Frequent health care utilisation and avascular necrosis are associated with cannabis use in adults with sickle cell disease
Prior studies have identified high rates of cannabis use in people with sickle cell disease (SCD).1-3 However, prior studies were underpowered to identify if cannabis use was associated with any SCD-related comorbidities, especially comorbidities such as pulmonary complications or stroke, which have been associated with smoked cannabis use in the general population. To address this gap, we conducted a large cohort study to examine the association between patient demographics and comorbidities of SCD associated with cannabis use. We hypothesised that cannabis users would have more comorbidities related to pain, which may drive use. We had no a priori hypothesis as to whether use would correlate with rates of acute chest syndrome (ACS) or stroke as it has been shown that over half of cannabis users with SCD use infrequently (weekly, monthly, or less than monthly), which may mitigate this association.1
The study was conducted at the Adult Sickle Cell Center at Montefiore Medical Center in Bronx, NY after obtaining approval from the Institutional Review Board of the Albert Einstein College of Medicine. Inclusion criteria were: (i) SCD with any genotype, (ii) age ≥21 years, (iii) presented for routine outpatient SCD care during 8/2018–1/2019 period and (iv) able/willing to complete a survey in English or Spanish.
Patients completed a paper survey instrument created by the investigators (see Supplement S1) in English or Spanish. Patients completed surveys independently and then reviewed them with a clinician. Questions asked about demographics, comorbidities of SCD, frequency of Emergency Department (ED) use, hospital admissions, and opioid use. Reported demographics, SCD diagnosis and comorbidities, healthcare utilisation, hydroxycarbamide (hydroxyurea) and opioid use were all confirmed and corrected if necessary via a detailed electronic medical records (EMR) review performed by two clinicians after the survey was completed. Current cannabis users were defined as patients with self-reported use in the previous 30 days, prior users were defined as those with any self-reported use, non-users were defined as those who reported no previous use. No differentiation was made between illicit and medical cannabis. Patients were asked to identify their reason for cannabis use and preferred modality. Multiple answers were allowed in each category. Patients were classified as having frequent pain crisis if they reported experiencing pain crisis monthly or more. They were classified as having high healthcare utilisation if they reported three or more ED visits and/or hospital admissions during the prior 12 months or if three or more were present in the EMR.
Clinical characteristics were summarised as means for continuous variables and counts and percentages for categorical variables. Clinical characteristics of prior cannabis users were compared to non-users using the chi-square or Fisher’s exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Data were analysed using Statistical Analysis System (SAS) software, version 9.2 (SAS Institute Inc., Cary, NC, USA).
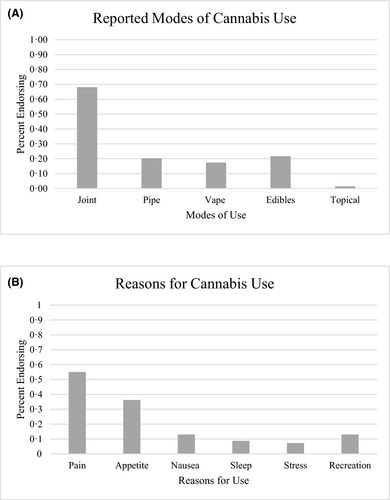
Of 224 patients meeting eligibility criteria, the mean age was 37·1 years, 53·6% were female, 66% were born in the United States, genotype was 75% HbSS/HbSB0 and 25% HbSC/HbSB+. Of the 224 patients, 69 (30·8%) were prior cannabis users, 43 (19·2%) were current users and 155 (69%) were non-users. The most common mode of use reported was smoking cannabis cigarettes (i.e. ‘joints’ or ‘blunts’) and the most common reason reported for use was pain treatment (Fig 1).
Prior cannabis users were more likely to be male (P = 0·04) and to have been born in the United States (P = 0·001) than non-users, there were no differences in age, genotype race, or hydroxycarbamide use. When SCD comorbidities were examined, prior users were more likely to have avascular necrosis (AVN) of the bone that non-users (P = 0·03), there were no differences in rates of ACS, stroke, venous thromboembolism, retinopathy, liver disease, pulmonary hypertension, leg ulcers and priapism, Finally, users were more likely to have frequent pain crisis (P = 0·04), to have high healthcare utilisation (P = 0·02), and to report home opioid use (P = 0·002) (Table I).
| Characteristic | Cannabis non-user | Cannabis user | P * |
|---|---|---|---|
| N | 155 | 69 | |
| Genotype, HbSS/Hbβ0, n (%) | 118 (76·1) | 49 (71·0) | 0·4 |
| Sex, Female, n (%) | 90 (58·1) | 30 (43·5) | 0·04 |
| Age, years, mean (SD) | 36·7 (12·2) | 38·0 (13·9) | 0·5 |
| Race, Black, n (%) | 113 (72·9) | 58 (84·1) | 0·07 |
| Birthplace, USA, n (%) | 80 (52·6) | 50 (75·8) | 0·001 |
| Retinopathy, n (%) | 39 (25·2) | 15 (21·7) | 0·6 |
| VTE, n (%) | 26 (16·8) | 15 (21·7) | 0·4 |
| Acute chest, n (%) | 117 (75·5) | 55 (79·7) | 0·5 |
| AVN, n (%) | 55 (35·5) | 35 (50·7) | 0·03 |
| Leg ulcer, n (%) | 38 (24·5) | 15 (21·7) | 0·7 |
| Priapism: male only, n (%) | 24 (36·9) | 16 (41·0) | 0·7 |
| Frequent pain crisis, n (%) | 98 (63·2) | 53 (76·8) | 0·04 |
| Frequent ED/admission, n (%) | 21 (15·2) | 19 (28·8) | 0·02 |
| Home opioid use, n (%) | 108 (71·1) | 62 (93·9) | 0·002 |
| Hydroxycarbamide use, n (%) | 117 (75·5) | 49 (71·0) | 0·5 |
- Bold indicates statistically significant values.
- AVN, avascular necrosis; ED, Emergency Department; VTE, venous thromboembolism.
- * Chi-squared test, Fisher’s exact test or t-test.
We found that pain-related outcomes such as AVN, frequent pain crisis, and home opioid use were associated with cannabis use, but cannabis use was not associated with ACS or stroke. While previous studies that examined cannabis use in people with SCD showed that it was common, none were able to detect significant differences in SCD related comorbidities between users and non-users.3 By examining a large cohort we were able to show that the presence of AVN was associated with cannabis use, a novel finding. This suggests that comorbidities related to pain may be associated with cannabis use but cannabis use may not negatively impact respiratory comorbidities such as ACS or central nervous system comorbidities such as stroke.
The present study has limitations. Given the cross-sectional nature of this and prior studies it is not possible determine if cannabis use causes pain-related complications, or if patients with pain are using cannabis for pain management. However, prior studies showed illicit cannabis users with SCD had fewer episodes of admission compared to non-users with a similar severity of pain, patients with SCD who obtained medical cannabis had a subsequent reduction in healthcare utilisation, and in a study of 5 days of inhaled cannabis or placebo for chronic pain in SCD, cannabis was associated with an increase in mood and no worsening of pain.4-7 In regards to AVN is it possible that the anti-inflammatory properties of cannabis may help in mitigating inflammatory pain in AVN.8 Therefore, we suggest pain-related outcomes drive cannabis use. Rates of cannabis use in our present cohort were lower than those reported in other studies, which may be because we defined use by self-report and had a large percentage of foreign born individuals in our cohort, the stigma associated with cannabis use in those countries could lead to low rates of use and/or of reporting use.1-3, 9-11 We did not obtain descriptions of pain quality experienced or date of first cannabis use, and reasons for cannabis use was by self-report. Further, all cause ED visits and admissions were examined, although we suspect the majority were due to acute pain exacerbation based on prior studies.
Our present study provides reassurance that while patients with SCD who have more pain-related comorbidities are more likely to use cannabis, users do not show increased rates of other SCD comorbidities. Prospective trials are needed to further describe the safety and efficacy of cannabinoids in patients with SCD.
Funding
Dr Curtis is supported by K23HL151884, Dr Cunningham is supported by K24DA036955, Dr Starrels is supported by K24DA046309, and Drs Starrels, Cunningham, and Arnsten are supported by R01DA044171.
Conflicts of interest
Drs Miodownik, Bradford, Cunningham, Arnsten, Choi, and Eisenberg report no conflicts of interest or competing interests. Dr Curtis declares consultancy for Global Blood Therapeutics. Dr Ogu declares Consultancy for Vertex Pharmaceuticals and Novo-Nordisk, Speaker Bureau for Global Blood Therapeutics. Dr Minniti declares Advisory roles to Novartis, Global Blood Therapeutics, Roche, Forma, Bluebird Bio, Emmaus Life Science, CSL- Behring, Chiesi, Novo-Nordisk and Research support from Global Blood Therapeutics and Bayer. Dr Starrels declares research support from the FDA-mandated Opioid Post-Marketing Requirement Consortium.
Author contributions
Conceptualisation: Drs Caterina P. Minniti, MD, Ugochi Olivia Ogu, MD, Hope Miodownik, MD, Christopher Bradford, MD; Methodology: Caterina P. Minniti, MD Ugochi Olivia Ogu, MD, Hope Miodownik, MD; Formal analysis and investigation: Hope Miodownik, MD, Christopher Bradford, MD, Jaeun Choi, PhD, Ruth Eisenberg, MS, Susanna Curtis MD PhD; Writing: Hope Miodownik, MD, Susanna Curtis, MD PhD, Christopher Bradford, MD; Writing - review and editing: Susana Curtis, MD PhD, Ugochi Olivia Ogu, MD, Caterina P. Minniti, MD, Joanna L. Starrels, MD, MS, Chinazo O. Cunningham, MD, MS, Julia H. Arnsten, MD, MPH; Supervision: Caterina P. Minniti, MD Ugochi Olivia Ogu, MD Susanna Curtis MD PhD.
Ethics approval
This study was performed in line principals of the Declaration of Helsinki and approval was granted by the Albert Einstein College of Medicine Institutional Review Board.
Consent to participate
Informed consented was not obtained as all information was anonymised.
Consent for publication
Consent for publication was not obtained as all information was anonymised.




