Interleukin 6-blockade treatment for severe COVID-19 in two patients with multiple myeloma
Coronavirus disease 2019 (COVID-19) presents with a broad clinical spectrum, varying from asymptomatic infection to severe pneumonitis, leading to acute respiratory distress syndrome and death.1 Accumulating evidence suggests that in severe COVID-19, an acute hyperinflammatory syndrome characterised by fever, hypoxia and increased serum inflammatory markers, occurring 5–10 days from the first symptoms, is the major driver of morbidity and death.2 Hyperinflammation is not specific to COVID-19. Similar syndromes were previously described in respiratory disease associated with other coronaviruses, including the severe acute respiratory syndrome–coronavirus (SARS-CoV) in 2003 and Middle East respiratory syndrome–coronavirus (MERS-CoV) in 2012.3, 4 However, hyperinflammation in COVID-19 is more severe and, although it primarily affects the lungs, it shares many features with the cytokine release syndrome (CRS) after chimeric antigen receptor (CAR) T-cell therapy in patients with B-cell acute lymphoblastic leukaemia and B-cell lymphomas.2, 5
Central to pathogenesis of the CRS-like syndrome in COVID-19 is the increased secretion of interleukin 6 (IL-6) by pathogenic CD4+ T cells, activated monocytes, macrophages and dendritic cells.2, 6 IL-6 is a pleotropic mediator of inflammatory signals, including induction of acute phase protein production, such as C-reactive protein (CRP), fibrinogen and certain complement members (C3 ad C5R). IL-6 also activates the vascular endothelium, induces production of pro-thrombotic mediators and orchestrates migration of activated monocytes and neutrophils to the infected lungs.7
Tocilizumab is a monoclonal antibody against the IL-6 receptor (IL-6R), approved by the USA Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of CRS after CAR-T-cell therapy.8 Tocilizumab engages both the soluble and membrane-bound IL-6R with complete IL-6 signalling blockade, resulting in rapid resolution of fever, respiratory distress and hypotension in CRS.9 A favourable safety profile further supports the rationale for its use in COVID-19.8, 10
In small series of patients with COVID-19, IL-6 blockade therapy with tocilizumab has been reported.11 Currently, several clinical trials are exploring cytokine blockade in severe COVID-19; however, patients with myeloma or other active malignancy are usually excluded.
We report treatment with tocilizumab in two patients with myeloma with confirmed COVID-19, who developed CRS-like syndrome and respiratory failure.
The first patient is a 66-year-old man with relapsing myeloma, on third-line lenalidomide-based therapy, admitted with a 3-day history of fever (39 °C), dry cough and hypoxia (oxygen saturation 93%). The chest X-ray was compatible with COVID-19. He deteriorated suddenly after 3 days with a ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) of 175 and respiratory rate of 36 breaths/min, and was deemed not to be a candidate for intensive care unit (ICU) admission. He received two doses of intravenous tocilizumab 800 mg given 12 h apart, with rapid clinical improvement, resolution of fever and reduction of oxygen requirements to minimal support after 24 h. Gradual decrease of inflammatory markers levels, including CRP and serum ferritin ensued Fig 1. He was discharged from hospital after 8 days.
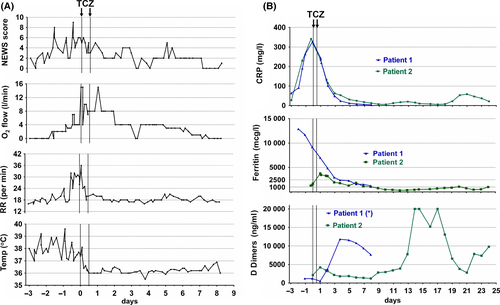
The second patient, a 59-year-old man with relapsing myeloma on fourth-line therapy with bortezomib and panobinostat, was urgently admitted after a history of COVID-19 symptoms for 7 days and rapid deterioration within 24 h with fever 38.3 °C, hypoxia with a PaO2/FiO2 of 126 and respiratory rate of 38 breaths/min. The patient was intubated and ventilated and received tocilizumab as above, on admission to the ICU. There was rapid resolution of inflammatory markers Fig 1, but slower improvement of the respiratory function. The patient received a tracheostomy after mechanical ventilation for 24 days and remains in ICU with reducing organ support requirements.
Respiratory failure and mortality in COVID-19 cluster in older age and comorbid groups.1, 12 Patients with myeloma are usually older with complex humoral and cellular immunodeficiency and comprise a particularly vulnerable population.13 Paradoxically, this group is increasingly finding themselves in a precarious position during the COVID-19 epidemic: services are shifted away from their care and for the first time, treatments are modified for reasons other than to improve outcome.14 They are further disadvantaged because many clinical trials for COVID-19 exclude patients with cancer.
Our present report suggests that patients with myeloma can be successfully supported for severe COVID-19 and respond to biological therapies. Early intervention with IL-6 blockade may prevent progression to critical disease and escalation to invasive ventilation. Patients with cancer should not face the double disadvantage from adjustments in their usual care and an unjustified ceiling in COVID-19 therapy. Instead, inclusion to clinical trials with active treatment arms or liberal compassionate use of promising treatments should be encouraged.




