A phase II study of zandelisib in patients with relapsed or refractory indolent non-Hodgkin lymphoma: ME-401-K02 study
ClinicalTrials.gov identifier: NCT04533581.
Summary
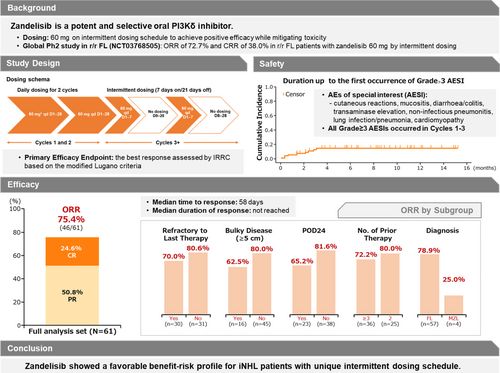
Zandelisib, a selective, potent PI3Kδ inhibitor, demonstrated favourable outcomes in patients with relapsed or refractory follicular lymphoma in a global phase II study. This phase II study evaluated the efficacy and safety of zandelisib for relapsed or refractory follicular lymphoma or marginal zone lymphoma. Sixty-one patients received zandelisib orally at 60 mg daily continuously in the first two 28-day cycles, followed by intermittent dosing on Days 1–7 following each cycle until progressive disease or unacceptable toxicity. Objective and complete response rates were 75.4% (95% confidence interval [CI], 62.7%–85.5%) and 24.6% (95% CI, 14.5%–37.3%) respectively. Median time to response was 58 days; 70.5% (43/61) of patients achieved their first response by Week 8. At least one Grade ≥ 3 treatment-emergent adverse event (TEAE) occurred in 55.7% of patients: transaminase elevation (8.2%); cutaneous reactions (3.3%); and diarrhoea, enterocolitis and lung infection (1.6% each), defined as adverse events of special interest. The discontinuation rate due to any TEAE was 14.8%. No zandelisib-related death occurred. Zandelisib showed favourable efficacy and tolerability in Japanese patients with relapsed or refractory indolent non-Hodgkin B-cell lymphoma. This unique dosing schedule may maintain efficacy while mitigating the safety issues observed with other PI3Kδ inhibitors (ClinicalTrials.gov number, NCT04533581).
Graphical Abstract
Zandelisib, a selective, potent PI3Kδ inhibitor, demonstrated favourable outcomes in patients with relapsed or refractory follicular lymphoma in a global phase II study. This phase II study evaluated the efficacy and safety of zandelisib for relapsed or refractory follicular lymphoma or marginal zone lymphoma by a unique intermittent dosing schedule to mitigate its on-target toxicities. The objective response rate was 75.4%, with a 24.6% complete response rate. The cumulative incidence of Grade ≥ 3 AEs of special interests reached a plateau after cycle 3. Zandelisib showed a favourable benefit–risk profile for patients with relapsed or refractory indolent non-Hodgkin lymphoma.
INTRODUCTION
Follicular lymphoma (FL) and marginal zone lymphoma (MZL) are the two most common types of indolent B-cell non-Hodgkin lymphoma (iB-NHL).1 Both are heterogeneous and incurable and are treated based on the patient's condition through several relapses, leading to a decline in response and survival rates over time.2-6 Recently, novel treatment options (e.g. anti-CD19 CAR-T therapy, EZH2 inhibitor and bispecific antibodies) have been approved for relapsed or refractory (r/r) FL/MZL.7 However, most patients with FL/MZL relapse even when treated with these, so new treatment options for r/r FL/MZL are needed.
Phosphatidylinositol 3-kinase (PI3K) is a lipid kinase with a catalytic subunit that has four different isoforms (α, β, γ and δ) and is involved in the mobilisation and activation of multiple intracellular enzymes that regulate cell proliferation, survival and motility. In particular, PI3Kδ is highly expressed in lymphocytes and plays an essential role in malignant B-cell function, so PI3Kδ has been considered a potential target molecule for these tumours.8-10
Although several PI3Kδ inhibitors have demonstrated clinically meaningful efficacy for FL, chronic lymphocytic and small lymphocytic leukaemia, these agents have been associated with several on-target class-specific adverse effects, including infectious and autoimmune toxicities such as colitis, pneumonitis and rash,11 in which regulatory T-cell (Treg) dysregulation due to continuous PI3Kδ inhibition may be involved.12 As a consequence, the approvals of all drugs in this class have now been withdrawn for FL/MZL indications.11
Zandelisib (ME-401) is a selective oral inhibitor of PI3Kδ that has been investigated for the treatment of FL or chronic lymphocytic leukaemia/small lymphocytic lymphoma in a phase Ib dose-escalation study (NCT02914938) at three doses (60, 120 and 180 mg once daily) using continuous schedule (CS) or intermittent dosing (ID).13 No dose-limiting toxicities occurred, and the maximum tolerated dose was not reached. The recommended phase II dose was 60 mg based on the safety and pharmacokinetic profile and comparable objective response rate (ORR) at the three doses evaluated. The ORR was comparable between the two dosing schedule groups, and most patients in each group responded after the first two cycles. The safety profile was better in the ID group, with incidences of Grade ≥ 3 treatment-emergent adverse events (TEAEs) and treatment discontinuation rates of 76% and 16% in the CS group and 44% and 10% in the ID group respectively. Such a treatment break in ID dosing is expected to contribute to Treg recovery. These findings led to the investigation of zandelisib monotherapy with ID for patients with r/r FL/MZL in the global phase II study, TIDAL (NCT03768505).14
A phase I study (ME-401-K01, NCT03985189) was conducted to confirm the safety, tolerability and optimal clinical dose of zandelisib in Japanese patients with r/r iB-NHL.15 Nine Japanese patients with r/r FL/MZL received zandelisib on CS (45 and 60 mg) until progression or intolerability. Overall, the efficacy and safety profile of zandelisib in Japanese patients was consistent with that in the previous phase Ib study. In particular, eight of nine patients achieved a response after the first two cycles of administration, without dose-limiting toxicities. Based on these results, it was considered that the administration of zandelisib 60 mg daily for the first two cycles, followed by ID from the third cycle onwards, would achieve positive efficacy while mitigating toxicity. Therefore, the present study (NCT04533581) evaluated the efficacy and safety of zandelisib 60 mg ID in Japanese patients with r/r FL/MZL.
MATERIALS AND METHODS
Study design
This phase II multicentre, open-label, single-arm study was conducted from 17 September 2020 to 2 May 2022 at 30 facilities across Japan. This study adhered to the principles of the Declaration of Helsinki and followed the Pharmaceuticals and Medical Devices Law, the Good Clinical Practice Ministerial Ordinance and its partial amendments. The Institutional Review Board of each institution approved the study protocol and study documents. Before screening, patients provided written informed consent to participate.
Study population
Patients ≥20 years of age with histologically confirmed FL (grade 1–3a), MZL (splenic, nodal and extranodal) or indolent B-NHL not otherwise specified, according to the World Health Organization classification,16 and with ≥2 lines of therapy, including anti-CD20 antibody and alkylating agents, were included. For detailed inclusion and exclusion criteria, see the Supplementary Methods.
Intervention
Each treatment cycle lasted 28 days (Day 1–Day 28) with zandelisib administered (60-mg capsules orally) once daily on a CS during the first two cycles. Cycles 3+ were administered per ID (60-mg capsules orally once daily for the first 7 days, then rest for the following 21 days) (Figure S1).
Study drug administration was continued until disease progression or intolerability. Safety was managed with dose interruption, but an absence from medication for >8 consecutive weeks led to discontinuation.
If a patient experienced PD during ID, based on the Lugano Classification lymphoma response criteria17 modified for proper evaluation using computed tomography or positron emission tomography/computed tomography, they could be switched back to CS to recapture disease control. If PD was observed again, the patient was discontinued. Additional methods, including details of Pneumocystis jirovecii pneumonia prophylaxis, appear in the Supplementary Methods.
Study end-points
The primary end-point was the best overall response, defined as the percentage of patients achieving complete response (CR) or partial response (PR). The tumour assessment was performed by the Independent Response Review Committee (IRRC: Calyx, Relias, NC). Best overall response assessment was based on the modified Lugano criteria.17 The secondary efficacy end-points included the duration of response (DOR), progression-free survival (PFS), CR rate and time to treatment failure.
Safety was evaluated based on adverse events (AEs); changes in laboratory values, vital signs and electrocardiography parameters; percentages of AEs of special interest (AESI), that is, cutaneous reactions, mucositis, diarrhoea/colitis, transaminase elevation, non-infectious pneumonitis, lung infection/pneumonia, cardiomyopathy; and time to the first occurrence of AESI expression. AESI were defined based on a past report and prior global phase II study, TIDAL (NCT03768505).11, 14 Other end-points are detailed in the Supplementary Methods.
Statistical analysis
Given the small size of the MZL patient population, the number of patients needed was estimated by considering FL patients after ≥2 lines of therapy as the main patient population. Forty-nine patients were required under the assumption of a threshold response rate of 40%, to an expected response rate of 60% with one-sided significance level of 2.5% and a power of ≥80%. The sample size was then increased to 60 patients to consider for drop-outs and to collect additional safety information. The analysis populations were the safety, full, pharmacodynamics and immune analysis sets (see Supplementary Methods for more details).
RESULTS
Patient characteristics
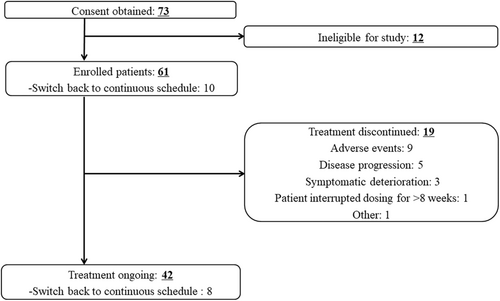
Of the 73 screened patients, 12 were excluded, and 61 were enrolled. Of these, 19 discontinued the study (Figure 1). At the data cut-off date, 42 patients (68.9%) remained in the study.
Patients (N = 61) had a median age of 70.0 years (range: 46–80), 38 (62.3%) were aged >65 years, 36 (59.0%) were male and 54 (88.5%) had an Eastern Cooperative Oncology Group Performance Status of 0 (Table 1). The histopathological diagnosis was FL in 57 (93.4%) patients, and MZL in four (6.6%), 46 (75.4%) had Ann Arbor stage III–IV disease and 16 (26.2%) had a tumour bulk ≥5 cm. Patients had received a median of 3 (range: 2–9) prior lines of therapies, and 30 (49.2%) were refractory to the last treatment received. All patients had received treatment with anti-CD20 antibody and alkylating agents. A total of 29 (47.5%) patients had progression of disease within 24 months of initiating first-line chemoimmunotherapy (POD24).
| iB-NHL patients (N = 61) | |
|---|---|
| Age | |
| Median, years (range) | 70.0 (46–80) |
| Age ≥ 65 years, n (%) | 38 (62.3) |
| Sex, n (%) | |
| Male/female | 36 (59.0)/25 (41.0) |
| ECOG performance status, n (%) | |
| 0/≥1 | 54 (88.5)/7 (11.5) |
| Tumour diagnosis, n (%) | |
| FLa/MZL | 57 (93.4)/4 (6.6) |
| Stage at enrolment, n (%) | |
| I–II | 15 (24.6) |
| III–IV | 46 (75.4) |
| Tumour bulk (largest lymphoid mass), n (%) | |
| ≥5 cm | 16 (26.2) |
| No. prior therapies | |
| Median, n (range) | 3 (2–9) |
| 2/≥3 | 25 (41.0)/36 (59.0) |
| Refractory to last therapy, n (%) | 30 (49.2) |
| POD24, n (%) | 23 (37.7) |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; iB-NHL, indolent B-cell non-Hodgkin lymphoma; MZL, marginal zone lymphoma; POD24, progression of disease within 24 months of initial chemoimmunotherapy.
- a One patient diagnosed with FL at pre-trial examination was found to have MZL at the end-study examination (after the data cut-off).
Study endpoints
Efficacy—Primary and secondary end-points
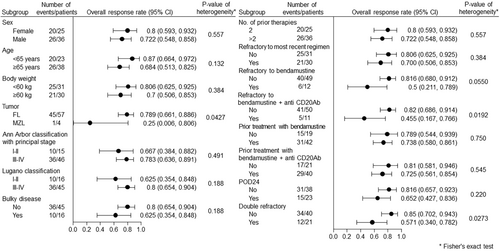
Based on the IRRC review, the ORR was 75.4% (95% confidence interval [CI]: 62.7%–85.5%), and the CR rate was 24.6% (95% CI, 14.5%–37.3%) in the full analysis set (Table 2). The ORR was consistent across all subgroups regardless of prior treatment and poor prognostic factors, including patients with POD24 (65.2%), ≥3 prior lines of therapy (72.2%) and refractory disease (70.0%) (Figure 2).
| All patients (N = 61) | Follicular lymphoma (n = 57) | Marginal zone lymphoma (n = 4) | |
|---|---|---|---|
| Objective response rate, n (%) | 46 (75.4) | 45 (78.9) | 1 (25.0) |
| 95% CI | 62.7–85.5 | 66.1–88.6 | 0.6–80.6 |
| Best overall response, n (%) | |||
| Complete response | 15 (24.6) | 15 (26.3) | 0 |
| Partial response | 31 (50.8) | 30 (52.6) | 1 (25.0) |
| Stable disease | 10 (16.4) | 8 (14.0) | 2 (50.0) |
| Progressive disease | 1 (1.6) | 1 (1.8) | 0 |
| Not evaluable | 4 (6.6) | 3 (5.3) | 1 (25.0) |
| Duration of response, median | Not reached | — | — |
| Progression-free survival, median | Not reached | — | — |
- Note: Best overall response is defined as the best of all overall responses evaluated between the first intermittent dosing administration and switching back to the continuous schedule (2nd continuous schedule). 95% CI: Clopper–Pearson 95% confidence interval.
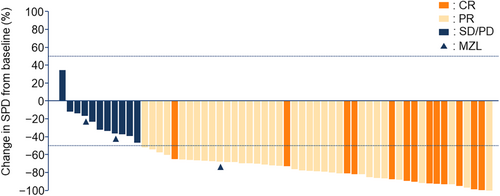
All patients except one had tumour size reductions with zandelisib, and 50 patients showed ≥50% reduction from baseline (Figure 3).
The median time to response was 58.0 (95% CI: 57.0–71.0) days, and 43 patients (70.5%) achieved their first response by Week 8. Eight patients (13.1%) obtained CR by Week 8, and 13 (21.3%) obtained CR by Week 24. The median follow-up duration was 9.5 (95% CI: 8.0–11.1) months, and the median DOR and PFS were not reached (Table 2).
Of the 10 patients who switched back to CS from ID, disease control was achieved in eight patients (80.0%), including one CR (10.0%) and four PRs (40.0%). The response was not evaluable in two patients.
Figure S2 shows the time course of zandelisib treatment for individual patients. Of the 60 patients followed up for at least 6 months, 45 (75.0%) who responded to zandelisib treatment continued the study treatment for ≥6 months.
Safety
At the data cut-off date, the median (range) duration of exposure in the safety analysis set (N = 61) was 7.8 (0.3–15.0) months. TEAEs occurring in ≥10% of patients are listed in Table 3. Any-grade TEAEs occurred in 59 (96.7%) patients, Grade ≥ 3 TEAEs occurred in 34 (55.7%) patients, and these were assessed as related to drug treatment in 28 (45.9%). The most common TEAE, with ≥10% of patients, was neutrophil count decreased (42.6%), followed by diarrhoea (36.1%) and white blood cell count decreased and aspartate aminotransferase increased (14.8% each).
| All grades, n (%) | Grade ≥ 3, n (%) | |
|---|---|---|
| Patients with any TEAE | 59 (96.7) | 34 (55.7) |
| Diarrhoea | 22 (36.1) | 1 (1.6) |
| Nausea | 7 (11.5) | 0 |
| Stomatitis | 7 (11.5) | 0 |
| Pyrexia | 8 (13.1) | 0 |
| Neutrophil count decreased | 26 (42.6) | 17 (27.9) |
| Aspartate aminotransferase increased | 9 (14.8) | 2 (3.3) |
| White blood cell count decreased | 9 (14.8) | 5 (8.2) |
| Alanine aminotransferase increased | 8 (13.1) | 2 (3.3) |
| Blood creatinine increased | 8 (13.1) | 0 |
| Platelet count decreased | 8 (13.1) | 2 (3.3) |
| Hypokalaemia | 7 (11.5) | 1 (1.6) |
| Back pain | 8 (13.1) | 0 |
| Rash | 8 (13.1) | 1 (1.6) |
- Note: Coding Dictionary: MedDRA version 25.0. If a TEAE of the same preferred term and system organ class was recorded for a patient more than once, the patient was counted only once by the maximum grade.
- Abbreviations: MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse event.
In total, 43 (70.5%) patients experienced AESI. The incidence of diarrhoea/colitis was 39.3% (Grade ≥ 3 in 3.3%). Transaminase elevation and cutaneous reaction were observed in 29.5% (Grade ≥ 3 in 8.2%) and 26.2% (Grade ≥ 3 in 3.3%) respectively. Mucositis occurred in 11.5% of patients; no Grade ≥ 3 events were observed. Pulmonary infection/pneumonia occurred in 6.6% (Grade ≥ 3 in 1.6%). There were no cases of non-infectious pneumonitis or cardiomyopathy. Most AESI occurred in the first two cycles, and the expression of AESI reached a plateau after the end of Cycle 3, which was the transition to ID (Figure 4).

During this study, 19 patients discontinued treatment. The main reasons for discontinuation were AE in nine patients (14.8%), including three patients with AESI (two hepatic function abnormal and one erythema multiforme). Five patients discontinued because of PD, and three patients because of symptomatic deterioration. One patient interrupted the study treatment for >8 weeks. One event of sudden death occurred during the study but was not considered to be related to the treatment. A decision was made by the investigator for one patient to discontinue treatment due to the risk of AE recurrence.
In the present study, Grade ≥ 3 events occurred in six patients (9.8%), including two patients that discontinued due to gastroenteritis and cytomegalovirus enteritis. The cytomegalovirus event was treated with antiviral drugs. There were no deaths related to viral infection.
Three of the 10 patients who switched back to CS experienced AESI (two with diarrhoea/colitis and one with cutaneous reaction). Two patients discontinued treatment after switching back because of symptomatic deterioration, not because of AEs.
Exploratory and other endpoints
The proportions of Treg cells expressing Foxp3/CD25 in CD4+ lymphocytes decreased from Cycle 1 to Cycle 3 and then tended to recover after Day 15 of Cycle 3 (Figure S3A). Within the population of lymphocytes, proportions of B cells, T cells (CD3+CD8+ and CD3+CD4+) and NK cells (CD16+CD56+) did not change throughout the treatment period (Figure S3B–E).
Chemokine (C-X-C motif) ligand 13 levels were reduced from baseline by zandelisib administration throughout the study (Figure S4A,B).
DISCUSSION
This phase II trial evaluated the efficacy and safety of zandelisib 60 mg monotherapy with ID in Japanese patients with r/r FL/MZL, showing favourable efficacy and tolerable safety profiles. This suggests that this unique dosing schedule could be promising for managing safety while maintaining efficacy. Zandelisib showed favourable antitumour activity, with an ORR of 75.4% and a CR rate of 24.6%. All patients, except one, had a reduction in tumour size. The median DOR and PFS were not reached with a median follow-up of 9.5 months. High ORRs were maintained even in patients with a poor prognostic feature, such as those who had received >2 lines of prior therapy (72.2%), those with bulky disease (≥5 cm) (62.5%), those with tumours refractory to their last therapy (70.0%) and those with POD24 (65.2%). The safety profile was tolerable, with low discontinuation rates due to AEs (14.8%) and low Grade ≥ 3 AESI rates during ID. Zandelisib with ID was also evaluated in the global phase II study TIDAL, in which 121 patients with r/r FL demonstrated an ORR of 72.7%, CR rate of 38.0% and discontinuation rate due to TEAEs of 17.4%,14 which is consistent with the results of the present study.
While there is a limitation in making cross-trial comparisons, the ORR of zandelisib in this study was 75.4%, compared with other PI3K inhibitors yielding ORRs of around 50%–60% each (e.g. idelalisib at 56.8%,18 copanlisib at 59.2%,19 duvelisib at 47.3%20 and umbralisib at 47.1%21). Zandelisib has a different molecular structure to other PI3K inhibitors, resulting in higher isotype selectivity for PI3Kδ and longer residence in tumours (≥5 h),22 which may explain its favourable efficacy. Most patients (70.5%) treated with zandelisib had their first response by Week 8. The proportion of patients who achieved CR increased from 13.1% at Week 8 to 21.3% at Week 24, indicating that ID did not reduce activity but led to a durable remission. This trend is also suggested by the DOR and PFS results, although these are immature.
The PI3K pathway plays a vital role in cancer biology, and PI3Kδ inhibitors have produced clinical benefits for iB-NHL patients.23-25 However, this drug class has limitations related to fatal and/or serious infections and immune-mediated toxicity, which were defined as AESI in this study.11 The follow-up durations and target diseases differed in each study, but Grade 5 AEs occurred in 4%–6% of patients with iB-NHL receiving idelalisib, copanlisib or duvelisib.18-20 In a study investigating idelalisib, which is the representative of PI3Kδ inhibitors, diarrhoea and/or colitis occurred in 16.0%, hepatotoxicity in 20.8% and infection in 22.4% of cases.18 To mitigate these risks, based on previous findings, the ID method and infection management were implemented in this study and yielded positive outcomes.
Immune-mediated toxicity has been considered to be related to Treg suppression.12 A novel ID administration method was used in a phase Ib study (ME-401-002, NCT02914938), which showed similar efficacy with fewer safety concerns compared to CS dosing. Most patients in the ID and CS groups obtained an objective response after the first two cycles. Based on these data, this unique and novel dosing scheme, starting from CS and then changing to ID, was selected for a phase II study.14
In this study, zandelisib was administered by CS dosing and then ID. The discontinuation rate due to any AEs was low at 14.8%, and a Grade 5 AE occurred in only one patient (sudden death) despite this trial being conducted during the COVID-19 pandemic. The patient who experienced the Grade 5 AE completed 56 days of CS and interrupted treatment on Day 57 because of AEs. The patient died on Day 106. The investigator judged that there was no causal relationship with treatment because the death occurred 50 days after the last dose, and the patient was recovering from the AEs that led to the dose interruption before the death occurred. The incidence of Grade ≥ 3 AESI, such as diarrhoea/colitis (3.3%) and transaminase elevation (8.2%), was also low. Notably, the occurrence of AESI reached a plateau after the third cycle, which is after the transition to ID. Treg levels decreased during the first two cycles with daily administration but tended to recover at Day 15 of Cycle 3, after ID started. Intermittent administration may have contributed to the suppression of immune-mediated toxicity. In recent years, various ID schedules have been used to evaluate PI3K inhibitors, such as once daily for 8 weeks and then once weekly for parsaclisib.26 Although a direct comparison is difficult, the discontinuation rate in this study was lower (14.8% for zandelisib and 29.0% for parsaclisib26), which suggests that optimal ID dosing differs depending on the PI3K inhibitor.
Although there was concern about a decrease in efficacy due to a reduction in dosing intensity after switching to ID, this study suggests that the loss of efficacy after the transition to ID was limited. Continuous reduction of chemokine (C-X-C motif) ligand 13, a cytokine produced by PI3K signalling from tumours, was observed, suggesting that PI3Kδ inhibition in the tumour continues to occur with ID with zandelisib. These results support that ID is both effective and safe and that ID does not reduce efficacy and leads to durable remission.
For idelalisib treatment, the European Medicines Agency has issued comprehensive recommendations for measures to minimise risk of serious infections, including prophylaxis for Pneumocystis jirovecii pneumonia.27, 28 In this study, several recommendations for prophylaxis of infection were implemented in the protocol, including prophylaxis for P. jirovecii pneumonia, granulocyte-colony stimulating factor prophylaxis for neutropenia and monitoring cytomegalovirus and hepatitis B virus. The management of AEs by dose interruption or changing the dosing schedule to ID was also recommended. In the present study, there were two discontinuations caused by infectious events and no deaths related to infections. This shows that, with effective management, the occurrence of infections can be minimised, which is an important aspect for the PI3Kδ inhibitor class of drugs.
This study had several limitations. One was the short follow-up time, with a longer follow-up time needed to estimate the DOR, PFS and long-term safety profiles of zandelisib. Additionally, the number of patients who switched back from ID to CS dosing was small; thus, efficacy and safety of this strategy remains unclear. Finally, this study was a single-arm study without any comparators.
In conclusion, zandelisib showed a high efficacy in Japanese patients with r/r iB-NHL. The safety profile of zandelisib ID was manageable, with a relatively low number of AE-related discontinuations and low rates of AESI and infections. These results indicate that zandelisib could yield a favourable benefit–risk profile for this population.
AUTHOR CONTRIBUTIONS
Wataru Munakata, Miyoko Hanaya, Asuka Matsumoto and Masaaki Kuriki made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; all authors participated in drafting the work, revising it critically for important intellectual content and final approval of the version to be published; and Miyoko Hanaya, Asuka Matsumoto and Masaaki Kuriki agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
Zandelisib is being developed by Kyowa Kirin Co., Ltd., in collaboration with MEI Pharma, Inc. This study was funded by Kyowa Kirin Co., Ltd. The authors thank the patients who participated in this study, as well as their families and caregivers. The authors thank Keyra Martinez Dunn, MD, of Edanz (www.edanz.com), for providing medical writing support. The authors also thank Satoshi Hirano, Yukihiro Nishio and Ayaka Hanaki of Kyowa Kirin Co., Ltd., who provided writing support, and Mitsuhiko Odera of Kyowa Kirin Co., Ltd., the Project Leader of the zandelisib investigational team. Editorial support was provided in accordance with Good Publication Practice guidelines (ismpp.org/gpp-2022).
FUNDING INFORMATION
This research was funded by Kyowa Kirin Co., Ltd., 1-9-2, Otemachi, Chiyoda-ku, Tokyo.
CONFLICT OF INTEREST STATEMENT
WM declares receiving support for the present manuscript (e.g. funding, provision of study materials, medical writing, article processing charges, etc.) from Kyowa Kirin Co., Ltd.; grants or contracts from Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Genmab K.K., Janssen Pharmaceutical K.K. and Nippon Shinyaku Co., Ltd.; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen Pharmaceutical K.K., Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., SymBio Pharmaceuticals Ltd., Mundipharma K.K., Gilead Sciences, Inc., MSD K.K., Amgen K.K., Takeda Pharmaceutical Co., Ltd., Eisai Co., Ltd., Novartis Pharma K.K., AstraZeneca K.K., Genmab K.K., Nippon Shinyaku Co., Ltd. and AbbVie G.K. HG declares receiving support for the present manuscript (e.g. funding, provision of study materials, medical writing, article processing charges, etc.) from Kyowa Kirin Co., Ltd.; grants or contracts from Kyowa Kirin Co., Ltd., Sanofi K.K., Bristol-Myers Squibb K.K. and SymBio Pharmaceuticals Ltd.; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bristol-Myers Squibb K.K., Novartis Pharma K.K., Eisai Co., Ltd., SymBio Pharmaceuticals Ltd., MSD K.K., Kyowa Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Janssen Pharmaceutical K.K., Chugai Pharmaceutical Co., Ltd., AbbVie G.K., and Gilead Sciences, Inc.; and support for attending meetings and/or travel from Sanofi K.K. and Chugai Pharmaceutical Co., Ltd. T Kumode declares receiving payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Ono Pharmaceutical Co., Ltd. and Janssen Pharmaceutical K.K. NF received grants or contracts from AbbVie G.K., Chugai Pharmaceutical Co., Ltd., Chordia Therapeutics Inc., Genmab K.K., Incyte Biosciences Japan G.K., Kyowa Kirin Co., Ltd., Loxo Oncology, Inc., and Takeda Pharmaceutical Co., Ltd. as well as payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie G.K., AstraZeneca K.K., Bristol-Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd., CSL Behring K.K., Eisai Co., Ltd., Eli Lilly Japan K.K., Genmab K.K., Janssen Pharmaceutical K.K., Kyowa Kirin Co., Ltd., Nippon Shinyaku Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Sanofi K.K., SymBio Pharmaceuticals Ltd. and Takeda Pharmaceutical Co., Ltd. MS received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Kyowa Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., Pfizer Japan Inc., Astellas Pharma Inc., Nippon Shinyaku Co., Ltd., Ono Pharmaceutical Co., Ltd., MSD K.K., Bristol-Myers Squibb K.K., Asahi Kasei Pharma Corporation, Novartis Pharma K.K., Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Mundipharma K.K., AbbVie G.K., CSL Behring K.K., SymBio Pharmaceuticals Ltd., Janssen Pharmaceutical K.K., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Amgen K.K., Novo Nordisk Pharma Ltd., Nippon Kayaku Co., Ltd., as well as payment for participation on a Data Safety Monitoring Board or Advisory Board from Kyowa Kirin Co., Ltd. TU received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis Pharma K.K., Janssen Pharmaceutical K.K., AbbVie G.K., Nippon Shinyaku Co., Ltd., Meiji Seika Pharma Co., Ltd., Eisai Co., Ltd., Chugai Pharmaceutical Co., Ltd., Asahi Kasei Pharma Corporation, Sanofi K.K., Kissei Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., Kyowa Kirin Co., Ltd., Takeda Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd. and AstraZeneca K.K. YM received grants or contracts from Kyowa Kirin Co., Ltd., Taiho Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., Takeda Pharmaceutical Co., Ltd. and Eisai Co., Ltd.; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen Pharmaceutical K.K.; and payment for expert testimony from Chugai Pharmaceutical Co., Ltd. and Roche Diagnostics K.K. MI received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sumitomo Pharma Co., Ltd., PharmaEssentia Japan K.K., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co., Ltd. and Bristol-Myers Squibb K.K. K Ishizawa declares receiving support for the present manuscript (e.g. funding, provision of study materials, medical writing, article processing charges, etc.) from Kyowa Kirin Co., Ltd.; grants or contracts from AbbVie G.K., Zenyaku Holdings Co., Ltd., IQVIA Services Japan K.K. and Novartis Pharma K.K.; as receiving payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K. and Bristol-Myers Squibb K.K.; and payment for participation on a data safety monitoring board or advisory board from Ono Pharmaceutical Co., Ltd. T Seike, AM and MK are employees of Kyowa Kirin Co., Ltd. T Shimoyama, KK, MT, T Kawakita, MH and K Izutsu have no conflicts of interest to declare in relation to the current work.
ETHICS STATEMENT
The institutional review board of each institution approved the study protocol and study documents.
PATIENT CONSENT STATEMENT
Before screening, patients provided written informed consent to participate.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated and/or analysed will be available in the Vivli repository, https://vivli.org/ourmember/kyowa-kirin/, as long as the conditions of data disclosure specified in the policy section of the Vivli website are satisfied.