to completely unravel the PECAM-1 cytoplasmic domain. Since we found that the PECAM-1 cytoplasmic domain can be readily phosphorylated in vitro although rather slowly or inefficiently, it is possible that PECAM-1 may not have unravelled enough for phosphorylation to occur. Further studies are necessary to determine the precise mechanism for the force-dependent PECAM-1 phosphorylation observed in ECs.
Phosphorylation of the cytoplasmic region was observed in solution without the application of force. This indicates that the construct does not achieve a fully physiological state in its purified form. This may be due to the lack of the normal connections formed as a complex with other molecules (Newman, 1997; Ilan et al., 2000) and/or the influence of the membrane (Paddock et al., 2011) in vivo. Since the addition of lipid to the purified protein reduced phosphorylation in solution, an interaction with the plasma membrane may serve to induce structural elements that block PECAM-1 from being phosphorylated in the absence of applied force. In our future studies, such a system will be used to investigate whether in the presence of lipid, PECAM-1 attached to a stretchable surface could be phosphorylated upon stretch, using an in vitro approach developed earlier by Sawada et al. (2006).
In this study, we have also shown that the EC response to cyclic stretch is a reversible process. This suggests that ECs are constantly surveying their force environment. PECAM-1 seems to be a molecule well-suited to provide this type of surveillance. Once PECAM-1 is phosphorylated, SHP2 which is a protein tyrosine phosphatase, binds PECAM-1 (Masuda et al., 1997; Osawa et al., 2002). This binding not only activates certain signalling cascades such as the extracellular signal-regulated kinase1/2 activation pathway (Osawa et al., 2002) but also de-phosphorylates PECAM-1. De-phosphorylated PECAM-1 is then added back to a ready-to-respond pool of unphosphorylated PECAM-1. At cell–cell contact regions, PECAM-1 constantly cycles from the cell membrane through a submembranous vesicular system and back again (Mamdouh et al., 2003). The result is that at any one time, roughly half the PECAM-1 molecules are unavailable for binding to neighbouring cells. This would give PECAM-1 time to dephosphorylate within the cell and provide a constant availability of new, unphosphorylated PECAM-1 coming to the cell surface, making the system resistant to habituation and allowing for continued sensitivity to mechanical stresses. We suggest that this cyclic nature of the PECAM-1 mechanosignalling allows it to continuously survey the mechanical environment in which the EC monolayer is present. Our Pearson's correlation analysis on PECAM-1 and protein phosphotyrosine localisation at the cell–cell contact gave a value of 0.55, which supports the observation made by Mamdouh et al. (2003) of PECAM-1 recycling. The presence of anti-PECAM-1 staining without corresponding anti-phosphotyrosine labelling is consistent with the idea that a certain fraction of PECAM-1 is unphosphorylated since it is in the submembranous vesicular systems.
For our stretch experiments, we used cyclic stretch (1 Hz) because large arteries are known to cyclically stretch and recoil with the same frequency as the heartbeat. However, according to our hypothesis of PECAM-1 mechanotransduction, the same PECAM-1 response should be seen when an EC monolayer is stretched once and held in the stretched position. Indeed, in our in vitro model stretching, we used this type of stretch which we called a single stretch (Chiu et al., 2008). However, when we applied a single stretch to an EC monolayer, PECAM-1 phosphorylation was observed initially but to a lesser extent and less consistently than that observed in cyclically stretched cells (data not shown). When ECs are kept on a substrate that remains in a constantly stretched state, it may be possible for cells to remodel their substrate attachment points in a manner that reduces the applied forces, leading the cells themselves to eventually return to an unstretched state. Thus, once dephosphorylated, PECAM-1 would not then be re-phosphorylated, resulting in a reduction in PECAM-1 phosphorylation. For this reason, we used cyclic stretch rather than a single stretch. When ECs are stretched in a uniaxial manner, they align perpendicular to the direction of stretch. Indeed, in vivo, in the straight portion of arteries, the vessel stretches radially with each heart beat and ECs are elongated perpendicular to this directional stretch. In contrast, it has been suggested that the direction of stretch around vessel branches is not uniaxial (Chien, 2007). When ECs were stretched biaxially, we found that the EC shape became polygonal, similar to the cell shape found in vessel branches. It appears that ECs in large arteries exhibit the cell shape that is consistent with the local flow and stretch environment.
Mechanotransduction in ECs is a complex process, with structural changes within PECAM-1 contributing to the signalling process. The activation of PECAM-1 by environmental forces and the subsequent response by the cell highlights the intricate system by which cells sense and respond to their mechanical environment.
Materials and methods
EC response to cyclic stretch
BAECs were purchased from Clonetics and cultured in Dulbecco's Modified Eagle's medium (Mediatech, Inc.) supplemented with 10% foetal bovine serum (Equitech-Bio, Inc.), 50 IU/mL penicillin (Mediatech, Inc.) and 50 μg/mL streptomycin (Mediatech, Inc.) at 37°C in a humidified CO2 (5% in air) incubator. Cells were used between passages six and nine.
The one-dimensional (uniaxial) stretch apparatus has been described previously (Shi et al., 2007; Reinhart-King et al., 2008). Rectangular silicone chambers were coated by adding and then evaporating 2 mL of 100 μg/mL type I collagen in 0.02 N acetic acid at room temperature (RT) overnight, UV-sterilised for 20 min in a biosafety cabinet and rinsed five times with sterile PBS. BAECs were plated on the collagen-coated surface (30 × 30 mm) and cultured for 2–3 days to reach confluence. The silicone chamber was mounted on the stretch apparatus in a humidified CO2 incubator and stretched by 15% at 1 Hz for various lengths of time.
A two-dimensional (biaxial) stretch apparatus was manufactured in house with the same basic design as the one-dimensional apparatus except with two motorised bars that are perpendicular to each other and can be operated synchronously or one at a time. Biaxial 40 × 40 mm cell stretch chambers (ST-CH-04.0-XY) were purchased from STREX and prepared for culturing cells in the same manner described for the one-dimensional chamber. To obtain 15% overall bidirectional stretch, we stretched 10.6% in X and Y directions: 15 = square root of (10.62 + 10. 62).
EC response to stretch was studied morphologically as well as by determining the extent of PECAM-1 phosphorylation. Cell shape changes were recorded by removing a chamber from the stretcher at various times and photographing cells in the centre region of the chamber using an Olympus phase contrast microscope (IMT-2; 10× objective lens with 0.3 N.A.) equipped with an Olympus digital camera (CAMERIA c-5060). A routine immunofluorescence technique was performed by fixing ECs in stretch chambers with 4% paraformaldehyde in PBS for 10 min at RT followed by permeabilisation with PBS containing 0.1% Triton-X 100. PECAM-1 and phosphotyrosine were localised with rabbit anti-PECAM-1 (C-20) from Santa Cruz Biotechnology, Inc. and mouse anti-protein phosphotyrosine (4G10) from Millipore. Secondary antibody staining was done with Alexa Flour 488-labelled goat anti-mouse IgG (Invitrogen) and Alexa Flour 546-labelled goat anti-rabbit IgG (Invitrogen). Cells were observed using an Olympus epi-fluorescence microscope (BX51) with a 60× water-dipping objective lens (N.A. 0.9) and images were recorded with a Spot camera (RT colour Spot; Diagnostic Instruments, Inc.) operated by the camera software (Version 3.5.9.1 for Mac OS). For the PECAM-1 phosphorylation assay, ECs were stretched by 15% at 1 Hz for 5, 10 and 15 min, lysed, and PECAM-1 immunoprecipitated with anti-PECAM-1 (C-20). Phosphorylation was detected and quantified by Western blotting using C-20 and 4G10 primary antibodies followed by IRDye 680-conjugated anti-rabbit IgG (Rockland) and IRDye 800-conjugated anti-mouse IgG (Rockland) secondaries, respectively and imaging with the two-colour Odyssey Infrared Imaging System (Li-COR Biosciences) as described earlier (Chiu et al., 2008). The level of PECAM-1 phosphorylation was determined by the ratio of phosphotyrosine to PECAM-1 staining using the software of the Odyssey system. The value obtained from unstretched confluent cells was taken as 1 for each set of experiments and other values were expressed relative to it. Student's t test (one-tailed distribution, equal variance, pairwise comparisons) was used to determine levels of significance. P values < 0.05 were considered to be significant.
Protein constructs
An Escherichia coli codon-optimised sequence containing the 121 amino acids from the bovine PECAM-1 cytoplasmic tail (Uniprot P51866) flanked by an N-terminal 6xHis and a C-terminal AviTag was purchased from GenScript (Figure 4a). This construct was subcloned into the pQE-12 expression vector (Qiagen) and the protein produced in E. coli. The cells were lysed using lysozyme and sonication, and the protein purified on HiTrap nickel-chelating columns (GE Healthcare Life Science). Residual imidazole from the purification process was removed using dialysis. I27-PECAM-1 constructs (Figure 6b) were prepared by replacing one to three of the Ig-like titin I27 domains within a recombinant construct with the cytoplasmic PECAM-1 domain by restriction digest and ligation (Best et al., 2001). E. coli were transformed and the protein was purified on HiTrap columns.
Functional study of construct
The function of the purified 6xHis-PECAM-1 cytoplasmic tail-Avitag (HPA) construct was tested by mixing 5 μg of the protein with 100 ng of Fyn (Sigma) and 1.25 nM ATP for 60 min at RT or 15 min at 37°C. After phosphorylation, the samples were run on 16% polyacrylamide gels and blotted with anti-protein phosphotyrosine. An 800 nm IR dye labelled secondary antibody was used to visualise phosphorylation using the Odyssey IR imager. The influence of the lipid DPC on phosphorylation was studied by adding 0.3–30 μM of the lipid to the protein during incubation with Fyn.
Force spectroscopy
Force spectroscopy experiments were performed with an Asylum MFP 3D AFM. The I27-PECAM-1 constructs were bound at low concentration on a gold coated coverslip through a self-assembly interaction with 2 terminal cysteines for 30 min. The sample was rinsed and hydrated with PBS. The AFM laser was positioned on the end of a triangular cantilever and the system was allowed to rest for 30 min before force spectroscopy was performed. The cantilever was first brought to the cover slip for photodiode calibration. Spring constants were then determined, after moving the cantilever away from the surface, through the thermal method. A typical spring constant in fluid was 30 pN/nm. Force spectroscopy measurements were made by approaching the surface with a force of 300 pN at a rate of 50 nm/s. After resting for 1 s at the surface and allowing the protein to passively adsorb to the tip, the cantilever was withdrawn at 50 nm/s to 1000 nm away from the surface. The tip position was moved around the substrate using computerised controls and multiple force extension curves were obtained. Positive curves were determined by having an expected number of characteristic I27 motif signatures. On average, due to the low concentrations needed for single molecule actuation, only 10 positive curves would be obtained for 100 total test points.
Author contribution
The study was developed by all the authors. J.L.S. and E.M. performed experiments using the cytoplasmic tail of PECAM-1 (Figures 4-7); T.N.T., Y.J.C. and E.M. performed the cell stretch experiments (Figures 1-3); and R.L.C. and K.F. were responsible for overseeing and supporting the project.
Funding
A grant-in-aid from the American Heart Association (11GRNT5850001) to K.F. was used to manufacture the 2D stretch apparatus. J.L.S. and this study were supported by a grant from National Institute of Health R21 (HL113703) to R.L.C. and K.F.
Acknowledgements
We thank Gregory Fedorchak, Rebecca Slater and Xiao Wu for making the 2D stretch apparatus as their senior design project in the Department of Bioengineering, University of Rochester. This project was supervised by Dr. Danielle Benoit and K.F. We also thank the reviewers who made important suggestions. I27 constructs were provided by Dr. Piotr Marszalek of Duke University and Jane Clarke of the University of Oxford.
Conflict of interest statement
The authors have declared no conflict of interest.