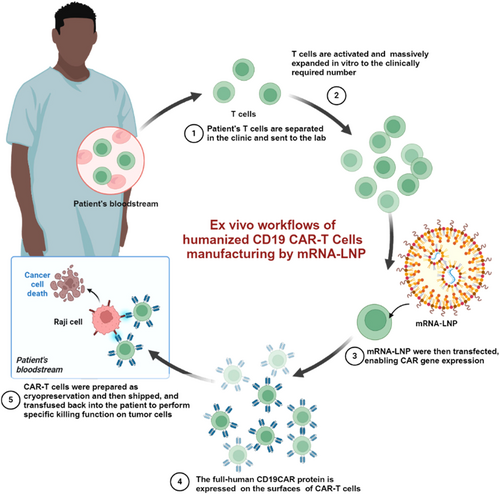
mRNA-laden lipid nanoparticle-enabled humanized CD19 CAR-T-cell engineering for the eradication of leukaemic cells
[Correction added on 14 February 2025, after first online publication: The subcategory has been changed.]
Summary
Chimeric antigen receptor T-cell (CAR-T) therapy has shown transformative potential in treating malignant tumours, with increasing global approval of CAR-T products. However, high-production costs and risks associated with viral vector-based CAR-T cells—such as insertional mutagenesis and secondary tumour formation—remain challenges. Our study introduces an innovative CAR-T engineering approach using mRNA delivered via lipid nanoparticles (LNPs), aiming to reduce costs and enhance safety while maintaining strong anti-tumour efficacy. We developed an LNP-based transfection protocol for efficient delivery of mRNA encoding full-human CAR constructs, achieving high CAR expression and significant cytotoxicity against leukaemic cells in vitro. Co-culture with Raji cells showed increased cytokine secretion and tumour cell killing by mRNA-LNP CAR-T cells. Therapeutic efficacy was further demonstrated in an NOD-scid-IL2Rγnull (NSG) mouse model with Raji engraftment, where treated mice exhibited marked tumour regression and extended survival. These findings underscore the potential of mRNA-LNPs as a non-viral, effective CAR-T engineering platform, offering a promising alternative to traditional methods that could improve CAR-T safety, efficacy and accessibility in clinical cancer immunotherapy.
Graphical Abstract
We introduce a novel CAR-T-cell engineering strategy utilizing mRNA-laden lipid nanoparticles (mRNA-LNPs) to address the challenges of viral vector-based approaches, including insertional mutagenesis and high production costs. Humanized CAR mRNA targeting CD19 was encapsulated into LNPs using microfluidic mixing, achieving efficient transfection and robust CAR expression. Engineered CAR-T cells exhibited potent cytotoxicity against leukaemic cells in vitro, demonstrated increased cytokine secretion and enhanced tumour killing in co-culture systems. In vivo studies in NSG mice with Raji cell engraftment confirmed significant tumour regression and prolonged survival, highlighting mRNA-LNP technology as a safer, scalable alternative for CAR-T therapy.
INTRODUCTION
Chimeric antigen receptor T-cell (CAR-T) immunotherapy has achieved significant breakthroughs in recent years, revolutionizing the treatment landscape for certain haematological malignancies.1 Both the U.S. Food and Drug Administration (FDA) and China National Medical Products Administration (NMPA) have, respectively, approved six CAR-T products for clinical use, targeting CD19+ B-cell malignancies, such as diffuse large B-cell lymphoma (DLBCL) and acute lymphoblastic leukaemia (ALL), and BCMA+ multiple myeloma,2-10 offering hope to patients with relapsed or refractory disease who have limited treatment options.
As research in this area progresses, the potential applications of CAR-T therapy are rapidly expanding beyond haematological cancers.11, 12 Investigators are now exploring its use in non-malignant conditions, with a particular focus on autoimmune diseases such as systemic lupus erythematosus (SLE),13-15 immune thrombocytopenia (ITP),16 rheumatoid arthritis (RA)17 and multiple sclerosis (MS).18 Preclinical and early clinical studies suggest that CAR-T cells could be engineered to target and eliminate autoreactive immune cells, potentially offering a long-lasting therapeutic effect for patients with refractory and relapsed autoimmune disorders. The evolving landscape of CAR-T therapy is thus poised to broaden its impact across a wide range of diseases, heralding a new era of precision immunotherapy.
Viral-based transduction is the most common CAR transgene alternation approach, accounting for 94% of the assessable products, with a majority of 53.3% utilizing lentiviral vectors.19 At present, all CAR-T products that have gained regulatory approval and reached the commercial market are developed using lentiviral transduction technology. This method involves the introduction of a CAR gene into T cells via lentiviral vectors, which integrate into the host genome, leading to stable CAR expression.20 Lentiviral transduction has been a cornerstone in the production of CAR-T cells due to its high efficiency and ability to achieve long-term gene expression. Nevertheless, one limitation of lentiviral vectors is their potential to cause insertional mutagenesis, as they integrate randomly into the host genome, potentially disrupting essential genes or activating oncogenes.21, 22 According to reports, the FDA has required additional black box warnings to be added to the labels of the six approved CAR-T-cell therapy products. The FDA believed that all CAR-T-cell therapies targeting BCMA or CD19 that had been approved for marketing carry the risk of secondary T-cell malignancies.23 As of 31 December 2023, a total of 22 cases of T-cell malignancies have been reported in patients treated with CAR-T-cell therapies.24 These malignancies include T-cell lymphoma, T-cell large granular lymphocytic leukaemia, peripheral T-cell lymphoma and cutaneous T-cell lymphoma, and the reported cases involve five out of the six currently marketed CAR-T products. Another study systematically investigated a patient who developed secondary CD4+ T-cell indolent lymphoma following treatment with Cilta-cel CAR T-cell therapy.25 Targeted mRNA sequencing revealed the presence of CAR mRNA within the tumour cells, and the malignant T cells exhibited monoclonal expansion. This clone was detected in biopsy samples and peripheral blood after treatment. Further whole-genome sequencing identified the insertion of the CAR construct within the second intron of the SSU72 gene in this clone.25 Therefore, we have concerns about the potential insertion of lentivirus as a carcinogenic risk that could cause CAR-T-cell-derived secondary malignancies.
Ongoing research is exploring alternative approaches, such as non-viral delivery vectors to further improve the safety, scalability and cost-effectiveness of CAR-T-cell manufacturing. mRNA-LNP technology represents a ground-breaking advancement in the field of molecular medicine, particularly in the areas of vaccine development and gene therapy.26, 27 In the era of the COVID-19 pandemic, mRNA-LNP-based vaccines have achieved remarkable and unprecedented success.28-30 This platform utilizes LNPs to encapsulate and protect mRNA, facilitating its delivery into target cells. Once inside the cell, the mRNA is translated into proteins, allowing for the expression of therapeutic or immunogenic proteins that can either treat diseases or elicit immune responses, as seen in mRNA-based vaccines.31 The key advantages of mRNA-LNP technology include its ability to induce rapid and potent protein expression without the need for viral vectors, which avoids the risk of insertional mutagenesis. LNPs are crucial for stabilizing the otherwise fragile mRNA, enhancing its cellular uptake and ensuring its release into the cytoplasm.
In the context of cancer immunotherapy, mRNA-LNP is being explored for the development of personalized cancer vaccines and as a tool for immune cell engineering. The flexibility, rapid production and scalability of mRNA-LNP systems make them a promising platform for future innovations in precision medicine.27 In this study, we encapsulated full-human CAR mRNA targeting CD19 into mRNA-LNP using microfluidic mixing devices and optimized the LNP-based transfection protocol to efficiently deliver mRNA into T cells, resulting in robust expression of the CAR molecules on the cell surface. The engineered CAR-T cells were then evaluated for their ability to eradicate tumour cells in vitro and in vivo.
METHODS
Cell lines and cell culture
The human cell lines Raji, Nalm6, Daudi and K562 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The Raji-luciferase and Nalm6- luciferase cell lines were purchased from Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (Shanghai, China). The identities of the cell lines were confirmed using short tandem repeat (STR) DNA sequencing analysis. The cell lines were maintained in 1640 Roswell Park Memorial Institute (RPMI) medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and 1% penicillin–streptomycin (Sigma-Aldrich, St. Louis, MO, USA). Cultures were incubated at 37°C with 5% CO2 and were regularly tested for mycoplasma contamination.
Human primary CD3+ T-cell isolation and expansion
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors by density gradient centrifugation, and CD3+ T cells were isolated by using positive magnetic selection (human CytoSinct™ CD3 Nanobeads, Genescript, Nanjing, China). The anti-CD3 monoclonal antibody (OKT3, 5 μg/mL, Biolegend, San Diego, CA, USA) and anti-CD28 monoclonal antibody (CD28.2, 5 μg/mL, Biolegend, San Diego, CA, USA) were precoated in 48-well plate overnight at 4°C to activate CD3+ T cells for 48 h. The activated CD3+ T cells were cultured in the X-VIVO15 medium (Lonza, Basel, Switzerland) containing recombinant human IL-2 (50 ng/mL, T&L Biotechnology, Beijing, China).
In vitro synthesis of CAR mRNA
The CAR mRNA was produced by in vitro transcription methods according to One-pot mRNA Co-transcription Kit (Bsa I) (Jiangsu Synthgene biotechnology Co., Ltd., Nanjing, China). The structure of DNA template contains a T7 promoter, 5′-untranslated regions (UTR) (AGGTCCAACACAACATATACAAAACAAACGAATCTCAAGCAATCAAGCATTCTACTTCTATTGCAGCAATTTAAATCATTCTTTTAAAGCAAAAG), 3′-UTR (GCTGCAGAATTCGTCGACG GATCCGATCTGGTACTGCATGCACGCAATGCTAGCTGCCCCTTTCCCGTCCTGGGTACCCCGAGTCTCCCCCGACCTCGGGTCCCAGGTATGCTCCCACCTCCACCACCTCTGCTAGTTCCAGACACCTCCCAAGCACGCAGCAATGCAGCCAAAACGCTTAGCCTAGCCACACCCCCACGGGAAACAG), and a 3′-polyA tail with a length of 112 bp. Briefly, the plasmid DNA encoding humanized second-generation CD19 CAR bearing CD3ζ and 4-1BB costimulatory domains was used as the starting template and the linearizing enzyme (BsaI), nucleotide/modified nucleotide (ATP, CTP, GTP and N1-Me-pUTP), cap analogues (CAP GAG) were coculture in 37°C for 1 h. Subsequently, once the reaction mixture returned to room temperature, T7 RNA polymerase was added, and the reaction was allowed to proceed at 37°C for 2–3 h. Upon completion, DNase I (RNase-free) was added, followed by incubation at 37°C for 15 min to degrade the template DNA. The transcribed RNA was then purified using RNA Cleaner magnetic beads.
Preparation and characterization of mRNA-LNP
mRNA-LNP was prepared by microfluidic mixing technique according to previous study.32-34 To prepare the aqueous phase, the mRNA was dissolved in a citrate-citric acid aqueous solution (50 mmol/L, pH 4.0) at a concentration of 33.3 μg/mL. To prepare the ethanol phase, O-(Z,Z,Z,Z-heptatriaconta-6,9,26,29-tetraen-19-yl)-4-(N,N-dimethylamino) (D-LIN-MC3-DMA) (Avanti, Alabaster, Alabama, USA), 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) (MedChemExpress, Shanghai, China), 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) (Ponsure Biotechnology, Shanghai, China), cholesterol (Sigma, St. Louis, Missouri, USA) were combined at a molar ratio of 50%, 10%, 1.5% and 38.5% respectively. The lipid-ethanol solution and the mRNA-citrate buffer were filtered through a 0.22-μm membrane filter. 1 millilitre volume of ethanol phase and 3 mL of the mRNA aqueous phase were drawn separately into syringes. These were subsequently mixed using a microfluidic chip (NEXSTAR, Shanghai, China), with the organic and aqueous phases combined at a 1:3 ratio (ethanol to buffer) and flow rates of 3 and 9 mL/min, respectively, to produce mRNA-LNPs. After sample collection, 15 volumes of PBS were immediately added to dilute the ethanol concentration to below 1%. Ultrafiltration was then performed using a 30-kDa ultrafiltration tube (Milipore, Billerica, Massachusetts, USA) at 3000 g for 20 min. The final obtained mRNA-LNP was stored in PBS containing 10% sucrose and frozen at −80°C.
The encapsulation rate of mRNA-LNP was determined according to RiboGreen RNA quantitative detection reagent kit (Solarbio, Beijing, China).
The dynamic light scattering (DLS), polydispersity index (PDI) and zeta potential of mRNA-LNPs were measured by NanoBrook ZetaPlus (Brookhaven Instruments, Holbrook, New York). The particle size distribution was also detected by nano-flow cytometry (NanoFCM, Xiamen, China). The morphology of mRNA-LNPs was observed using transmission electron microscopy (TEM) (H-7000FA, Hitachi, Japan) after placing a drop of NP solution on the copper mesh and staining with 0.2% (w/w) phosphotungstic acid for 60 s. The stability was investigated by monitoring particle size and PDI during 3 weeks of storage in 10% sucrose + PBS at 4°C.
Cellular uptake and lysosomal escape experiments
To visualize LNP-mediated mRNA delivery to cells, the mRNA was labelled with 5-aminofluorescein (FAM). Human primary T cells were seeded in glass bottom confocal dishes. Subsequently, the FAM-mRNA-LNPs were added into the dishes. At predetermined times, the cells were washed three times with PBS to remove free LNPs, stained with Lysotracker Red (Beyotime Biotechnology, Shanghai, China) for 30 min, fixed with 4% paraformaldehyde (Servicebio, Wuhan, China), and then stained with DAPI (Servicebio, Wuhan, China). The cells were washed with PBS three times, and eventually, the cells were observed using Dragonfly/CR-DFLY-201-40 turntable laser confocal microscopy (Andor Technology, UK). Additionally, we further quantified the fluorescence intensity using flow cytometry (BD, Franklin Lakes, New Jersey, USA).
In vitro cell cytotoxicity assay
The cytotoxicity of mRNA-LNPs to human primary T cells was evaluated by CCK-8 kit (MedChemExpress, Shanghai, China). The cells were seeded in 96-well plates, treated with various concentrations of LNPs for 24 h and then incubated with 10 μL/well CCK-8 agents for 1–4 h at 37°C. Finally, the absorbance values (optical density, OD) were measured at 450 nm by a microplate reader (Bio Tek, Winooski, Vermont, USA).
In vitro transfection experiments
The isolated human primary T cells were activated according to above protocols for 48 h, and subsequently seeded in a six-well plate at 5 × 105 cells/well. Then, these cells were incubated with mRNA-LNPs for 24 h. At determined time points, the dramatic changes of the transfection efficiency of CD19 CAR mRNA were measured with APC-labelled human CD19 protein (ACROBiosystems, Beijing, China) for 30 min in room temperature and washed with PBS for three times. Then, the CAR-positive rate was detected by flow cytometry (BD, Franklin Lakes, New Jersey, USA).
In vitro proliferation assay performed with CAR-T cells
mRNA-LNP-based CAR-T cells were labelled with CytoTellTM Red 650 (AAT Bioquest, Sunnyvale, California, USA) and then mixed with Raji or K562 cells. At selected time points, cells were analysed on a flow cytometer.
Degranulation assay
1 × 105 target cells of Raji, Nalm6, Daudi, K562 and 1 × 105 mRNA-LNP-based CD19 CAR-T cells were suspended in 200 μL of X-VIVO 15 without IL-2 and co-cultured in a U-shaped 96-well plate. Then, CD107a-FITC antibody (BioLegend, San Diego, CA, USA) (5 μL/well) were added into the wells, followed by incubation at 37°C in 5% CO2 for 4 h. When incubated to 1 h, BFA solution (2 μL/test; containing 1:1000 Monensin from MedChemExpress and 1:1000 Brefeldin A from MedChemExpress) was added and the co-culture systems were continued for 3 h. Subsequently, these cells were collected and washed with PBS for three times and stained by CD8-PerCP antibody for 30 min. Finally, the samples were detected by flow cytometry (BD, Franklin Lakes, New Jersey, USA).
In vitro cytotoxicity evaluation
Lactate dehydrogenase (LDH) release assay and bioluminescence imaging were performed to measure cytotoxicity of CAR-T cells. The CAR-T cells prepared by transfecting primary T cells with mRNA-LNP were co-incubated with CD19+ Raji cells, Nalm6 cells and CD19− K562 cells in 96-well plates for 24 h at different effector/target ratios. Subsequently, the cell culture plates were centrifuged at 400 g for 5 min, and 120 μL of supernatant was collected from each well. The medium supernatants were collected and measured immediately for LDH activities following the manufacturer's instructions (Beyotime Biotechnology, Shanghai, China). The percentage cytotoxicity was calculated according to the following formula: Cytotoxicity % = 100 × [((CAR-T cell + target cell) − (CAR-T cell alone + target cell alone))/(max target cell lysis − target cells alone)]. The bioluminescence signal was quantified by detection of the radiance in the region of interest (ROI) with an IVIS Spectrum in vivo imaging system (IVIS Spectrum, PerkinElmer, Waltham, Massachusetts, USA).
Viable and dead cell observations
In order to visually assess cellular viability after treating with the various doses of mRNA-LNPs, the Calcein/PI cell viability assay kit (Beyotime Biotechnology, Shanghai, China) was used according to the manufacturer's protocol. Cells were incubated with different doses for 24 h and washed with PBS. Following that, cells were incubated with calcein AM and PI solutions for 30 min at 37°C, then observed with a fluorescence microscope (Olympus, Tokyo, Japan).
Labelling-free living cell dynamic observation
The CAR-T cells prepared by mRNA-LNP and Raji cells were co-cultured in a glass bottom confocal dish at a ratio of 1:1, and non-engineered human primary T cells were incubated with Raji cells as control group. Then, these cells were dynamically observed with a tomographic microscope (3D Cell Explorer, NanoLive, Switzerland).
3D spheroid cell model
The 3D spheroid Raji cell models were generated according to SpheroX™ 96Ukit cell sphere culture 96U plate set (EFL, Suzhou, China). Briefly, the well plate is first treated by adding 100 μL anti-adhesion coating solution to each hole of the SpheroX 96Ukit well-plate and then placing the plate in an incubator at 37°C overnight. Finally, after discarding the coated solution, 200 μL of Raji cell suspension labelled by carboxyfluorescein diacetate succinimidyl ester (CFSE) was added to each well (2 × 104 cells/well). Raji cells were allowed to form spheroids by incubating the plates at 37°C and 5% CO2 for 48 h.
To assess specific binding and infiltration of mRNA-LNP-based CD19 CAR-T cells, after the formation of the 3D spheroid Raji cell models, the CytoTell™ Red 650 (AAT Bioquest, Sunnyvale, California, USA) labelled CAR-T cells or non-engineered T cells were added and co-cultured with the spheroids for 6 h. Then, wells were washed by PBS for three times to remove free effector cells. Wells were imaged and assessed using a confocal high-content imaging system (ImageXpress Micro Confocal, Molecular Devices, San Jose, California, USA).
To evaluate the specific cytotoxicity of mRNA-LNP-based CD19 CAR-T cells towards 3D spheroid Raji cell models, the CFSE-labelled Raji cells were formed into spheroids in the plate, and then, CAR-T and non-engineered T cells were added and incubated for 12 h. Subsequently, 2 μL of PI working solution was added into each well and stained for 15 min at room temperature. Wells were imaged and assessed using a confocal high-content imaging system (ImageXpress Micro Confocal, Molecular Devices, San Jose, California, USA). The 3D tumour spheres were also collected and blown into single-cell suspensions to detect the destruction of Raji cells by flow cytometry (BD, Franklin Lakes, New Jersey, USA).
Cytokine release assay
The CAR-T cells prepared with mRNA-LNP were co-incubated with Raji, Nalm6 and K562 cells in 96-well plates for 24 h at different effector/target ratios. Subsequently, the cell culture plates were centrifuged at 400 g for 5 min, and the supernatant was collected. We used the cytometric bead array (CBA) (BD, Franklin Lakes, New Jersey, USA) to measure the cytokines TNF-α, IFN-γ and IL-2 in the supernatant.
Xenograft mouse B-cell leukaemia models for antitumour efficacy study
Approximately 7- to 8-week-old male NOD-Prkdcscid IL2rgem1/Smoc (M-NSG) mice were purchased from Shanghai Model Organisms Center, Inc (Shanghai, China). After a week of adaptive breeding under pathogen-free conditions, on day −5, mice were intravenously injected with 5 × 105 Raji-luc cells diluted in 200 μL of PBS. The mice were randomized into three study groups 7 days post inoculation and treated with systematic injection of PBS, 1 × 107 mRNA-LNP-based CAR-T cells or 1 × 107 non-engineered T cells on day 7, 14 and 21. Mice were euthanized when body weight loss exceeded 20% of their pre-dosing weight, or animals exhibited signs of severe distress or became moribund.
In vivo bioluminescence imaging
D-Luciferin potassium salt (Meilunbio, Dalian, China) suspended in PBS (15 mg/mL) was injected (150 mg/kg) intraperitoneally 10 min before acquisitions. Tumour burdens were monitored and quantified by an IVIS spectrum in vivo imaging system (IVIS Spectrum, PerkinElmer, Waltham, Massachusetts, USA). Bioluminescence imaging (BLI) signals are presented as the total counts of radiance and acquired by LivingImage software (IVIS Spectrum, PerkinElmer, Waltham, Massachusetts, USA).
Histological staining
For H&E staining, major tissues including hind limb, liver and spleen were collected and washed three times with PBS, fixed in 4% paraformaldehyde and embedded in paraffin. The H&E staining of organs was performed according to standard procedures and observed with a microscope (Olympus BX41, Tokyo, Japan). For immunohistochemical (IHC) staining, these tissues were embedded in OCT and cut into 8 μm sections, followed by stained with anti-CD19 antibodies (ABclonal Biotechnology, Wuhan, China); then, these sections were incubated with horseradish peroxidase (HRP)-labelled secondary antibody and react with DAB under specific conditions to form brown precipitates and finally observed under a microscope (Olympus BX41, Tokyo, Japan).
Statistical analysis
All experimental data are presented as the mean ± standard deviation (SD) (n = 3–5). All data were statistically analysed and visualized using GraphPad Prism software (version 8.0.2, San Diego, California, USA). All experiments were repeated independently at least twice. All comparisons between two groups were performed using either a two-tailed unpaired Student's t-test or the Mann–Whitney U-test. Comparisons between more than two groups were performed by one-way analysis of variance (ANOVA), two-way ANOVA and Tukey's multiple comparisons test. Kaplan–Meier curves were used to estimate the survival rates. A p-value of less than 0.05 was considered to indicate a statistically significant difference (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns: not significant).
RESULTS
Synthesis and characterization of mRNA-LNPs
First, we synthesized the mRNA of second-generation full-human CD19 CAR bearing CD3ζ and 4-1BB costimulatory domains (Figure 1A). To improve the delivery of CAR mRNA molecules to human primary T cells, we encapsulated CAR mRNA into mRNA-LNPs by microfluidic mixing devices (Figure 1B). Transmission electron microscopy (TEM) revealed that the obtained nanoparticles exhibited a uniform spherical morphology with a diameter of approximately 80 nm (Figure 1C), in agreement with the results of size distribution detected by nanoflow meter (Figure 1D).
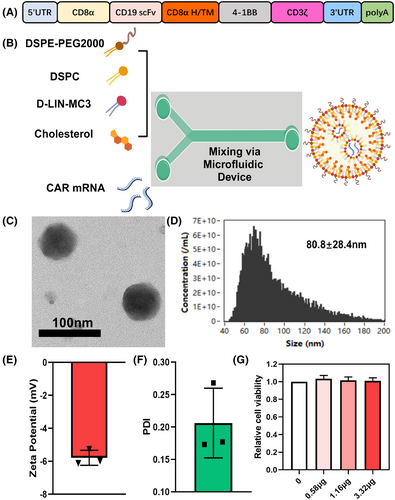
We also measured the hydrodynamic diameter distribution of these LNPs by DLS, the average size was 88.20 ± 4.038 nm (Figure S1A,B), which was slightly larger than the particle size observed by TEM, possibly due to the formation of a hydration film by PEG on the LNP surface. In addition, a distinct Tyndall effect was observed in mRNA-LNP solutions upon exposure to a 680-nm laser (Figure S2). The zeta potential of mRNA-LNPs approached −6 mV at pH 7.4, indicating a negative surface charge (Figure 1E). The PDI of mRNA-LNPs was 0.206 ± 0.0537 (Figure 1F), suggesting the size of these LNPs was relatively uniform and concentrated. The encapsulation efficiency of mRNA-LNPs was 97.33 ± 2.082% in Day 0 (Figure S1C). Also, the stability of the LNPs was evaluated. As shown in Figure S1C–E, these particles remained stable in 10% sucrose + PBS, with negligible encapsulation rate, size and PDI differences, for 3 weeks at 4°C, which demonstrated that these mRNA-LNPs were of excellent stability. In order to evaluate the viability of human primary T cells after treatment with LNPs, LNPs containing different doses of mRNA were incubated with T cells for 24 h and compared with the PBS-treated control group, and we found no difference in cell activity among all groups (Figure 1G). Live & dead analysis was also performed to evaluate the biocompatibility of LNPs, and no significant cell death was found under the current doses (Figure S3). Taken together, these findings suggest that the synthesized mRNA-LNPs demonstrate distinguished performance and hold significant potential for mediating gene delivery of CAR.
Cellular uptake of mRNA-LNPs in human primary T cells
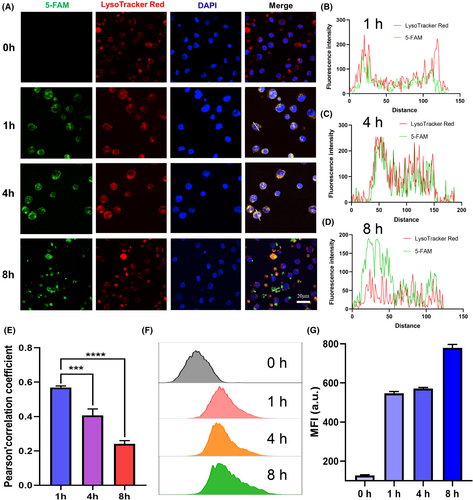
Subsequently, we used FAM-labelled mRNA to visualize its cellular uptake and intracellular behaviours in human primary T cells. We observed that FAM-labelled mRNA was internalized by T cells, exhibiting strong green fluorescence at 0, 1, 4 and 8 h (Figure 2A). Additionally, late endosomes and lysosomes were labelled with LysoTracker Red, followed by fluorescence colocalization analysis of FAM-RNAs with LysoTracker Red-labelled endo/lysosomes. As shown in Figure 2B–D, the colocalization between the green fluorescence of FAM and the red fluorescence of endo/lysosomes progressively diminished with extended incubation time, which was confirmed by quantitative analysis of Pearson's correlation coefficient (Figure 2E), demonstrating the successful escape of mRNA-LNPs from the endo/lysosomal compartments. We also further quantitatively analysed FAM-mRNA-LNPs at different incubation times by flow cytometry; the results showed that the green fluorescence intensity of the cells increased gradually with the prolong of time (Figure 2F,G). Collectively, these findings indicate that the obtained mRNA-LNPs possess significant potentials for biomedical applications involving the intracellular delivery and subsequent cytosolic release as well as expression of CD19 CAR-mRNA molecules.
mRNA-LNP-mediated CAR expression in T cells
To further confirm the capacity of this LNP-based delivery system to enable CAR expression in human primary T cells, we encapsulated mRNA encoding a full-human second-generation CD19 CAR within LNPs and conducted transfection experiments. T cells were transfected with LNPs containing varying doses of mRNA, and subsequent flow cytometry analysis at 24 h revealed that as little as 0.29 μg of mRNA could induce a CAR positivity rate of 51.7%, with higher mRNA concentrations leading to progressively increased CAR expression levels (Figure 3A). At 48 h, we observed a slight decrease in CAR positive rate at the lower mRNA concentrations of 0.29 and 0.58 μg, while higher concentrations of 1.16 and 3.32 μg resulted in an increase in CAR expression levels (Figure 3A). Thus, we hypothesize that higher mRNA transfection doses require more time to induce a higher CAR positive rate, as sufficient mRNA expression may take longer to achieve compared to lower doses. In addition, flow cytometry analysis at 72 h revealed a substantial decline in CAR positivity across all dose groups. For instance, the CAR positive rates in the two lower dose groups dropped to 28.5% and 40%, while the rates in the two higher dose groups decreased to 47.5% and 60.9% respectively (Figure 3A). To assess the expression kinetics of CD19 CAR mRNA, we performed continuous monitoring at multiple time points and observed a gradual decline in CAR positivity across all dose groups over time. In the dynamic change curve of positive rate of CAR, prior to day 10, the curve showed a steep decline, indicating a rapid reduction in CAR positivity, while after day 10, the curve became more gradual (Figure 3B), suggesting a slower decline in CAR expression. These results correspond to the transient nature of mRNA expression following intracellular delivery. The sharp decrease in CAR positivity during the first 10 days is likely due to the vigorous and rapid proliferation of activated T cells, whereas the slower decline observed later aligns with the reduced rate of T-cell proliferation in the following days.
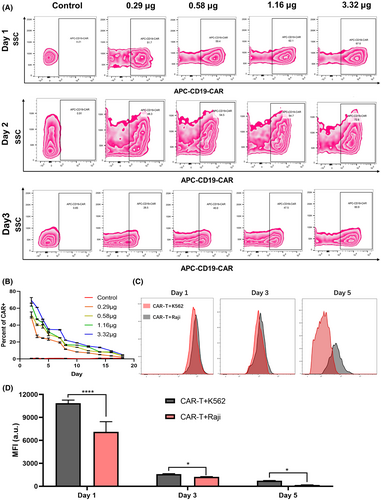
Moreover, we evaluated the proliferative response of mRNA-LNP-based CD19 CAR-T cells following co-incubation with target cells. Flow cytometry results indicated that CytoTellTM Red 650-labelled CAR-T cells exhibited lower mean fluorescence intensity (MFI) after interacting with CD19+ Raji cells compared to CD19− K562 cells (Figure 3C,D). This suggests that, even after activation by anti-CD3 and anti-CD28 antibodies, these CAR-T cells can be further activated upon binding to their corresponding target cells, leading to a stronger proliferative response.
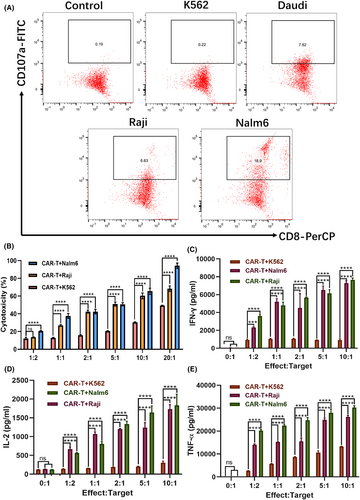
In vitro effector activity of mRNA-LNP-engineered CAR-T cells
In vitro experiments were also conducted to assess the effector function of mRNA-LNP-engineered full-human CD19 CAR-T cells against target cells. We specifically examined their cytotoxicity and cytokine secretion in response to CD19+ target cells, providing insights into the functional capabilities of these CAR-T cells following mRNA delivery. CD107a, also known as lysosomal-associated membrane protein-1 (LAMP-1), plays a key role in cytotoxic T-cell function and is commonly used as a marker of degranulation.35 During the process of target cell killing, cytotoxic T cells release cytolytic granules, which are essential for inducing apoptosis in target cells. When these granules fuse with the plasma membrane, CD107a is transiently expressed on the cell surface. We co-incubated mRNA-LNP-engineered CAR-T cells with CD19+ Daudi, Raji and Nalm 6 cells, using CD19− K562 cells as control group, and assessed CD107a surface expression via flow cytometry. The results demonstrated varying levels of CD107a expression on the surface of CD19+ tumour cells, with Nalm 6 cells showing the highest response. The corresponding CD8+CD107a+ population exhibited a positive rate of 21.0 ± 1.167%, which was significantly higher than that observed in Daudi (7.367 ± 0.2385%) and Raji cells (6.163 ± 0.2368%). In contrast, K562 cells exhibited minimal to no CD107a expression (Figure 4A; Figure S5). Furthermore, the tumour cytotoxicity of mRNA-LNP-engineered CAR-T cells was studied using a lactate dehydrogenase (LDH) cytotoxicity assay and bioluminescence imaging. The experimental results demonstrated that mRNA-LNP-engineered CAR-T cells exhibited stronger cytotoxic activity against CD19+ Raji and Nalm6 cells after 24 h of incubation across various effector-to-target ratios, ranging from 1:1 to 20:1 (Figure 4B). The bioluminescence imaging also displayed that, with the increase of effector–target ratios, the bioluminescence signal of Raji-Luc cells decreased gradually (Figure S6). We also analysed the antitumour cytokine production capacity of the mRNA-LNP-engineered CAR-T cells. The results showed that mRNA-LNP-engineered CAR-T cells stimulated by CD19+ Raji and Nalm6 cells exhibited significantly higher expression of IFN-γ, IL-2 and TNF-α at various effector-to-target ratios ranging from 1:2 to 10:1, compared to those incubated with CD19− K562 cells (Figure 4C–E). Collectively, these experimental findings likely result from the interaction between the CD19 protein on target cells and the mRNA-LNP-engineered CAR-T cells, leading to enhanced tumour cytotoxicity and increased production of antitumour cytokines.
Tumour infiltration and cytotoxicity of mRNA-LNP-engineered CAR-T cells in 3D spheroid models, and dynamic monitoring of killing process
In this study, we further utilized 3D tumour spheroid models to evaluate the infiltration and cytotoxicity of mRNA-LNP-engineered CAR-T cells in a more physiologically relevant tumour microenvironment. As shown in Figure 5A, we successfully prepared 3D spheroid model of Raji cells using a 96-well plate with ultra-low adhesion U-bottom. As shown in Figure 5B,C, after 24 h of co-culture, the fluorescence intensity of CytoTellTM Red 650-labelled CAR-T cells was significantly higher than that of non-engineered T cells, and a substantial number of CAR-T cells infiltrated the inner regions of the green Raji cell spheroids, while non-engineered T cells predominantly remained at the spheroid periphery, with only a few detected in the interior. In addition, the quantitative colocalization analysis of CytoTellTM Red 650 fluorescence intensity in the largest cross section of 3D tumour spheroid models (as outlined with the white lines) also revealed that the red fluorescence of CytoTellTM Red 650-labelled CAR-T cells and CFSE-labelled Raji cells has significant higher overlap, and however, non-engineered T cells overlap to a lesser extent (the plot profile in Figure 5B,C). The cytotoxic effects were also assessed in 3D spheroid model of Raji cells as shown in Figure 5D,E. After 24 h of co-culture, we added PI to detect the dead Raji cells. The results demonstrated that the magenta fluorescence intensity of PI in mRNA-LNP-engineered CAR-T-cell group was obviously stronger than that of non-engineered T cells. Correspondingly, the plot profile of quantitative colocalization analysis further indicated that the magenta fluorescence intensity of PI was of a strong distribution from the edge of the sphere to the inside, whereas the magenta fluorescence alone existed in the spheroid with few cells detected in the edge and interior (the plot profile in Figure 5D,E; Figure S8). Moreover, flow cytometry results showed that 3D tumour spheroids treated with mRNA-LNP-engineered CAR-T cells exhibited an over 4.13-fold increase in dead Raji cells when compared to non-engineered T cells (Figure S7A,B). Additionally, real-time imaging and dynamic monitoring of the killing process were conducted with a tomographic microscope to assess the temporal progression of CAR-T cell-mediated tumour eradication. As shown in Figure 5F and Movies S1 and S2, we selected six representative images at different time points, respectively, for the two groups. mRNA-LNP-engineered CAR-T cells exhibited continuous deformation, movement and migration towards the Raji cells, eventually forming an enclosing cellular cluster. Consequently, the targeted Raji cells began to undergo morphological changes, including vacuolization and cell shrinkage, displaying typical features of cell apoptosis. In contrast, the non-engineered T cells exhibited random movement and failed to form cellular clusters.
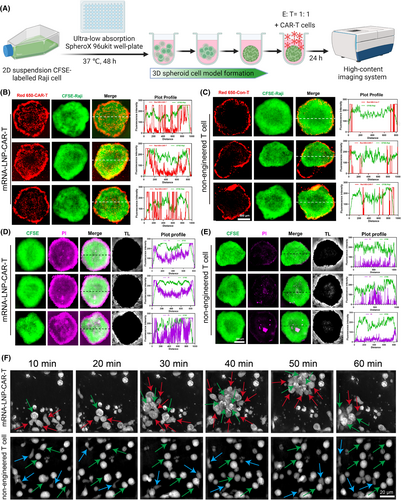
Taken together, for mRNA-LNP-engineered CAR-T cells, our results of penetration and cytotoxic behaviour in such a 3D environment further validated the effectiveness of the LNP delivery system in arming CAR-T-cell function, thereby providing crucial data for more effective preclinical and clinical applications. The dramatic monitoring of killing process towards Raji cells offered insights into the efficiency of cytotoxic activity, further contributing to comprehensively understand the therapeutic potential of mRNA-LNP-CAR-T cells.
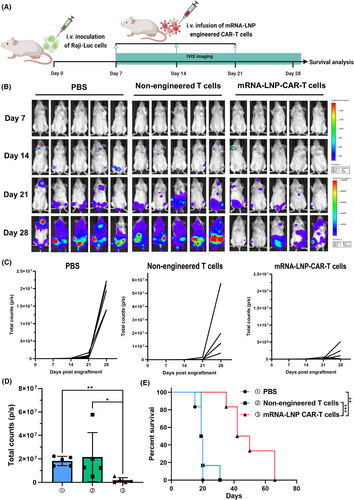
Antitumour activity of mRNA-LNP-engineered CAR-T cells in B-cell leukaemia mouse models
Encouraged by the robust in vitro cytotoxic activity of mRNA-LNP-engineered CAR-T cells, we further examined their antitumour efficacy in a murine model of B-cell malignancy. Immunodeficient NSG mice were intravenously injected with 5 × 105 luciferase-expressing Raji cells. On day 7, the mice with established Raji tumours were randomly assigned to three groups (n = 5 per group). On day 7, 14 and 21, the three groups received intravenous injections of either PBS, non-engineered T cells or mRNA-LNP-engineered CAR-T cells. The tumour growth was monitored by using in vivo bioluminescence imaging with an IVIS system. As shown in Figure 6B,C, Raji tumours in the PBS group grew rapidly, showing that PBS was unable to inhibit tumour growth. In parallel, there was also no apparent tumour growth restriction effect in the non-engineered T-cell group, even though the mice had been administered intermittently three times. It indicated that, although non-engineered T cells exhibited moderate antitumour activity in vitro, they failed to effectively eliminate Raji cells in vivo, resulting in an inability to delay tumour progression. In comparison with the two groups, the mRNA-LNP-engineered CAR-T cells significantly controlled the tumour growth. As shown in Figure 6D, the quantitative statistical analysis of tumour load of mice in different treatment groups on day 28 revealed that these mRNA-LNP-engineered CAR-T cells were effectively trafficked and homed into tumours and exhibit potent killing activity. Moreover, mice treated with mRNA-LNP-engineered CAR-T cells demonstrated significantly extended survival, with 50% of the cohort remaining alive at day 40 (Figure 6E). The mice in each group were dissected and the livers were obtained. We found that the livers of PBS group and non-engineered T-cell group had more Raji tumour infiltrating lesions, while the livers of mice treated with mRNA-LNP-CAR T cells had fewer lesions (Figures S9 and S10). Consistent with in vivo bioluminescence imaging results, histological sections of the liver, spleen and bone marrow from the mRNA-LNP-CAR-T-cell group revealed scarce Raji cells, while massive leukaemic cell infiltration was observed in the PBS and non-engineered T-cell groups (Figures S11 and S12).
DISCUSSION
In this study, we demonstrated the efficacy of an mRNA-LNP-based approach for engineering full-human CD19 CAR-T cells, offering a non-viral alternative to traditional lentiviral vector systems. Our findings highlight the potential of mRNA-LNP technology to overcome key limitations associated with viral-based CAR-T-cell production, such as the risk of insertional mutagenesis and the high costs of manufacturing,21, 22 while maintaining robust antitumour activity both in vitro and in vivo.
The synthesis and characterization of our mRNA-LNP formulations confirmed that these nanoparticles were efficiently taken up by human primary T cells, resulting in rapid and potent CAR expression on the cell surface. This was further corroborated by our in vitro effector activity assays, where mRNA-LNP-engineered CAR-T cells exhibited significant cytotoxicity against CD19+ leukaemic cells, as evidenced by enhanced cytokine secretion and tumour cell killing. Notably, the dynamic monitoring of tumour cell elimination and the capacity of the tumour infiltration and cytotoxicity in 3D cell spheroid models revealed that mRNA-LNP-engineered CAR-T cells could effectively infiltrate the tumour microenvironment and exert cytotoxic effects in a manner comparable to, or even exceeding, that of traditional viral-engineered CAR-T cells. These results underscore the therapeutic potential of mRNA-LNPs as a platform for CAR-T-cell engineering, particularly in complex tumour environments where effective infiltration is critical for therapeutic success.
Importantly, our in vivo experiments using an NSG mouse model engrafted with Raji cells further validated the antitumour efficacy of mRNA-LNP-engineered CAR-T cells. Bioluminescence imaging and survival analysis demonstrated significant tumour regression and prolonged survival in mice treated with these CAR-T cells. These results are in line with previous studies indicating the capacity of mRNA-LNP-based systems to induce robust and sustained therapeutic effects,36, 37 without the integration risks associated with lentiviral vectors. Given the increasing reports of secondary T-cell malignancies linked to viral CAR transduction,24, 25, 38, 39 our non-integrative mRNA-LNP platform offers a safer alternative, eliminating concerns over oncogenic insertional mutagenesis. Clinical trials have typically utilized electroporation (EP) to deliver mRNA for CAR engineering of immune cells.40-43 However, Kitte et al. conducted a comprehensive comparison between conventional EP and LNPs.44 In contrast to EP, the transfection method based on LNPs enhanced the activity of T cells in vitro, prolonged the expression of CAR, exhibited a more gradual proliferation rate and significantly augmented the sustained therapeutic efficacy of CAR-T cells.44
While our study provides compelling evidence for the efficacy of mRNA-LNP-enabled CAR-T cells, several challenges remain. First, the transient nature of mRNA expression, while advantageous in terms of safety, may limit the duration of CAR-T-cell activity. One limitation associated with transient expression is the observed decline in CAR MFI and the percentage of CAR-positive cells over time in our study. This reduction may impact the sustained cytotoxic function of CAR-T cells, especially in scenarios requiring prolonged tumour eradication. Compared to lentiviral stable expression, which ensures long-term CAR expression, transient CAR expression presents both opportunities and challenges for clinical applications. On the one hand, transient expression minimizes the risk of insertional mutagenesis, which could potentially lead to secondary tumour formation, as well as off-tumour toxicities associated with prolonged CAR signalling. It also allows for iterative dosing strategies, offering a dynamic control over CAR-T-cell activity. On the other hand, the need for repeated administrations may pose logistical and economic challenges in clinical settings. Despite these limitations, the transient expression strategy remains highly promising for applications where temporary CAR activity suffices, such as acute control of tumour burden before definitive therapies or in cases requiring CAR-T-cell modulation to minimize adverse effects. Future work should focus on optimizing the balance between transient expression and long-term persistence, potentially through sustained release mRNA delivery systems,45 sequence optimization, self- and trans-amplifying RNA and circular RNA46-48 or enhancing the stability of the LNP formulations. Additionally, although our data demonstrate the feasibility of mRNA-LNP-mediated CAR-T-cell engineering, further studies are needed to assess the scalability and clinical applicability of this approach, particularly in the context of large-scale manufacturing for patient-specific therapies.
In conclusion, our study demonstrates that mRNA-LNP technology offers a promising, efficient and safe alternative to viral vector-based CAR-T-cell engineering. This non-viral platform holds significant potential for improving the safety, scalability and accessibility of CAR-T-cell therapies, thereby advancing the field of cancer immunotherapy. With further optimization and clinical validation, mRNA-LNP-enabled CAR-T cells could play a pivotal role in the next generation of precision immunotherapies for haematological malignancies and beyond.
AUTHOR CONTRIBUTIONS
Conceptualization, H.M.; methodology, Z.C., J.S., A.R. and Y.L.; software, L.L. and J.W.; validation, Z.C., Y.H. and H.M.; formal analysis, Z.C., H.H., and Y.L.; investigation, Z.C.; resources, H.M.; data curation, H.M.; writing—original draft preparation, Z. C and H.M.; writing—review and editing, H.M.; visualization, Z.C., Y.L. and H.H.; supervision, H.M; project administration, H.M.; funding acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
We thank all researchers for their contribution to this article. All the schematic illustrations were created by using the website tool BioRender (https://www.biorender.com/, accessed on 10 Oct 2024).
FUNDING INFORMATION
This work was supported by grants from the National Natural Science Foundation of China (No. 82350103 and 82425003) and the National Key R&D Program of China (No. 2023YFC2507805).
CONFLICT OF INTEREST STATEMENT
All the authors declare no actual or potential conflict of interest with this study.
ETHICS APPROVAL STATEMENT
All mice were housed in a pathogen-free animal facility according to institutional guidelines. All experiments were carried out according to the regulations and standards of the ethics committee of Huazhong University of Science and Technology (approval number: 3893). The authors have received permission to publish the research data from all the participants.
Open Research
DATA AVAILABILITY STATEMENT
We agree to share the publication-related data by e-mailing the corresponding author.