Plasma inflammatory and angiogenic protein profiling of patients with sickle cell disease
Summary
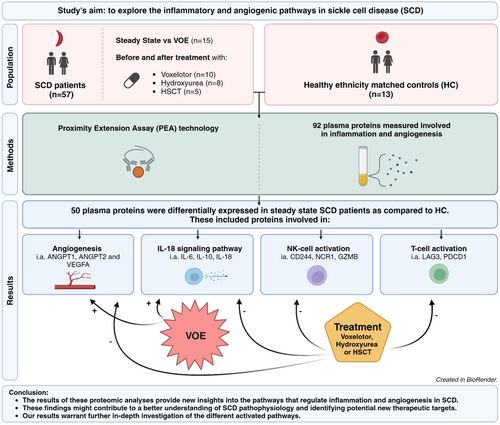
In this study, we aimed to explore the inflammatory and angiogenic pathways in sickle cell disease (SCD). We used proximity extension assay technology (Olink) to measure 92 plasma proteins involved in inflammation and angiogenesis. Plasma samples were collected from 57 SCD patients (sickle cell anaemia/HbS-β0 thalassaemia-thalassaemia) in steady-state and 13 healthy ethnicity-matched healthy controls (HCs). From 15 patients, paired samples were collected during both steady-state and vaso-occlusive episodes (VOEs) and from 23 SCD patients longitudinal samples were collected before and after treatment with either voxelotor (n = 10), hydroxyurea (n = 8) or allogeneic haematopoietic stem-cell transplantation (n = 5). Fifty plasma proteins were differentially expressed in steady-state SCD patients as compared to HC. These included proteins involved in angiogenesis (i.e. ANGPT1, ANGPT2 and VEGFA), the IL-18 signalling pathway (i.e. IL-6, IL-10, IL-18), T-cell activation (i.e. LAG3, PDCD1) and natural killer (NK)-cell activation (CD244, NCR1, GZMB). While proteins involved in angiogenesis and the IL-18 signalling pathway were further upregulated during VOE, levels of several proteins involved in the IL-18 pathway, T-cell and NK-cell activation and angiogenesis, restored towards levels detected in HCs after curative or disease-modifying treatment. These findings might contribute to a better understanding of SCD pathophysiology and identifying potential new targets for therapeutic interventions.
Graphical Abstract
The results of these proteomic analyses reveal 50 proteins involved in inflammation and angiogenesis that are differentially expressed in patients with sickle cell disease in steady state as compared to healthy ethnicity-matched controls. These included proteins involved in angiogenesis (i.e. ANGPT1, ANGPT2 and VEGFA), the IL-18 signalling pathway (i.e. IL-6, IL-10, IL-18), T-cell activation (i.e. LAG3, PDCD1) and NK-cell activation (CD244, NCR1, GZMB). While proteins involved in angiogenesis and the IL-18 signalling pathway were further upregulated during a vaso-occlusive episode, levels of several proteins involved in the IL-18 pathway, T-cell and NK-cell activation and angiogenesis, restored towards levels detected in HCs after curative or disease-modifying treatment.
Abbreviations
-
- ADA
-
- adenosine deaminase
-
- ANGPT1
-
- angiopoietin-1
-
- ANGPT2
-
- angiopoietin-2
-
- ARG1
-
- arginase-1
-
- CA9/CAIX
-
- carbonic anhydrase 9
-
- CASP8
-
- caspase-8
-
- CCL13/MCP-4
-
- C-C motif chemokine 13
-
- CCL17
-
- C-C motif chemokine 17
-
- CCL19
-
- C-C motif chemokine 19
-
- CCL2/MCP-1
-
- C-C motif chemokine 2
-
- CCL20
-
- C-C motif chemokine 20
-
- CCL23
-
- C-C motif chemokine 23
-
- CCL3
-
- C-C motif chemokine 3
-
- CCL4
-
- C-C motif chemokine 4
-
- CCL7
-
- C-C motif chemokine 7
-
- CCL8/MCP-2
-
- C-C motif chemokine 8
-
- CD244
-
- natural killer cell receptor 2B4
-
- CD27
-
- CD27 antigen
-
- CD274/PD-L1
-
- programmed cell death 1 ligand 1
-
- CD28
-
- T-cell-specific surface glycoprotein CD28
-
- CD4
-
- T-cell surface glycoprotein CD4
-
- CD40
-
- tumour necrosis factor receptor superfamily member 5
-
- CD40LG
-
- CD40 ligand
-
- CD5
-
- T-cell surface glycoprotein CD5
-
- CD70
-
- CD70 antigen
-
- CD83
-
- CD83 antigen
-
- CD8A
-
- T-cell surface glycoprotein CD8 alpha chain
-
- CRTAM
-
- cytotoxic and regulatory T-cell molecule
-
- CSF1
-
- macrophage colony-stimulating factor 1
-
- CXCL1
-
- growth-regulated alpha protein
-
- CXCL10
-
- C-X-C motif chemokine 10
-
- CXCL11
-
- C-X-C motif chemokine 11
-
- CXCL12
-
- stromal cell-derived factor 1
-
- CXCL13/MCP-4
-
- C-X-C motif chemokine 13
-
- CXCL5
-
- C-X-C motif chemokine 5
-
- CXCL8
-
- interleukin-8
-
- CXCL9
-
- C-X-C motif chemokine 9
-
- DCN
-
- decorin
-
- EGF
-
- pro-epidermal growth factor
-
- FASLG
-
- tumour necrosis factor ligand superfamily member 6
-
- FGF2
-
- fibroblast growth factor 2
-
- GZMA
-
- granzyme A
-
- GZMB
-
- granzyme B
-
- GZMH
-
- granzyme H
-
- HGF
-
- hepatocyte growth factor
-
- HMOX1
-
- heme oxygenase 1
-
- ICOSLG
-
- ICOS ligand
-
- IFNG
-
- interferon gamma
-
- IL10
-
- interleukin-10
-
- IL12A_IL12B
-
- interleukin-12
-
- IL12RB1
-
- interleukin-12 receptor subunit beta-1
-
- IL13
-
- interleukin-13
-
- IL15
-
- interleukin-15
-
- IL18
-
- interleukin-18
-
- IL1A
-
- interleukin-1 alpha
-
- IL2
-
- interleukin-2
-
- IL4
-
- interleukin-4
-
- IL5
-
- interleukin-5
-
- IL6
-
- interleukin-6
-
- IL7
-
- interleukin-7
-
- IQR
-
- interquartile range
-
- KDR/VEGFR-2
-
- vascular endothelial growth factor receptor 2
-
- KIR3DL1
-
- killer cell immunoglobulin-like receptor 3DL1
-
- KLRD1
-
- natural killer cells antigen CD94
-
- LAG3
-
- lymphocyte activation gene 3 protein
-
- LDH
-
- lactate dehydrogenase
-
- LGALS1/Gal-1
-
- galectin-1
-
- LGALS9/Gal-9
-
- galectin-9
-
- MCV
-
- mean corpuscular volume
-
- MMP12
-
- macrophage metalloelastase
-
- MMP7
-
- matrilysin
-
- NCR1
-
- natural cytotoxicity triggering receptor 1
-
- NOS3
-
- nitric oxide synthase, endothelial
-
- NPX
-
- Normalised Protein eXpression
-
- PDCD1
-
- programmed cell death protein 1
-
- PDCD1LG2/PD-L2
-
- programmed cell death 1 ligand 2
-
- PDGFB
-
- platelet-derived growth factor subunit B
-
- PGF
-
- placenta growth factor
-
- TEK/TIE2
-
- angiopoietin-1 receptor
-
- TGFB1
-
- transforming growth factor beta-1 proprotein
-
- TNF
-
- tumour necrosis factor
-
- TNFRSF12A
-
- tumour necrosis factor receptor superfamily member 12A
-
- TNFRSF21
-
- tumour necrosis factor receptor superfamily member 21
-
- TNFRSF4
-
- tumour necrosis factor receptor superfamily member 4
-
- TNFRSF9
-
- tumour necrosis factor receptor superfamily member 9
-
- TNFSF10/TRAIL
-
- tumour necrosis factor ligand superfamily member 10
-
- TNFSF12/TWEAK
-
- tumour necrosis factor ligand superfamily member 12
-
- TNFSF14
-
- tumour necrosis factor ligand superfamily member 14
-
- VEGFA
-
- vascular endothelial growth factor A
INTRODUCTION
Sickle cell disease (SCD) is marked by chronic haemolysis and vascular occlusion, leading to painful vaso-occlusive episodes (VOEs) and chronic organ damage.1, 2 SCD is also considered a chronic inflammatory disease, in which leukocytes, platelets and endothelial cells are continuously activated.3 Chronic intravascular haemolysis and recurrent ischaemia–reperfusion are the two major driving forces behind chronic inflammation in SCD.4 Excessive haemolysis leads to the release of free haemoglobin, haem and iron, all catalysing the production of reactive oxygen species (ROS). Haemolysis-induced oxidative stress plays a central role in endothelial activation, hypercoagulability and inflammation in SCD.5 Both ischaemia due to vaso-occlusion and reperfusion with the restoration of oxygen supply drive chronic inflammation in SCD by the generation of ROS.6 Hypoxia and chronic inflammation also drive angiogenesis in SCD.7, 8 Angiogenesis contributes to several clinical manifestations of SCD, such as proliferative retinopathy, pulmonary hypertension and moyamoya syndrome.9-11
Earlier studies have shown that haemolysis and ischaemia–reperfusion can activate the NOD-like receptor protein-3 (NLRP3) inflammasome in SCD, leading to the production and secretion of IL-1β and IL-18.12-16 The upregulation of IL-18 can result in the initiation of downstream signalling and induction of other inflammatory proteins such as IL-6, IL-8 and IL-10. Upregulation of this IL-18 pathway has been reported in SCD and is associated with a worse clinical outcome.12, 17, 18 Furthermore, several studies have reported increased levels of proteins involved in angiogenesis in steady-state SCD patients (e.g. ANGPT1, ANGPT2, PGF and VEGFA) and a further increment of VEGFA during VOE.9, 19-24 Although inflammation and angiogenesis seem to play an important role in SCD pathophysiology, a comprehensive evaluation of various activated inflammatory and angiogenic pathways involved in SCD is lacking. Moreover, how these pathways are affected during VOE or after curative or disease-modifying therapies has not been evaluated yet.
While the currently available disease-modifying therapies, such as hydroxyurea and voxelotor, primarily exert their effect through reduction of haemolysis, their ameliorating effects may also be mediated by downstream suppression of inflammatory pathways. As chronic haemolysis and ischaemia–reperfusion injury are the main drivers of inflammation and angiogenesis in SCD, therapies targeting haemolysis (e.g. hydroxyurea, voxelotor or cure by allogeneic haematopoietic stem-cell transplantation [HSCT]) may ameliorate the activation of pro-inflammatory and pro-angiogenic pathways.
In this study, we aimed to explore the inflammatory and angiogenic pathways in SCD by measuring a large targeted panel of plasma proteins involved in inflammation and angiogenesis, both in steady state and during VOE. We also investigated the impact of three different therapies: hydroxyurea, voxelotor and HSCT, on the plasma levels of the different targets of this panel. The results of this study might contribute to a better understanding of the activated inflammatory and angiogenic pathways in SCD pathophysiology and the downstream effects of currently available treatments for SCD. Furthermore, these new insights may lead to the identification of potential new targets for therapeutic interventions in SCD.
METHODS
Study population
In this prospective study, ethylenediaminetetraacetic acid (EDTA) plasma was collected from SCD patients with the more severe genotype of sickle cell anaemia (HbSS) and HbS-β0 thalassaemia (HbSβ0). Blood was drawn during steady state in all patients, and in a subset of patients also within 48 h after hospital admission for VOE. Steady state was defined as a clinically stable period with no signs of an ongoing VOE. To be included in the steady-state group, patients were required to not have experienced a VOE in the preceding 4 weeks. In a subgroup of patients, we collected paired samples before and after the initiation of treatment with either hydroxyurea, voxelotor or HSCT. For patients included in the hydroxyurea and voxelotor arm, blood sampling was performed before initiation of the treatment and after at least 3 months of treatment. In the HSCT arm, blood samples were taken at baseline and approximately 1 year after transplantation. Patients included in the HSCT cohort were still treated with post-transplant immunosuppression (sirolimus) at the time of the follow-up blood draw. Ethnicity-, sex- and age-matched healthy controls (HCs) were included for comparison. Participants were considered a match if at least one parent originated from an SCD-endemic region. This study was approved by the Institutional Review Board of Amsterdam University Medical Center and performed in accordance with the Declaration of Helsinki 2013. Written informed consent was obtained from all participants before blood collection.
Experimental analysis
An EDTA tube containing whole blood (6 mL, K3EDTA; Greiner Bio-One) was centrifuged at 2000 g for 10 min at 4°C within 1 h after sampling. Collected plasma was stored at −80°C until further use. Proteins were measured in 1 μL plasma using the Olink® Target 96 Immuno Oncology panel (Olink Proteomics AB, Uppsala, Sweden) according to the manufacturer's instructions. The Proximity Extension Assay (PEA) technology used for the Olink protocol has been described previously and enables 92 analytes to be analysed simultaneously.25 In brief, pairs of oligonucleotide-labelled antibody probes bind to their targeted protein, and if the two probes are brought in close proximity, the oligonucleotides will hybridise in a pair-wise manner. The addition of a deoxyribonucleic acid (DNA) polymerase leads to a proximity-dependent DNA polymerisation event, generating a unique polymerase chain reaction (PCR) target sequence. The resulting DNA sequence is subsequently detected and quantified using a microfluidic real-time PCR instrument (Biomark HD, Fluidigm). In quantitative polymerase chain reaction (qPCR), the cycle threshold (Ct) represents the number of cycles that is required to replicate enough DNA to be detected. The Ct values generated by qPCR are translated into a relative quantification unit, Normalised Protein eXpression (NPX), which is an arbitrary unit on a log2-scale where a high value corresponds to a higher protein expression. A positive NPX difference (estimate) reflects an upregulation of protein expression and thus higher plasma levels and a negative estimate reflects a downregulation of protein expression and thus lower plasma levels. All assay validation data (detection limits, intra- and inter-assay precision data) are available on manufacturer's website (http://www.olink.com).
Statistical analyses
RStudio version 4.2.1 was used for data analysis and visualisation. Data are presented as mean ± standard deviation, or median and interquartile range (IQR) depending on the distribution of data. Differences between subgroups were tested using an unpaired or paired t-test as appropriate. t-test results were corrected for multiple testing using the Benjamini–Hochberg method. Results with a corrected p < 0.05 were considered significant. Volcano plots were used to visualise the differential expressed proteins as identified by a t-test. In these volcano plots, the significance is presented on the y-axis and the NPX difference between two groups (estimate) on the x-axis. Proteins of which the plasma levels were significantly different between the two compared groups after correction for multiple testing are shown in red. Pearson correlation was used to assess the correlations between protein levels and clinical laboratory test results in SCD patients in steady state.
RESULTS
Baseline characteristics
Plasma samples were collected from 57 SCD patients in steady state (median age 27 years [IQR 21–34]) and 13 HC (median age 29 [IQR 28–39]). From 15 SCD patients, plasma samples were collected both in steady state and during VOE (within 48 h after admission). In a subgroup of 23 patients, we collected paired samples before and after the initiation of treatment: hydroxyurea (n = 8), voxelotor (n = 10) and HSCT (n = 5) (Table 1). Changes in blood counts and haemolysis parameters after therapeutic interventions are described in Table 2. There were no significant differences in protein levels between SCD patients at baseline grouped by sex or hydroxyurea use, and there was no correlation between protein levels and age in steady-state patients.
| Control | SCD patients in steady state | VOE | Hydroxyurea | HSCT | Voxelotor | |
|---|---|---|---|---|---|---|
| Participants (n) | 13 | 57 | 15 | 8 | 5 | 10 |
| Gender (M/F) | 7/6 | 38/19 | 10/5 | 3/5 | 4/1 | 7/3 |
| Age median (IQR) | 29 (28–39) | 27 (21–34) | 27 (19–34) | 21 (19–32) | 27 (25–27) | 34 (31–44) |
| HbSS/HbSβ0 | - | 48/9 | - | - | - | - |
| Hydroxyurea use, n (%) | - | 25 (44%) | 4 (27%) | 0 (100%)a/8 (100%)b | 4 (80%)a/0 (0%)b | 3 (30%) |
- Abbreviations: HbSS/ HbSβ0, sickle cell anaemia/HbS-β0 thalassaemia; HSCT, haematopoietic stem-cell transplantation; IQR, interquartile range; SCD, sickle cell disease; VOE, vaso-occlusive episode.
- a Before initiation of treatment.
- b After initiation of treatment.
| Steady state (n = 57) | VOE (n = 15) | Hydroxyurea (n = 8) | HSCT (n = 5) | Voxelotor (n = 10) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |||
|
Haemoglobin (g/dL) median (IQR) |
8.4 (7.6–9.7) |
7.6 (6.9–9.2) |
8.2 (7.6–9.7) |
9.0a (8.9–10.0) |
9.7 (9.0–10.3) |
12.2a (11.4–13.1) |
7.6 (7.3–8.2) |
10.0a (8.9–11.5) |
|
HbF % median (IQR) |
7.5 (3.7–10.9) |
4.4 (2.6–6.2) |
7.7 (2.5–10.9) |
22.9a (6.2–27.2) |
10.2 (7.3–16.4) |
- | - | - |
|
MCV (fL) median (IQR) |
87 (77–97) |
89 (77–102) |
86 (74–94) |
104a (97–112) |
101 (93–103) |
84a (76–85) |
82 (75–88) |
74 (69–94) |
|
Reticulocytes (×109/L) median (IQR) |
260 (209–313) |
252 (204–291) |
215 (177–397) |
105a (67–213) |
336 (207–370) |
91a (83–139) |
252 (227–324) |
166a (130–225) |
|
Leukocyte count (×109/L) median (IQR) |
9.3 (7.5–10.7) |
15.2b (13.7–16.3) |
9.1 (7.5–11.3) |
6.1a (4.8–8.7) |
6.6 (5.8–6.7) |
2.8a (2.7–3.2) |
8.7 (7.5–11.4) |
7.2 (6.4–8.6) |
|
LDH (U/L) median (IQR) |
422 (337–582) |
520b (439–606) |
415 (376–577) |
316a (292–347) |
437 (402–473) |
257a (176–311) |
575 (421–663) |
314a (282–419) |
| Bilirubin total (μmol/L) median (IQR) |
53 (32–92) |
61 (29–65) |
63 (45–91) |
36a (28–37) |
27 (20–35) |
4.0a (4.0–5.0) |
59 (44–124) |
39a (17–65) |
- Abbreviations: HSCT, haematopoietic stem-cell transplantation; IQR, interquartile range; LDH, lactate dehydrogenase; MCV, mean corpuscular volume; SCD, sickle cell disease; VOE, vaso-occlusive episode.
- a Significantly different compared to before the intervention.
- b Significantly different compared to steady state.
Steady-state SCD versus HCs
Comparison of inflammatory protein levels between HC and SCD patients in steady state identified 50 out of 92 proteins that were differentially expressed, defined as a significant difference in NPX value (estimate) (Figure 1). These included cytokines (IL-6, IL-7, IL-8, IL-10, IL-18), chemokines (CCL3, CCL4, CCL17, CCL19, CCL23, CXCL9, CXCL10, CXCL11, MCP-4, MCP-2), growth factors and other proteins involved in angiogenesis (ANGPT2, HGF, ANGPT1, PDGF subunit B, VEGFA, EGF, FGF2, TIE2, PGF, LAP TGF-beta-1) and members of the tumour necrosis factor [TNF] superfamily (TNF, TNFRSF4, TNFRSF9, TNFSF14, tumour necrosis factor ligand superfamily member 6 [FASLG]). Furthermore, also significant differences in the plasma levels of granzymes (GZMA, GZMH, GZMB), soluble receptors and ligands (PD-L1, PDCD1, PD-L2, CD27, CD70, CD4, CD244, CD40-L, CD83, LAG3, CRTAM, NCR-1) and other immune modulators (LGALS9, ADA, HO-1, ARG1, CASP-8) were measured when comparing protein levels between HC and SCD patients in steady state. The proteins that significantly differed between HC and SCD patients in steady state could also be categorised into proteins involved in angiogenesis/tissue development (ANGPT1, ANGPT2, TIE2, HGF, FGF2, EGF, PGF, VEGFA, PDGF subunit B, LAP TGF-beta-1), the IL-18 signalling pathway (IL-6, IL-8, IL-10, IL-18, CCL3, CCL4, CCL19, CD83, FASLG, TNF), T-cell activation (CD4, CRTAM, TNFSF14, TNFRSF9, LAG3, CD27, PDCD1, GZMA, GZMH, FASLG) and natural killer (NK)-cell activation (CD244, NCR1, GZMB) (Figure 2). Of the 50 differentially expressed plasma proteins only levels of CD70, which is a member of the TNF superfamily expressed primarily on activated T-cells and B cells, were significantly lower. Plasma levels of the other 49 proteins were significantly higher in steady-state SCD patients compared to HCs.
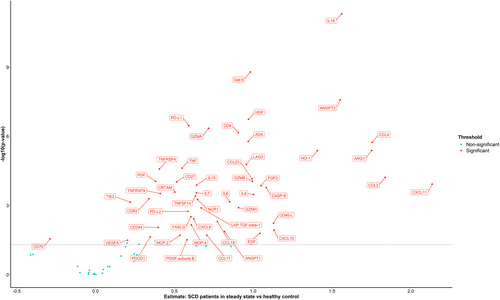
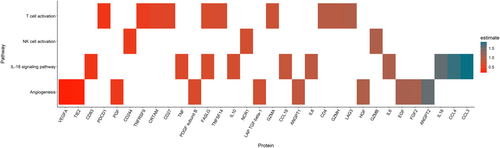
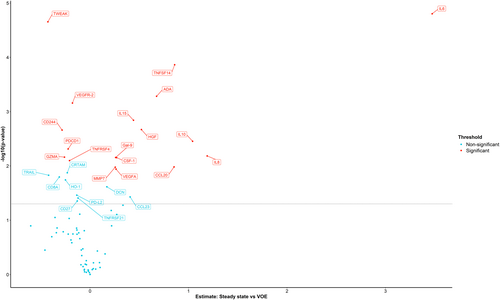
Steady state versus VOE
Pair-wise comparison of protein levels in 15 patients between steady state and VOE revealed 12 proteins (IL-6, TNFSF14, ADA, IL-15, HGF, IL-10, IL-8, LGALS9, CSF-1, CCL20, MMP7, VEGFA) that were significantly higher and six proteins (TWEAK, VEGFR-2, CD244, PDCD1, GZMA, TNFRSF4) that were significantly lower during VOE compared to steady state (Figure 3). Furthermore, tumour necrosis factor ligand superfamily member (FASLG) was significantly higher in SCD patients with two or more hospitalisations due to VOEs compared to those with less than two hospitalisations due to VOEs during the previous year (estimate: 0.39, confidence interval [CI]: 0.19–0.60).
Correlation analyses
In steady-state SCD patients, markers of haemolysis (low haemoglobin level, high reticulocyte count and high bilirubin and lactate dehydrogenase [LDH] levels) correlated with proteins involved in angiogenesis and tissue development (ANGPT2, VEGFA and PGF) and proteins involved in T-cell activation (CD4, LAG3). Plasma levels of LDH correlated with hepatocyte growth factor (HGF), a protein involved in tissue development and angiogenesis, proteins involved in T-cell activation (TNFRSF9 and PDCD1) and proteins involved in NK-cell activation (NCR1, GZMB). Plasma levels of proteins involved in tissue development and angiogenesis (HGF and PDGF subunit B) correlated positively with the leukocyte counts (Figure S4A–D).
Before and after therapeutic intervention
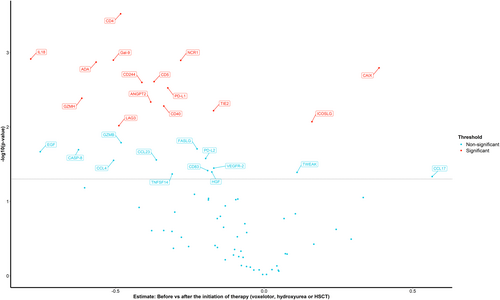
Comparison of protein levels before and after initiation of therapies which are known to reduce haemolysis (voxelotor, hydroxyurea and HSCT) showed that the plasma levels of IL-18, ANGPT2, TIE2, CD4, LAG3, GZMH, PD-L1, CD244, NCR1, ADA, CD40, CD5 and LGALS9 significantly decreased while on therapy (Figure 4). Except for CD5 and CD40, plasma levels of all the proteins that decreased significantly following therapy were significantly higher in steady-state SCD patients compared to HC. A separate comparison of protein levels before and after HSCT showed a decrease in proteins involved in the IL-18 signalling pathway (IL-18, CCL3, CCL4), angiogenesis (ANGPT2, TIE2, VEGFR-2), NK-cell activation (CD244) and T-cell activation (CD4, LAG3). However, these changes did not remain statistically significant after correcting for multiple testing. Furthermore, a trend towards a decrease in IL-18, NCR1, CD4, CD40, PD-L1, GZMH, LGALS1 and TRAIL was observed after initiation of voxelotor, and a trend towards a decrease in adenosine deaminase (ADA) was observed after initiation of hydroxyurea. The results of the comparisons of protein levels before and after the initiation of each therapy separately are shown in Figures S1–S3.
DISCUSSION
Using innovative highly sensitive multiplex PEA analysis of 92 inflammation- and angiogenesis-related proteins, we identified 50 plasma proteins that were differentially expressed in SCD patients in steady state as compared to ethnicity-matched HC. These included proteins involved in angiogenesis and tissue development, the IL-18 signalling pathway and NK-cell and T-cell activation. While proteins involved in angiogenesis and the IL-18 signalling pathway were further upregulated during VOE, levels of proteins involved in IL-18 pathway (IL-18), T-cell activation (CD4, LAG3, GZMH, PD-L1), NK-cell activation (CD244, NCR1) and angiogenesis (ANGPT2, TIE2) restored towards levels detected in HCs after curative or disease-modifying therapies. To our knowledge, this is the first study that comprehensively assessed the inflammatory and angiogenic protein profile in SCD patients in steady state, during VOE and following curative and disease-modifying therapies.
A total of 10 proteins involved in the regulation of angiogenesis and tissue development (ANGPT1, ANGPT2, HGF, FGF2, EGF, PGF, TIE2, VEGFA, PDGF subunit B, LAP TGF-beta-1) were significantly higher in plasma of steady-state SCD patients as compared to HC, suggesting a pro-angiogenic state in SCD patients in steady state with further increment during VOE.9, 19-24 While plasma levels of the angiogenesis markers PGF, VEGFA and HGF in steady-state SCD patients correlated with markers of haemolysis, cure by HSCT or treatment with hydroxyurea or voxelotor led to significant reduction in these markers, suggesting a significant role of haemolysis in the angiogenic process in SCD and its restoration by therapies targeting haemolysis.
The ANGPT-TIE2 pathway, one of the key pathways regulating angiogenesis, seems to be impaired in SCD patients. The ANGPT1/ANGPT2 ratio plays an essential role in vessel stability and regression.26 Decreased ANGPT1-TIE2 signalling impairs endothelial cell–cell junction integrity, whereas ANGPT2 signalling via endothelial integrins promotes actin stress fibres and pericyte dropout, leading to the formation of more leaky vessels.26 In the current study, the increase in ANGPT2 was more pronounced, resulting in a decreased ANGPT1/ANGPT2 NPX ratio. The lower ANGPT1/ANGPT2 ratio in SCD patients might lead to reduced ANGPT1-TIE2 signalling, thereby impairing vessel stability in these patients. Recently, increased ANGPT2 levels have been associated with a history of several SCD-related complications including dactylitis, acute chest syndrome, and VOE.27 The same study also reported a correlation between ANGPT2 levels and increased transcranial doppler velocities, which reflects an increased risk of developing stroke in children with SCD. The ANGPT-TIE2 pathway may also play a role in the development of retinopathy in SCD patients.28 In our cohort, the levels of ANGPT2 and TIE2 decreased in SCD patients following therapies that reduce haemolysis, suggesting that these therapies abrogate and potentially restore pathological angiogenesis towards the healthy situation.
We observed significantly higher plasma levels of ADA in steady-state SCD patients with further increments during VOE. ADA catalyses the degradation and salvage of adenosine, a multifunctional molecule playing a central role in the physiological response to hypoxia by promoting angiogenesis, vasodilatation and nitric oxide (NO) release from vascular endothelial cells and by reducing inflammation.29, 30 Since erythrocytes contain relatively high concentrations of ADA, chronic haemolysis is likely to be the main source of increased levels of ADA in SCD. However, the upregulation of ADA could also be an innate metabolic response to chronically elevated adenosine levels resulting from chronic hypoxia in SCD.31 In the current study, levels of ADA significantly decreased following therapies that resulted in reduction of haemolysis. Treatment of SCD mice with polyethylene glycol-modified adenosine deaminase (PEG-ADA), an already clinically available drug to treat ADA-deficient patients, was demonstrated to reduce HbS polymerisation, haemolysis and organ damage.32 Furthermore, incubation of erythrocytes of SCD patients with PEG-ADA significantly reduced the sickling of these cells in vitro.32, 33
In the current study, plasma levels of several proteins involved in the regulation of T-cell activation, including the plasma levels of soluble PDCD1 and LAG3 (sPDCD1 and sLAG3), were increased in steady-state SCD patients compared to HC. Although the definitive mechanisms of action of these soluble inhibitory immune checkpoints are still being explored, they seem to play an important immunomodulatory role in (chronic) inflammation, auto-immunity and cancer by competitively regulating the activation of their membrane-bound counterparts.34-37 In our cohort, plasma levels of LAG3 correlated positively with markers of haemolysis and plasma levels of PDCD1 correlated positively with LDH only. Previously, levels of sLAG3 and sPDCD1 have been associated with disease severity in COVID-19 patients, with higher levels of these proteins measured in severe and critical cases compared to mild and moderate cases.38 Together these results imply that levels of sLAG3 and sPDCD1 may also reflect disease severity in SCD.
In SCD, haemolysis and ischaemia–reperfusion can activate the NLRP3 inflammasome, leading to the production and secretion of IL-1β and IL-18.12-16 Steady-state SCD patients in our study had significantly higher plasma levels of IL-18. The upregulation of IL-18 in SCD patients has been described and associated with SCD-related cardiomyopathy and VOE previously.12, 17, 39 In our cohort, several other proteins involved in the IL-18 signalling pathway (IL-6, IL-8, IL-10, CCL3, CCL4, CCL19, FASLG, CD83, TNF) were significantly upregulated in SCD patients in steady state, with a further upregulation of IL-6, IL-8, IL-10 and VEGFA during VOE. While IL-6 is a multifunctional cytokine that plays a role in inflammation and haematopoiesis, IL-8 is a chemokine that attracts neutrophils to sites of inflammation.40, 41 High levels of both IL-6 and IL-8 have been associated with a poor clinical outcome in SCD.18 The role of the anti-inflammatory cytokine IL-10 in SCD is more complex and may have both protective and pathogenic effects.42 In this study, IL-6 was highly upregulated during VOE. Interestingly, recent studies also found increased IL-6 levels in sputum of SCD patients who suffered from acute chest syndrome, and several case reports have described the positive effects of the IL-6 receptor blocker tocilizumab in the treatment of posttransfusion haemolysis in SCD.43-45 The use of tocilizumab for the treatment of acute chest syndrome in SCD is currently investigated in a randomised controlled trial (NCT05640271). Our results suggest that it may also be interesting to investigate the use of tocilizumab for the treatment of VOE. Targeting the NLRP3 inflammasome pathway represents a potential therapeutic strategy for managing inflammation and improving outcomes in SCD patients. A randomised, placebo-controlled, double-blind trial of canakinumab, a monoclonal antibody targeting IL-1β showed promising results. Patients receiving canakinumab had shorter hospital stay and significant reduction in fatigue and absence from school or work.46 Unfortunately, the level of IL-1β was not part of the predefined panel of proteins measured in this study. In our cohort, the plasma level of IL-18 decreased significantly after initiation of treatments that reduce haemolysis in SCD (hydroxyurea, voxelotor and HSCT). Based on the findings of this study, future studies should evaluate a panel of selected proteins that are disease-specific. It would be interesting to further explore the potential of IL-1β, IL-18 or inflammasome inhibition for the treatment of SCD.
Increased plasma levels of CD244, NCR1 and GZMB in steady-state SCD patients suggest a role for NK-cells in SCD pathophysiology.47, 48 CD244 and NCR1 are cell surface receptors expressed primarily by NK-cells. While these receptors are mainly known for their activating functions, the role of (the soluble form of) these receptors under chronic inflammatory conditions such as SCD remains unknown. GZMB is an abundant protease in the cytosolic granules of NK-cells, and its levels are elevated in chronic inflammatory diseases, contributing to inflammation, vascular dysfunction and permeability and the release of growth factors from the extracellular matrix.49-51
Interestingly FASLG was significantly higher in SCD patients with ≥2 hospitalisations due to VOE during the previous year compared to those with <2 hospitalisation due to VOE during the previous year (estimate: 0.39, CI: 0.19–0.60). FASLG can be produced by activated T-cells and other immune cells and plays an important role in the induction of apoptosis by binding to FASLG. The upregulation of FASLG detected in patients with frequent VOEs may reflect increased induction of apoptosis of damaged (endothelial) cells in these patients.52 The exact role of FASLG in vaso-occlusion needs to be evaluated further.
Although our cohort of SCD patients in steady-state and during VOE were large enough to assure an adequate power to detect significant differences, the sample sizes of the treatment groups were smaller, precluding robust conclusions regarding the separate effects of voxelotor, hydroxyurea and HSCT on the various inflammatory and angiogenic pathways. For this reason, we combined these treatment cohorts to investigate the effects of therapies that reduce haemolysis in SCD on the plasma proteome. Furthermore, the comparison of protein levels before and after HSCT was limited by the fact that patients were still on sirolimus when the post-transplant samples were collected, which can potentially impact both inflammatory and angiogenic pathways. Larger prospective studies are needed to evaluate the effects of various therapies for SCD on various inflammatory and angiogenic proteins. Other disease-modifying therapies that might be interesting to evaluate are Crizanlizumab and l-glutamine. By inhibiting vaso-occlusion, the P-selectin inhibitor Crizanlizumab might reduce ischaemia–reperfusion damage and thereby both inflammatory and angiogenic proteins. The anti-oxidant l-glutamine might have anti-inflammatory properties that can potentially reduce the levels of the inflammatory proteins.53, 54
The circulating plasma proteins measured in this study may have multiple sources, which makes the interpretation of the results challenging. Furthermore, protein levels in this study were measured by relative quantification. Although relative differences between groups in our cohort were evident, we cannot draw conclusions regarding absolute protein levels and the clinical relevance of relative changes in protein levels. While enzyme-linked immunosorbent assay (ELISA) tends to be less specific and sensitive compared to PEA technology, clinical application of PEA technology is often limited by its costs and accessibility, making high-sensitivity ELISA an important alternative technique for measuring these inflammatory and angiogenic proteins.
In conclusion, the results of these proteomic analyses provide new insights into the pathways that regulate inflammation and angiogenesis in SCD, both during steady state and VOE. These findings might contribute to a better understanding of SCD pathophysiology and identifying potential new targets for therapeutic interventions in SCD patients. Our results warrant further in-depth investigation of the different activated pathways.
AUTHOR CONTRIBUTIONS
AEG and EN designed the study. AEG, KK, LAdL and EN gathered clinical data. LAdL analysed data. LAdL, TWK, KF, BJB, RvB and EN interpreted the data. LAdL and EN drafted the manuscript. All authors critically reviewed the manuscript for important intellectual content.
ACKNOWLEDGEMENTS
The authors extend their gratitude to all the patients and healthy individuals who participated in the study.
FUNDING INFORMATION
The authors declare no sources of funding.
CONFLICT OF INTEREST STATEMENT
BJB: Research funding: Novartis, Pfizer and BMS; Consultancy: Pfizer, Novo Nordisk, Vertex and BMS; Honoraria: Novo Nordisk and Sanofi. EN: Research funding: Novartis; Speaker's bureau: Vertex. KF: Research funding: SOBI, Novo Nordisk and CSL Behring; Consultancy: SOBI, Sanofi, Novo Nordisk and Roche; Membership on an entity's Board of Directors or advisory committees: ISTH SSC. For the remaining authors, no relevant conflicts of interest were declared.
ETHICS APPROVAL STATEMENT
This study was approved by the Institutional Review Board of Amsterdam UMC and performed in accordance with the Declaration of Helsinki 2013.
PATIENT CONSENT STATEMENT
Written informed consent was obtained from all patients included in this study.
Open Research
DATA AVAILABILITY STATEMENT
Data are available on request.