Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily
Recent evidence suggests that signs and symptoms of severe COVID-19 infection resemble more the pathophysiology and phenotype of complement-mediated thrombotic microangiopathies (TMA), rather than sepsis-induced coagulopathy or disseminated intravascular coagulation (DIC).1, 2 Since effective treatment is available for complement-mediated TMA,3 we aim to systematically describe relevant features (clinical phenotype, pathophysiology and management) in patients with severe COVID-19 infection.
Clinical phenotype of complement-mediated TMA
TMA is characterized by microangiopathic haemolytic anaemia, thrombocytopenia and organ damage, such as neurological, renal and cardiac dysfunction. Patients described by Zhang et al. presented with anaemia, increased lactate dehydrogenase (LDH), thrombocytopenia and organ damage (neurological in all patients and cardiac in one).2 Catastrophic antiphospholid syndrome (CAPS) is a plausible diagnosis that could not be confirmed, because of the requirement to repeat testing in 12 weeks. Even in this case, the clinical presentation, pathophysiology, and management that will be described below, would also match those of complement-mediated TMA.4
Indeed, increased LDH and thrombocytopenia have been reported in COVID-19 infection. Unfortunately, there are no reports of schistocytes to confirm microangiopathic haemolytic anaemia, probably due to the urgency of the pandemic and everyday difficulties in organizing sample transfer. Similarly, little is known on the nature of cardiac dysfunction in COVID-19. Myocarditis or inflammatory-induced cardiac damage have been postulated, but pathophysiological evidence points towards microvascular thrombosis, similar to that of TMA.1 Lastly, renal dysfunction, another characteristic of complement-mediated TMA, is also common in patients with severe COVID-19 infection.5
Pathophysiology of complement-mediated TMA
Complement activation plays a central role in the pathophysiology of TMAs. Complement-mediated TMAs are described by a two-hit disease model that applies for atypical haemolytic uraemic syndrome (aHUS), CAPS, transplant-associated thrombotic microangiopathy (TA-TMA) and HELLP (haemolysis, elevated liver enzyme and low platelet count) syndrome.6 The first hit results from germline mutations or acquired alterations in complement regulatory proteins or C3 convertase components. Commonly associated triggers of the second hit are pregnancy, inflammation, surgery or autoimmunity.3
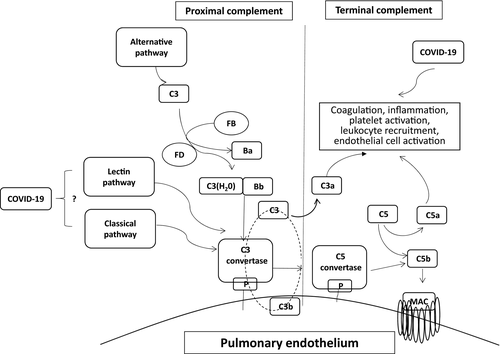
The complement system is part of the immune system providing innate defence against microbes and mediating inflammatory responses. Except for inflammation, a link also exists between the complement system and platelet activation, leukocyte recruitment, endothelial cell activation and coagulation (Fig 1). Briefly, proximal complement is activated through the alternative, classical, and lectin pathways. The three pathways lead to C3 activation and C3 convertase formation. C3 activation through the alternative pathway of complement amplifies this response, culminating in pronounced C3 fragment deposition on target cells. In the presence of increased surface density of deposited C3b, the terminal (lytic) pathway is triggered, leading to C5b-9 or membrane attack complex (MAC) formation on the surface of target cells.3
A common denominator of complement-mediated effects in TMAs is endothelial dysfunction and microvascular thrombosis. Recent evidence suggests that the same is true also for COVID-19 infection. Indeed, pericytes with high expression of angiotensin-converting enzyme 2 (ACE2) are target cells of COVID-19 resulting in endothelial cell and microvascular dysfunction. Since heart-failure patients have increased ACE2 expression, they are expected to be of high risk of cardiac injury due to COVID-19.7 Similarly, ACE2 is highly expressed on podocytes and tubule epithelial cells of the kidney, whereas a recent study has suggested viral tropism for the kidney.8
Furthermore, studies of previous coronaviruses have shown that blocking C3 activation significantly attenuates the lung-directed proinflammatory sequelae of infections. Both the genetic absence of C3 and blockade of downstream complement effectors have shown therapeutic promise by containing the detrimental proinflammatory consequences of viral spread mainly via inhibition of monocyte/neutrophil activation and immune cell infiltration into the lungs.9, 10 A recent study also revealed that coronaviruses’ (SARS-CoV, MERS-CoV and SARS-CoV-2) proteins bind to a key protein of the lectin pathway [MASP-2 (Mannan-binding lectin serine protease 2)], leading to complement-mediated inflammatory lung injury.11 In addition, Magro et al. have recently shown deposits of C5b-9, C4d, and MAPS-2 in the microvasculature of lung and skin biopsies of severe COVID-19 patients.12 These data are consistent with excessive activation of both the alternative and the lectin pathway of complement.
Taken together, these data suggest that the 'two-hit' hypothesis needs to be further investigated in patients with severe COVID-19 infections. Inflammatory states, such as diabetes and obesity, also activate complement and thus may exacerbate complement-mediated injury.13 Patients with severe lung injury may be the most likely to have a germline predisposition.
Management of TMA
There are two FDA-approved complement inhibitors: eculizumab (2007) and ravulizumab (2019). Both have been approved for the treatment of paroxysmal nocturnal haemoglobinuria (PNH), the disease model of complement activation, and have been also studied in TMAs.3 These monoclonal antibodies bind C5 and sterically hinder cleavage of C5 by the C5 convertase, blocking the generation of the proinflammatory C5a molecule and MAC formation (Fig 1).
Terminal complement inhibition with eculizumab has shown long-term efficacy and safety in complement-mediated TMAs. Off-label use has also been reported in COVID-19. Diurno et al. treated four severe COVID-19 patients with eculizumab. They observed an immediate reduction of C-reactive protein levels and clinical improvement leading to successful disease outcomes.14 Given the promising preclinical data and the severity of COVID-19 infections, eculizumab is currently studied in patients with severe COVID-19 infections (ClinicalTrials.gov Identifier: NCT04288713). In contrast to frequent intravenous infusions every two weeks that are required for eculizumab treatment, ravulizumab has the advantage of fourfold longer half-life. Using ravulizumab, a single intravenous dose should be sufficient in patients with COVID-19 infections.
Since C3 and the lectin pathway have been implicated in the pathophysiology of coronavirus infections, inhibitors of proximal complement pathways, under clinical development for complement-mediated TMAs, could also be efficacious in COVID-19 infections. The first case report of C3 inhibition with the compstatin-based inhibitor AMY-101 has shown safety and efficacy in severe COVID-19.15 Clinical data are needed to provide novel insights in these patients. Although gathered experience suggests a risk only for Neisserial infections with complement inhibitors, safety needs to be confirmed in COVID-19 patients also.
Conclusions and future perspectives
These data suggest that severe COVID-19 infections could be considered through the prism of a TMA. Functional complement assays to select patients that would benefit from complement inhibition need to be further explored in patients meeting clinical criteria. Both C3 and C5 inhibitors have shown early promising results.16 Importantly, there is an unmet clinical need of larger prospective trials using complement inhibitors in patients with severe COVID-19 infections. Results from clinical studies are eagerly expected to address this unprecedented morbidity and mortality rate with detrimental effects on every aspect of the global future.
Acknowledgements
Given the limited number of references allowed in this perspective, the authors thank colleagues who are not specifically cited for their contribution and their understanding.
Funding information
EG is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning 2014–2020» (MIS 5033021), enabled through the State Scholarships Foundation. RAB receives funding support from NIH/NHLBI R01HL133113.
Conflicts of interest
EG declares to have no potential conflicts of interest regarding the present work. RAB is a member of the scientific advisory board for and receives grant funding from Alexion Pharmaceuticals, Inc.
Author contributions
EG drafted and edited the manuscript. RAB edited and approved the final manuscript.