Successful prevention and screening strategies for COVID-19: focus on patients with haematologic diseases
Haematologic patients are immunocompromised and particularly susceptible to life-threatening viral infections.1 Regarding the worldwide outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the first case was diagnosed in Korea on January 20, 2020.2, 3 With COVID-19 spreading, the number of new COVID-19 cases had increased exponentially with a peak of 909 new infections on 29 February in Korea.4 The World Health Organization declared the COVID-19 pandemic on 11 March, and as of 6 May 2020 more than 3·5 million cases have been confirmed around the world.
In-hospital outbreaks of SARS-CoV-2 infection can have a major negative impact on providing essential medical services, and temporary hospital closures may be necessary to prevent further transmission.5, 6 The European Society for Blood and Marrow Transplantation recommends that, in this pandemic situation, non-urgent haematopoietic stem cell transplantation (HSCT) should be deferred if possible.7 However, if the medical use of HSCT in severely ill patients is restricted, there may be a worsening of their underlying diseases. Thus, appropriate screening strategies are needed for triaging patients to block the influx and nosocomial spread of COVID-19 while continuing to provide essential medical services for haematologic patients.8, 9
Seoul St. Mary’s hospital, which serves as a national referral hospital, has 1 365 beds. Our haematology hospital, which is part of Seoul St. Mary’s Hospital, is the largest medical institute for haematologic patients in Korea. We have four buildings in use: the main hospital, and an annex, college, and research institute (Fig 1). The main hospital contains all the facilities, including outpatient clinics, imaging departments, a clinical laboratory, a stem cell processing facility, and about 250 beds, for haematologic patients.
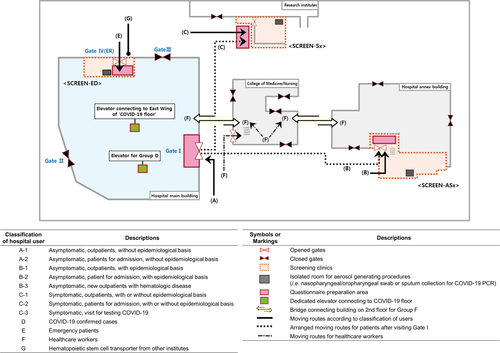
We classified hospital users based on their symptoms, potential epidemiological risk factors, and the purpose of their hospital visit, and applied stringent moving route control within the hospital according to their classification. All outpatients, inpatients, and medical staff were grouped according to the purpose of their hospital visit using a questionnaire (Fig 1). The questionnaire (Data S1) was created and updated based on the national and global epidemic information.10
Gates
We changed patient flow and hospital services as follows: Gates II and III of the main hospital were closed. All visitors should enter through Gate I, except for patients needing emergency care, who entered through Gate IV. All individuals were required to state whether they had COVID-19-related symptoms and/or epidemiological risk factors by answering the questionnaire. At Gate I, security personnel measured visitors’ body temperature with a non-contact thermometer. Thermal imaging cameras were also installed in the main connecting passages and monitored by security staff.
New screening clinics, patient flow, and the ‘COVID-19 floor’
We set up three screening clinics (SCREENs) in separate areas: (i) for asymptomatic but at risk patients (SCREEN-ASx) in the annex; (ii) for symptomatic patients (SCREEN-Sx) in the research institute; and (iii) for critical patients in the emergency department (SCREEN-ED). Each SCREEN had a separate space to collect respiratory specimens. To avoid unnecessary visits to the main hospital, these clinics provided oral prescriptions as well as laboratory/imaging tests. We also provided other forms of alternative clinical services such as tele-clinics and clinic visits by the guardian without the patient. SCREEN-ASx also had beds to provide simple procedures including transfusion, administration of granulocyte colony-stimulating factor, and indwelling-catheter care to haematologic patients. The patient flow according to group is shown in Fig 1.
We remodelled the entire floor of the hospital (hereafter the ‘COVID-19 floor’, Figure S1) dividing it into three spaces: (i) intensive care unit for critically ill COVID-19 (West Wing) patients; (ii) buffer rooms for asymptomatic patients with epidemiological risk factors (East Wing–Zone A); and (iii) buffer rooms for patients with pneumonia who required further monitoring of signs related to COVID-19 (East Wing–Zone B). The heating/ventilation/air conditioning system of the COVID-19 floor was separated from that of the other floors. All patients’ rooms were set to negative pressure. For neutropenic patients, an anteroom is kept at negative pressure, and the room where the patient stays is set for positive pressure. Healthcare workers (HCWs) routinely reported their body temperature and any symptoms by a web-based system. HCWs used a separate route (F) to access the main hospital (Fig 1).
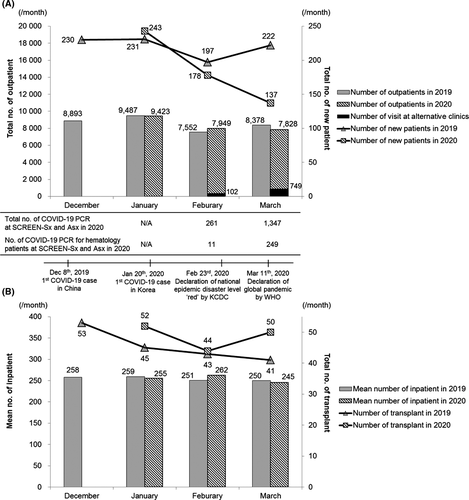
Until 24 April 2020, seven critically ill COVID-19 patients confirmed from outside were hospitalized in the West Wing of the COVID-19 floor, and there were four newly diagnosed COVID-19 patients in SCREEN-Sx and SCREEN-ED. There have been no cases of nosocomial onset or spread of COVID-19 in our hospital to date. The proportion of haematologic patients using alternative clinics increased from 1·3% in February to 9·6% in March 2020. In March 2020, we provided alternative consultations by tele-clinics (n = 194) and the guardian without the patient (n = 68) as well as SCREENs (n = 487). We performed 260 SARS-CoV-2 PCR tests for haematologic patients in February and March 2020. Among the haematologic patients, 1·3% were admitted to the East Wing of the COVID-19 floor. Despite the decreasing number of new haematologic patients over the course of the COVID-19 epidemic, the number of outpatient visit, mean number of inpatients each month, and the number of HSCT per month were comparable to those in the corresponding months of 2019 preceding the COVID-19 epidemic (Fig 2).
Several factors may have contributed to the successful prevention of in-hospital COVID-19 transmission without interruption of all treatments for haematologic patients: First, a screening questionnaire and measuring the body temperature were introduced at the hospital entrance and in each clinic. Second, patient groups and their moving routes were rigorously controlled. Third, SCREENs were housed in different buildings so that patients at risk were screened without entering the main hospital. Fourth, symptomatic patients were screened at SCREEN-Sx before entering the outpatient clinic area or hospitalization, and asymptomatic patients at epidemiological risk were also screened at SCREEN-ASx for hospitalization. However, the current mass-screening strategy is labour-intensive and requires dedicated cooperation from employees as well as visitors.
We have maintained our medical service for haematologic patients using the aforementioned systematic approaches. These could be valuable to avoid unnecessary scare about continuing treatments for immunocompromised patients. We hope that our experience may contribute to rapid ending of the COVID-19 pandemic.
Acknowledgements
We acknowledge the administrative support of Seoul St. Mary’s Hospital and the efforts of all the hospital employees. We also appreciate the excellent care of the patients provided by all the members of Catholic Hematology Hospital. We sincerely appreciate the support of all the families of the infection control team of Seoul St. Mary’s Hospital.
Author contributions
S-YC and S-SP contributed the conception and design of the study, and participated in data interpretation and drafting the article. D-GL and D-WK conceived the idea and planned the project, analyzed data, and revised the manuscript critically. J-YL, Y-JK, H-JK, C-KM, and BC reviewed and revised the paper. S-YC and S-SP contributed equally to this work. D-GL and D-WK contributed equally to this work.
Conflicts of interest
None of the authors have any conflicts of interest to report related to this work.
Ethical statement
The Institutional Review Board of Seoul St. Mary’s Hospital approved the research protocol and waived the need for informed consent due to the anonymous and retrospective design of the study (KC20RISI0273).




