Left-right asymmetry in the light of TOR: An update on what we know so far
Abstract
The internal left-right (LR) asymmetry is a characteristic that exists throughout the animal kingdom from roundworms over flies and fish to mammals. Cilia, which are antenna-like structures protruding into the extracellular space, are involved in establishing LR asymmetry during early development. Humans who suffer from dysfunctional cilia often develop conditions such as heterotaxy, where internal organs appear to be placed randomly. As a consequence to this failure in asymmetry development, serious complications such as congenital heart defects (CHD) occur. The mammalian (or mechanistic) target of rapamycin (mTOR) pathway has recently emerged as an important regulator regarding symmetry breaking. The mTOR pathway governs fundamental processes such as protein translation or metabolism. Its activity can be transduced by two complexes, which are called TORC1 and TORC2, respectively. So far, only TORC1 has been implicated with asymmetry development and appears to require very precise regulation. A number of recent papers provided evidence that dysregulated TORC1 results in alterations of motile cilia and asymmetry defects. In here, we give an update on what we know so far of mTORC1 in LR asymmetry development.
Abbreviations
-
- 4E-BP1
-
- eIF4E-binding protein 1
-
- AMPK
-
- AMP-activated protein kinase
-
- AP
-
- anterior-posterior
-
- CHD
-
- congenital heart defect
-
- DFC
-
- dorsal forerunner cell
-
- GRK5
-
- G protein-coupled receptor kinase 5
-
- Grk5l
-
- G protein-coupled receptor kinase 5-like
-
- KV
-
- Kupffer's vesicle
-
- LKB1
-
- liver kinase B1
-
- LR
-
- left-right
-
- mTOR
-
- mammalian (or mechanistic) target of rapamycin
-
- PDK1
-
- 3-phosphoinositide-dependent protein kinase 1
-
- Pras40
-
- proline-rich Akt substrate 40 kDa
-
- PTEN
-
- Phosphatase and Tensin homolog
-
- Raptor
-
- regulatory-associated protein of mammalian target of rapamycin
-
- Rheb
-
- small GTPAse RAS homologue enriched in brain
-
- S6K1
-
- ribosomal protein S6 kinase 1
-
- SHH
-
- Sonic Hedgehog
-
- ss
-
- somites stage
-
- TFEB
-
- transcription factor EB
-
- TORC1
-
- mTOR complex 1
-
- TORC2
-
- mTOR complex 2
-
- TSC
-
- tuberous sclerosis
Introduction
The mammalian (or mechanistic) target of rapamycin (mTOR) pathway is an evolutionary conserved signalling network. Integrating a multitude of environmental stimuli, it governs essential processes such as cell growth, proliferation and metabolism (Laplante and Sabatini, 2012; Shimobayashi and Hall, 2014). Controlling such basic processes, it is not surprising that mTOR signalling is also involved in human disease such as cancer, type 2 diabetes and neural disorders. Recently, however, mTOR signalling has also been implicated in the development of left-right (LR) asymmetry during early embryogenesis (DiBella et al., 2009; Yuan et al., 2012; Burkhalter et al., 2013). Here, we review the current knowledge of how the mTOR pathway influences asymmetric morphogenesis in different model organisms with special emphasis on LR asymmetry. Additionally, we give a summary of LR asymmetry development in the most commonly used model organisms.
The mTOR pathway
The mTOR network contains two main branches, each with its own mTOR complex at its center, TORC1 and TORC2, respectively. The two complexes were originally identified by their different sensitivity to rapamycin, with TORC1 being acutely sensitive (Brown et al., 1994), whereas TORC2 responds only after prolonged treatment (Sarbassov et al., 2006; Copp et al., 2009). Most what we know today about the mTOR network, however, relates to the action of the TORC1 with its core components mTOR, raptor and mLST8. In this review, we focus on this axis of the network and particularly on components that appear to be relevant for LR asymmetry development. For further reading about mTOR pathway regulation, we recommend two excellent reviews (Laplante and Sabatini, 2013; Shimobayashi and Hall, 2014). In addition and for ease of reading, we explain key factors of the TORC1 and 2 as well as regulators thereof in Table 1.
| Abbreviation | Name | Complex | Function | Regulation | Reference |
|---|---|---|---|---|---|
| mTOR protein kinase | Mechanistic target of rapamycin kinase | TORC1/TORC2 | Ser/Thr kinase | Core kinase | Brown et al. (1994); Sabatini et al. (1994) |
| Jacinto et al. (2004) | |||||
| mLST8, also known as GβL | Mammalian lethal with sec-13 protein 8 | TORC1/TORC2 | Binds mTOR kinase | Stimulates kinase activity | Jacinto et al. (2004) |
| Kim et al. (2003) | |||||
| Tti1 | Tel2 Interacting Protein 1 | TORC1/TORC2 | Complex with Tel2 | Assembly and stabilisation of core complex | Kaizuka et al. (2010) |
| Tel2 | Telomere maintenance 2 | TORC1/TORC2 | Complex with Tti1 | Assembly and stabilisation of core complex | Kaizuka et al. (2010) |
| Deptor | DEP domain containing mTOR-interacting protein | TORC1 | Inhibitory | Peterson et al. (2009) | |
| Raptor | Regulatory-associated protein of mammalian target of rapamycin | TORC1 | Scaffold assembly | Localisation and activity | Hara et al. (2002) |
| Kim et al. (2002) | |||||
| Pras40 | Proline-rich Akt substrate 40 kDa | TORC1 | Inhibitory | Wang et al. (2007) | |
| mSin1 | Mammalian stress-activated map kinase-interacting protein 1 | TORC2 | Scaffold | Assembly of complex | Yang et al. (2006) |
| Rictor | Rapamycin-insensitive companion of mTOR | TORC2 | Scaffold | Assembly of complex | Sarbassov et al. (2004)Pearce et al. (2007) |
| Protor 1 & 2 | Protein observed with rictor 1 and 2 | TORC2 | Activation of specific targets | ||
| Rheb | RAS homologue enriched in brain | Binds TORC1 | GTPase | Positive regulator of TORC1 | Inoki et al. (2003a) |
| Tee et al. (2003) | |||||
| Zhang et al. (2003) | |||||
| TSC1, also known as hamartin | Tuberous sclerosis 1 | Member of TSC complex | GTPase activating complex of Rheb | Negative regulator of TORC1 | Gao et al. (2002) |
| Inoki et al. (2002) | |||||
| Tee et al. (2002) | |||||
| TSC 2, also known as tuberin | Tuberous sclerosis 2 | Member of TSC complex | GTPase activating complex of Rheb | Negative regulator of TORC1 | Gao et al. (2002) |
| Inoki et al. (2002) | |||||
| Tee et al. (2002) |
- This table lists the core factors involved in the assembly as well as regulation of both mTOR complexes.
TORC1 is localised predominantly to the cytoplasm and its activity can be triggered by a vast and very diverse array of extracellular or membrane-bound stimuli including amino acids, growth factors or G protein-coupled receptors. In the cytoplasm reside also the most important negative regulators of TORC1, namely the TSC complex with its main components TSC1 and 2 (see Table 1) (Gao et al., 2002; Inoki et al., 2002; Tee et al., 2002; Dibble et al., 2012), which function as GTPase activating proteins for the small GTPase Rheb (Inoki et al., 2003a; Tee et al., 2003; Zhang et al., 2003). This entails an inactive GDP-bound state of Rheb, in which it is unable to bind to the catalytic domain of mTOR, and the inhibition of mTOR pathway activity. The TSC complex itself is controlled by a PI3K-PDK1-Akt axis, which can be activated by growth factors as well as a high cellular energy status. Akt phosphorylates TSC2 thereby causing inactivation of the TSC complex, what eventually permits TORC1 signalling to increase (Inoki et al., 2002; Manning et al., 2002). At the same time, Akt phosphorylates also Pras40. Pras40 is a direct TORC1 inhibitor, which functions through scaffolding of the essential TORC1 component Raptor (Sancak et al., 2007; Vander Haar et al., 2007). On the other hand, if a cell is in a low energy state TSC-mediated inhibition of TORC1 prevails. This inhibition is supported by activity of LKB1 (liver kinase B1), which activates AMPK (AMP-activated protein kinase) (Shaw et al., 2004). AMPK in turn phosphorylates Raptor as well TSC2 (at sites different than Akt), causing inactivation of TORC1 (Inoki et al., 2003b; Gwinn et al., 2008). In a similar fashion, DNA damage and hypoxia also activate AMPK and subsequently switch off TORC1 (Hwang et al., 2004; Liu et al., 2006; Alexander et al., 2010).
Intriguingly, TORC1 activity depends on a very distinct subcellular localisation within the cytosol, the lysosome. High amino acid levels within the lysosome prompt activation of the so-called ragulator complex of RAG GTPases and shuttling of TORC1 to the surface of the lysosome (Sancak et al., 2010; Shimobayashi and Hall, 2014). Moreover, the interaction of active Rheb and TORC1 occurs at the surface of the lysosome (Hwang et al., 2004; Liu et al., 2006; Sancak et al., 2010). Recent work revealed furthermore that even the TSC complex translocates from a general cytosolic localisation to the lysosome to inactivate TORC1 (Demetriades et al., 2014). The lysosome thus seems to work as a spatial platform for TORC1 activity. Interestingly, for TORC2, which is mainly activated by growth factors, the mitochondria-associated endoplasmatic reticulum has been postulated to be a similar platform (Boulbes et al., 2011; Betz et al., 2013).
Physiologically, TORC1 controls the most basic processes in any cell, such as protein and lipid synthesis, autophagy and energy metabolism. Here, the best-characterised targets and effectors of TORC1 are ribosomal protein S6 kinase 1 (S6K1) and eIF4E-binding protein 1 (4E-BP1). S6K1 becomes active through direct phosphorylation by mTOR at threonine 389. As a result, ribosome biogenesis is elevated (Hannan et al., 2003; Chauvin et al., 2014). Phosphorylation of 4E-BP1, on the other hand, prevents it from inhibiting translation initiation. Thus, both proteins generally increase protein production. In addition to its role on protein synthesis, 4E-BP1 modulates also the expression and efficiency of PPARγ in an mTOR-dependent manner. As PPARγ is a key factor controlling fatty acid uptake, synthesis and modification, it is well involved in adipogenesis (Kim and Chen, 2004; Le Bacquer et al., 2007). In a similar fashion, S6K1 induces lipogenesis. However, it does so by activating SREBP rather than by PPARγ (Hwahng et al., 2009). In parallel, lipogenesis is also promoted through mTOR-dependent, S6K1-independent inactivation of Lipin-1, an inhibitor of lipid synthesis (Peterson et al., 2011) and subsequent nuclear translocation of SREBP (Porstmann et al., 2008). Both processes together, protein as well as lipid biosynthesis, are responsible for the well-known increase in cell size upon TORC1 activation (Fingar et al., 2002) and the often therapeutically targeted cell cycle progression (Brown et al., 1994). Another important process regulated by TORC1 is autophagy (Noda and Ohsumi, 1998) and TORC1 inhibits it via at least two mechanisms. It firstly inactivates positive upstream modulators of autophagy (Ulk1 and ATG14) (Hara et al., 2008; Yan et al., 2012) and secondly prevents lysosome formation by phosphorylation of TFEB (Martina et al., 2012). In addition to that, the negatively regulated Lipin-1 governs autophagy (Zhang et al., 2014). Finally, energy metabolism is affected on the translational and the transcriptional level by induction of genes involved in glycolysis (Duvel et al., 2010) and through a positive effect of TORC1 on mitochondrial number (Morita et al., 2013). Taken together, TORC1 functions in a very unexpected localisation within the cytosol, the lysosome, from where it generally helps the cell to strive.
LR asymmetry development in model organisms
LR asymmetry is established very early during embryonic development. It describes the distinction into a left and a right side in our body. This discrimination between body halves determines not only where internal organs are positioned and how they are interlinked (i.e. by the vasculature), but also how their morphology is established. Consistently with this, deviations from regular LR asymmetry often result in serious conditions, such as isomerism or heterotaxy (for review see (Sutherland and Ware, 2009)). They are characterised by an apparently random organisation of organs, irregular gut looping and other detrimental complications. So are the majority of heterotaxy patients born with complex congenital heart defects (CHD) (Kennedy et al., 2007), which many times require immediate surgical attention (Ramsdell, 2005).
Importantly, the distinction of left versus right is not exclusive to humans or even mammalians. In the roundworm Caenorhabditis elegans, the first overall asymmetric arrangement of blastomers occurs at the six cell stage (Wood, 1991). Asymmetry with respect to left and right can be first observed from the 12 cell stage on with a second LR induction at the 24 cell stage, when certain genes are induced exclusively on the left side. This asymmetric development depends on a particular orientation of the mitotic spindle resulting in asymmetric positioning of the left and right daughter cells (for review see (Pohl, 2011)). Moreover, this process also triggers different cell number on either side of the AP axis (Pohl and Bao, 2010). In the fruit fly Drosophila melanogaster, LR asymmetry exists, too. Asymmetric organ arrangements can be observed in the brain (‘asymmetric body’), the gut and the malphigian tubes and most importantly the male genital disc (for review see (Coutelis et al., 2008)). The latter is the best-studied organ with respect to LR asymmetry in flies and arises from the directional rotation of the male genitalia that defines eventually the looping of the spermiduct (Adam et al., 2003). On the molecular level spermiduct rotation depends on JNK (Glise et al., 1995; Holland et al., 1997; Macias et al., 2004) and the expression of the Hox gene Abdominal-B, which leads to the induction of the myosin MyoID (Coutelis et al., 2013; Geminard et al., 2014).
In contrast to invertebrates, where LR asymmetry is restricted to certain substructures, a more pronounced and general LR asymmetry has developed in vertebrates. It is not fully clear by now, when vertebrates institute the very first step towards LR asymmetry, but a structure representing a temporal organ of laterality appears to play an important, yet not fully understood role. This temporal organiser represents the currently best characterised step regarding symmetry breaking, although it may potentially not be the initial event in the establishment of LR asymmetry (Vandenberg and Levin, 2009). However, as evidence for steps preceding the development of this organiser is still limited, we will refrain from explaining them in this review. Elaborate details for those steps in symmetry breaking can be found in a recent review (Vandenberg and Levin, 2013).
The organ of laterality has been identified in a number of model organisms. It is called gastrocoel roof plate in Xenopus (GRP) (Vodicka and Gerhart, 1995), Kupffer's vesicle (KV) in zebrafish (Melby et al., 1996; Essner et al., 2005) and posterior notochord (PNC) or node in mice (Blum et al., 1992; Beddington, 1994; Bellomo et al., 1996) (for review see also (Blum et al., 2009)). If the development of the organ of laterality is disturbed, LR asymmetry defects are common. Morphologically, the LR organiser consists of a mesodermal derived monociliated epithelium at the posterior end of the notochord. Motile cilia within this organiser perpetuate a leftward flow in mouse and frog and counter-clockwise flow in zebrafish of a so far not specified fluid that may transport morphogens to the left side of the embryo (Nonaka et al., 2002). This leftward flow accounts further through not fully understood processes for the transcription of Nodal pathway genes exclusively on the left side of the midline (Nonaka et al., 1998; Kawasumi et al., 2011; Shiratori and Hamada, 2014). Strikingly, in mice only two motile cilia are sufficient to trigger nodal flow and with such the whole cascade accounting for LR determination (Shinohara et al., 2012).
Although the complete mechanism has not been uncovered yet, there is growing evidence that this flow is sensed by non- or less motile cilia at the left edge of the organiser (McGrath et al., 2003). The ‘flow receptors’ in those cilia are polycystic kidney disease genes such as Pkd1l1 and Pkd2, which upon flow-sensing entail asymmetric calcium spiking towards the left side in the organiser itself or the adjacent endoderm (Pennekamp et al., 2002; Field et al., 2011; Yoshiba et al., 2012; Takao et al., 2013). But how does this calcium enrichment on one side account for discrete gene transcription on the left side? Up to date, this phenomenon has not been fully solved. What is clear is that disruption in the calcium distribution results in ambiguous expression of Nodal pathway genes and ultimately in LR asymmetry defects (Sarmah et al., 2005; Shu et al., 2007; Leung et al., 2008; Francescatto et al., 2010; Hatayama et al., 2011; Takao et al., 2013). Vice versa, abrogation of Nodal pathway gene expression or of Nodal itself causes severe asymmetry defects in chick (Logan et al., 1998; Yoshioka et al., 1998; Rodriguez Esteban et al., 1999; Yokouchi et al., 1999), mice (Meno et al., 1998; Gaio et al., 1999; Lowe et al., 2001; Meno et al., 2001) and zebrafish (Bisgrove et al., 1999; Yan et al., 1999; Essner et al., 2000; Long et al., 2003). Moreover, genetic variations in the Nodal pathway have also been associated with situs anomalies in patients (Mohapatra et al., 2009). Because of this, the analysis of Nodal pathway genes such as lefty2 or pitx2 has emerged as the gold standard to identify asymmetry deviations in experimental setups.
mTOR in asymmetry development
One of the earliest indications that mTOR governs asymmetry development was found in mice. The flat top mice, which were originally identified in the course of an ethylnitrosourea screen for genes affecting forebrain development rather than asymmetry defects (Hentges et al., 1999), fails to undergo embryonic turning (Hentges et al., 2001). The mutation responsible for the flat top phenotype is a mutation within the FRAP gene, the FKBP-rapamycin-associated protein (Brown et al., 1994), which is the original name for mTOR in higher eukaryotes. Most interestingly, however, embryonic turning represents one of the first signs of asymmetry development in mice (Collignon et al., 1996; Melloy et al., 1998). However, mTOR may not be exclusive to LR asymmetry. Two more studies indicate that the actions of mTOR not necessarily restrict to LR asymmetry only. Lee et al. (2012b) reported that mTOR steers polar body emission in mouse ovaries, which is a form of asymmetric cell division. In the same line, hemimegalencephaly, a condition characterised by overgrowth of one cerebral hemisphere, appears to be caused by mTOR-dependent one-sided hyperproliferation of the brain (Lee et al., 2012a). Although the latter two studies only indicate a general asymmetric effect by mTOR, it is still very likely that mTOR functions in LR asymmetry in mammals. The crucial piece of evidence, however, is still lacking. Node- or even cilia-specific loss-of-function models would be required as constitutive knockouts of mTOR or Raptor are lethal during the first few days of murine gestation (Gangloff et al., 2004; Guertin et al., 2006).
mTOR and LR asymmetry development in zebrafish
The greatest insight into how mTOR regulates symmetry breaking originates from studies in zebrafish. This is partly due to the fact that zebrafish have emerged as a very valuable tool to study the molecular mechanisms underlying asymmetry development. Moreover, zebrafish embryos are available in large numbers, develop very rapidly outside their mothers and allow facile methods of manipulation (Wixon, 2000) including LR organiser specific knockdowns (Essner et al., 2005). Before we go into details about mTOR in zebrafish LR development, we briefly explain the fundamentals of LR asymmetry development in zebrafish, which are also summarised in Figure 1.
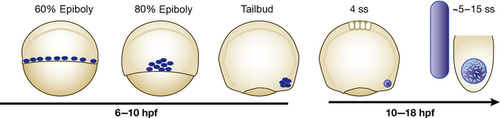
Development of the temporal organ of laterality in zebrafish
During gastrulation (6-10 hpf) DFCs (in blue) migrate and eventually coalesce into a rosette-like structure. By 4 ss, a simple, fluid filled vesicle has formed, in which the first cilia appear. Soon after, the cilia, which are asymmetrically distributed along the AP axis in the KV (Wang et al., 2012), start beating in a clockwise direction. Through that movement, a counterclockwise flow is induced, which is indispensable for the induction of laterality genes (blue bar). hpf, hours post fertilisation; ss, somite stage.
The laterality organiser in zebrafish forms at the posterior end of the notochord. It arises from a small number of dorsal forerunner cells (DFCs), which migrate ahead of other cells at the dorsal marginal zone (Cooper and D'Amico, 1996; Melby et al., 1996). Towards the end of gastrulation, the DFCs cluster deep in the future tailbud region. This aggregation depends on active FGF signalling, which in turn facilitates cadherin-1-mediated cell adhesion. Loss of a positive feedback regulator of FGF signalling, named canopy1, does consequently result in LR asymmetry defects (Matsui et al., 2011). Similarly, depletion of beta catenins, which besides mediating canonical Wnt signalling (Rao and Kuhl, 2010), are important regulators of cell adhesion, causes similar defects (Zhang et al., 2012). After clustering, the DFCs arrange into a simple epithelial rosette (Amack et al., 2007). Similar to other organs in zebrafish such as the neural tube (Strahle and Blader, 1994), a lumen is formed subsequently (Navis et al., 2013), which eventually turns into a fluid filled vesicle. Around the 4 somites stage (ss) the cells lining the KV develop single, motile cilia, which localise at the apical membrane of those cells facing the lumen (Amack et al., 2007). These cilia are able to sense and transduce molecular signals. In addition, just like in Xenopus or mice, these cilia rotate actively and produce a counterclockwise fluid flow in the KV (Kramer-Zucker et al., 2005; Kreiling et al., 2007). Furthermore, an asymmetric calcium signal is generated by this nodal flow (Sarmah et al., 2005; Shu et al., 2007; Gao et al., 2011), which most probably accounts for the transduction of a leftward signal to the left lateral plate mesoderm (LPM) (Matsui and Bessho, 2012). This in turn induces the expression of laterality genes such as southpaw, lefty2 and pitx2 specifically on the left side of the embryo (Bisgrove et al., 1999; Campione et al., 1999; Long et al., 2003; Matsui and Bessho, 2012) and causes the asymmetrically oriented organogenesis. Around 16–18 ss, the KV starts to disappear again (Francescatto et al., 2010), which is long before a heart tube or any other inner organ has developed.
Recently, TORC1 has been implicated in the formation and function of motile cilia (DiBella et al., 2009; Yuan et al., 2012; Burkhalter et al., 2013) and was shown to be modulated by cilia movements and ciliary transport, respectively (Boehlke et al., 2010; Umberger and Caspary, 2015). Interestingly, to facilitate correct symmetry breaking, TORC1 activity needs to be tightly regulated as both, hyperactivation as well as depletion result in altered ciliary length and function in the KV (DiBella et al., 2009; Yuan et al., 2012; Burkhalter et al., 2013). In zebrafish depleted for tuberous sclerosis 1a (TSC1a) (DiBella et al., 2009), nodal cilia are elongated and expression of southpaw, a lateralisation gene normally expressed in the left LPM (Long et al., 2003), occurs randomly (DiBella et al., 2009). Tsc1a is a negative upstream regulator of TORC1 and loss of its homolog in zebrafish leads to elevated TORC1 activity (Gao et al., 2002). Interestingly, knockout of either TSC1 or 2 increases also the length of primary cilia in mouse embryonic fibroblasts (Hartman et al., 2009). Expression of a dominant negative version of Glycogen synthase kinase 3B (Gsk3B), which regulates TSCs, too (Inoki et al., 2006), equally disrupts normal cilia form and asymmetry development (Yuan et al., 2012). Similarly, knockdown of G protein-coupled receptor kinase 5 (GRK5) in zebrafish augments TORC1 signalling and thus results in longer cilia and a failure in symmetry breaking (Burkhalter et al., 2013). This disruption of asymmetry is not only evident on the level of lateralisation genes during segmentation, but also on the level of the brain, pancreas and the heart. When Grk5l, the zebrafish homolog of GRK5 is lost, cardiac looping is randomised or not occurring at all. Additionally, early steps in valve formation are impaired, which together with the lateralisation defect strongly resembles the human condition heterotaxy. Grk5l, however does not act upstream of TORC1, but rather directly on it via interaction with Raptor. It dampens TORC1 activity towards its downstream effector S6K1 in zebrafish, but also in HEK293 cells as well as mouse hearts. The exact mechanism of GRK5-mediated inhibition of TORC1, however, remains to be investigated.
Vice versa, when TORC1 activity is inhibited by rapamycin treatment before gastrulation, KV cilia are shorter than in controls. Nevertheless, asymmetry determination is similarly disturbed as under conditions (i.e. overexpression of S6K1) with elevated mTOR signalling (Yuan et al., 2012). Thus, a very precise amount of TORC1 activity is necessary to ensure proper asymmetry development. But how would TORC1 exactly govern cilia formation? A simple, but nevertheless very elegant flagellar regeneration assay in Chlamydomonas reinhardtii demonstrated that it all comes down to protein synthesis. When deflagellated algae were treated with cycloheximide, an inhibitor of amino acid chain elongation during translation, flagella would almost not regrow. Concurrent treatment with rapamycin abolished flagellar regeneration completely indicating that flagellar regrowth depends on two pools of protein or flagella precursors: One, which is always ‘on stock’ and which is sensitive to cycloheximide. The second pool is acutely synthesised upon flagella amputation and requires the action of TORC1. Importantly, this appears to be an evolutionarily conserved mechanism as cycloheximide exerted the same effect on cilia length in zebrafish (Yuan et al., 2012).
Interestingly, this delicate regulation of TORC1 activity is required in the KV directly, as KV-specific knockdown of Grk5l or overexpression of S6K1 and dominant negative GSK3b, respectively in DFCs all resulted in equally elongated cilia (Yuan et al., 2012; Burkhalter et al., 2013). This is particularly striking, as the KV consists of less than a 100 cells, which basically dictate the position and morphology of all inner organs. Whether the cilium also serves a signalling platform for TORC1 assembly and function, however, remains to be tested. The fact that Grk5l localises to cilia may potentially indicate such integrating function. Moreover, a number of other signalling pathways such as the Sonic Hedgehog (SHH) pathway (Philipp and Caron, 2009) or the PDGF cascade (Schneider et al., 2005) rely on the spatial organisation in cilia. The mTOR pathway would just be another signalling pathway depending on this specialised organelle.
The question that persists, however, is how both, augmentation as well as blockade of TORC1 would have the same morphological outcome, a disturbance in LR asymmetry development. The answer is that there appears to be a certain ‘therapeutic window’ of cilia length that would allow for highest ciliary motility. Motile cilia in the KV beat with a certain frequency and in a circular motion rather than just forward and back. When TSC1a is knocked down or alternatively S6K1 overexpressed cilia still possess a circular beating pattern. However, the mean beat frequency of cilia is reduced in those fish. Similarly, the swimming speed of C. reinhardtii, which depends on flagellar beat frequency, declines with any aberration from a physiological TORC1 activity state (Yuan et al., 2012). In addition to that, rapamycin-mediated inhibition of TORC1 causes not only reduced velocity, but also non-directional fluid flow ultimately leading to a failure in the establishment of bilaterality. In the opposite case, when TORC1 is more active, the direction of the flow is not disturbed. However, it appears to be too slow to faithfully induce laterality in zebrafish (Yuan et al., 2012). Another reason how deregulated TORC1 leads to asymmetry defects may also lie within an impairment of KV cilia to sense molecular cues. SHH signalling for instance relies not only on properly formed cilia (Philipp and Caron, 2009), but has been linked to LR asymmetry (Levin et al., 1995). Thus, it would be important for further studies to analyse which downstream cascades are perturbed in ciliated cells with altered TORC1 activity. Moreover, so far we do not know, if other mTOR-dependent processes such as autophagy would be relevant for LR development (Figure 2). Last, but not least it remains unclear whether cells with deranged TORC1 affects the ultrastructure of cilia such as the inner or outer dynein arms of the microtubule doublets, which are indispensable for proper cilia function and hence physiology (Zariwala et al., 2011).
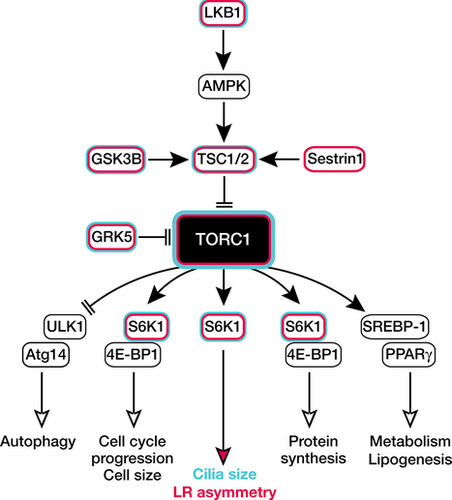
Key players of the mTOR complex1 cascade in LR asymmetry
Cartoon depicting a simplified model of the TORC1-dependent signalling pathway and its physiological outcome. Proteins, which have been associated with LR asymmetry are lined in red, whereas those that were shown to alter cilia size are additionally lined in blue. LKB1, liver kinase B1; AMPK, AMP-activated protein kinase; TSC1/2, tuberous sclerosis 1/2; GSK3B, glycogen synthase kinase 3B; TORC1, target of rapamycin complex 1; GRK5, G protein-coupled receptor kinase 5; ULK1, unc-51 like autophagy activating kinase 1; Atg14, autophagy related 14; S6K1, S6 kinase 1; 4E-BP1, Eukaryotic Translation Initiation Factor 4E Binding Protein 1; SREBP-1, Sterol Regulatory Element-Binding Protein 1; PPARγ, peroxisome proliferator-activated receptor gamma.
mTOR and situs anomalies in patients
How do these data from zebrafish relate to patients with an asymmetry-driven CHD? We have found three case reports of tuberous sclerosis patients, which also had situs anomalies (Neumann et al., 1995; Rallis et al., 2012; Meyer et al., 2013). This may be a coincidence, but both conditions are nevertheless likely to be connected. This assumption is based on the fact that a large number of tuberous sclerosis cases are due to a mutation in TSC1 or TSC2 (Jones et al., 1999; Niida et al., 1999; Dabora et al., 2001; Yamamoto et al., 2002), which again are important inhibitors of mTOR signalling.
Furthermore, if TSCs would serve as susceptibility genes in heterotaxy and thus CHD, other mTOR regulators might do so, too. As a matter of fact, a de novo translocation was found in a patient with dextrocardia and atrioventricular septal defect. This chromosomal translocation led to a breakpoint in the first intron of the SESTRIN1 gene (Peeters et al., 2003). Fibroblasts of this patient displayed a marked reduction of sestrin1, which was identified as an inhibitor of TORC1 upstream of TSC proteins (Budanov and Karin, 2008). Similar to other patients with unrestrained TORC1, this patient suffered from neurological abnormalities such as intellectual disabilities (Kato et al., 1996). Although those could be caused by a plethora of molecular cascades, additional similarities with Bannayan–Riley–Ruvalcaba syndrome were apparent such as hypotonia as well as the inability to speak and walk. Bannayan–Riley–Ruvalcaba syndrome is caused by mutations within the PTEN gene (Marsh et al., 1997), which in turn negatively regulates TORC1 (Sansal and Sellers, 2004). Since the available clinical data on SESTRIN1 patient is rather sparse, the connection between a potential abnormal TORC1 activity and LR asymmetry development must be drawn with great caution. However, when tested in zebrafish, it turned out that Sestrin1 does indeed function in LR asymmetry formation: Knockdown of Sestrin1 in zebrafish produced a phenotype of heart looping defects and randomised pancreas localisation (Peeters et al., 2006).
Conclusions
The establishment of LR asymmetry is one of the first key steps in embryonic morphogenesis and faithful heart development. Failure in symmetry breaking by any means results in serious complications, most of all in CHD. Although the intricate details of this process have not been fully understood, yet, it has become clear that at least in zebrafish fine-tuning of TOR signalling comprises a crucial prerequisite for it.
Funding
We would like to thank all funding bodies which have continuously supported our lab very generously: Marie Curie Reintegration Grant of the European Commission (268333), the Deutsche Stiftung für Herzforschung (F/09/11), the Boehringer Ingelheim Ulm University BioCenter and the Deutsche Forschungsgemeinschaft (PH 144/1). T.C.T. is a fellow of the International Graduate School in Molecular Medicine, which is funded by the German Excellence Initiative. We apologise to all colleagues whose work could not be cited due to space limitations.
Conflict of interest statement
The authors have declared no conflict of interest.